1. Introduction
Nature has always been a source of inspiration for humanity to solve all hurdles it has faced. This continues to be the case, with biomimetics appearing as a multi-disciplinary strategy that looks upon biologic structures and processes, with the objective of creating new materials or technologies that mimic those found in the biologic world [1], [2].
Currently, biomimetic approaches have been used in the biomedical area to develop materials with enhanced physical and mechanical properties, bioactivity, biofunctionalization and overall improved performance. This approach relies on the identification of problems that current materials have, research on the ways that nature has solved those issues and mimic those natural solutions as to create new better materials [1], [2].
One of the key problems that current structural synthetic biomaterials possess is low mechanical performance and stability. In fact, ceramic materials are generally too brittle [3], and biodegradable polymers tend to quickly lose their mechanical strength and resistance [4]. In both cases, the result can be the premature failure of the material. One solution for both problems can pass through the development of ceramic-polymer composites. Ideally, this would result in materials with higher toughness when compared to regular ceramics, and higher mechanical strength and resistance for biodegradable polymers [5], [6].
Nature has already developed a ceramic-polymer composite that shows those same desired properties [7], [8]. Nacre is a layered structure that naturally appears in the shell of several molluscs composed by aragonite tiles connected by an organic matrix, and is responsible for providing the shell with increased toughening, by allowing the dissipation of mechanical energy. It is the combination of its composition and of its layered and hierarchical structure that provides nacre with its outstanding features [9], [10].
Several different strategies have been proposed to develop nacre-like composites, using different processing techniques [6], [10]. Among these techniques, layer-by-layer (LbL) deposition appears as one of the most appealing due to its ability to create nanostructured layered structures using a wide range of different materials, with thickness controlled at the nanoscale level [6], [11], [12]. This review will focus on the identification of the different structural features and properties on nacre that should be considered in the design and production of nacre-inspired nanocomposites using the LbL deposition technique. Furthermore, we overview the potential of using such materials in the biomedical field.
2. Nacre
2.1. Definition and structure
In most molluscs of the bivalve and gastropods classes, the shell is composed by three main layers: the periostractum (outer layer, composed of hardened protein), the prismatic (middle layer, composed of columnar calcite) and nacre (inner layer, composed by aragonite and organic material). While the exterior layers are brittle and hard, providing the shell with resistance to penetration from external impact, nacre provides toughening, by allowing the dissipation of the mechanical energy [9], [13].
Nacre is composed of 95 wt.% of aragonite, a crystallographic form of CaCO3, and 5% (wt) of organic materials, such as proteins and polysaccharides. This organic matrix plays an important role in spatial and chemical control of the crystal nucleation and growth, microstructure and toughness enhancement [9], [10]. As an example, the presence of organic material in nacre provides an increase on fracture toughness up to 9 times of that of monolithic aragonite (3.3–9 MPa m1/2 and 1 MPa m1/2, respectively) [9], [14], [15].
In the broader sense, nacre is composed by a layered structure of aragonite tiles connected through an organic matrix, as shown in Fig. 1 A. Specifically, nacre is in fact composed by a hierarchy of structures that range from the nano to the macro-scale, similar to what is observed in other structural tissues [9], [10], [16]. Nevertheless, it is possible to distinguish two different nacre types, based on the organization of those structures. Columnar nacre, found in gastropods, has similar-sized tiles packed up concentrically, while sheet nacre, found in bivalves, consists of tiles stacked in a “brick wall” pattern. In columnar nacre, it is also important to distinguish the core areas (where tiles of the same column meet) and the overlap areas (were tiles from different columns overlap) due to the different stresses experienced on these areas [9], [16].
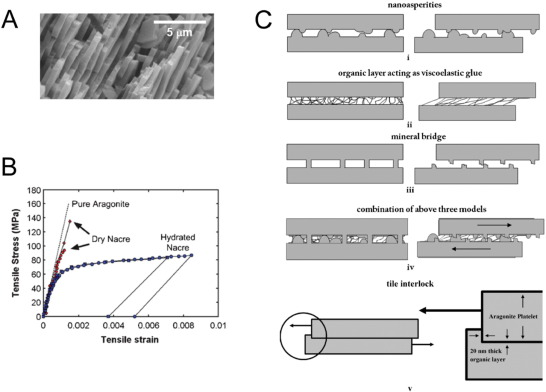
 Fig. 1. A – SEM micrograph of the fracture surface of nacre. Adapted from [7]; B - Tensile-strain curve for red abalone nacre and schematic showing the associated deformation mode. Adapted from [9]; C - Different models for sliding between tiles. (i) inter-tile layer formed by asperities, (ii) organic layer acting as a viscoelastic glue, (iii) mineral bridges, (iv) combination of the three mechanisms, (v) schematic showing the mechanism of loading through a cross-section cut across tiles and interlocks. Adapted from [17], [18].
Fig. 1. A – SEM micrograph of the fracture surface of nacre. Adapted from [7]; B - Tensile-strain curve for red abalone nacre and schematic showing the associated deformation mode. Adapted from [9]; C - Different models for sliding between tiles. (i) inter-tile layer formed by asperities, (ii) organic layer acting as a viscoelastic glue, (iii) mineral bridges, (iv) combination of the three mechanisms, (v) schematic showing the mechanism of loading through a cross-section cut across tiles and interlocks. Adapted from [17], [18].The building blocks of nacre are polygonal aragonite nanograins that are connected by biologic polymers, forming aragonite tiles or tablets. When these tiles are put under mechanical deformation, the nanograins suffer rotation and deformation, which allows for energy dissipation. This in return provides the aragonite tiles a ductile nature that is relevant for nacre's high fracture toughness [9].
In both nacre types it is also possible to observe mineral bridges connecting different tiles. These bridges, which protrude through the organic matrix, not only allows continued mineralization on the organic layers, but also improve their mechanical properties and prevent crack extension on nacre [9].
2.2. Mechanical properties
2.2.1. Micro-mechanical properties
Due to its structure, nacre behaves differently according to the direction of the applied compressive or tensile load.
While compressive and tensile stress values vary with the different existent nacres, in a general way nacre's compressive strength is higher when exerting perpendicular loads, while its tensile strength is higher when parallel loads are applied. Compressive strengths are also generally larger than tensile strengths. These results were obtained from sample cubes with 5 mm sides, cut from nacre of red abalone shells [9], [19], [20]. A similar compressive behaviour could also be observed in nacre-inspired composites produced by freeze casting, on sample with dimensions of 5 mm × 5 mm × 10 mm [21].
It is important to note that nacre behaves differently when under dry or hydrated conditions. Fig. 1 B represents a typical tensile behaviour of pure aragonite, dry nacre and hydrated nacre. As it is possible to observe, the tensile behaviour of dry nacre is more akin to that of pure aragonite, acting as a brittle material. In contrast, hydrated nacre shows a ductile behaviour. These results evidence not only that water is important for nacre's ductile behaviour, but also demonstrates that the organic materials are essential for nacre's properties, even if they only represent 5% of its composition. Such influence of water can also be observed in other calcium carbonate organizations such as crossed-lamellar sea shells [22]. After analysing this behaviour, Jackson et al. [14]concluded that water plasticizes the organic matrix, which affects the elastic modulus and tensile strength by reducing the matrix's modulus and shear strength, resulting in greater crack blunting and deflection abilities. This in turn results in a material with higher toughness.
2.2.2. Nano-mechanical properties
As nacre is composed by arrays of aragonite nanotiles, it is also important to understand the mechanical behaviour of nacre at the nanoscale. In 2006, Mohanty et al. showed that the aragonite tiles are viscoelastic in nature and can absorb energy [9], [23].
Dynamic nanomechanical tests were performed in both tiles and the organic matrix of nacre. Again, results vary according to different nacres, but tiles were found to show a value of elastic modulus (E) varying from 60 to 80 GPa [24], which are similar to the E value of monolithic aragonite (81 GPa). The aragonite tiles also reveal extensive plastic deformation, seen by the appearance of a clear residual indent and surrounding pile-up. In contrast, in monolithic aragonite there is only the appearance of the residual indent, with no pile-ups forming. Regarding the organic phase on nacre, values of E vary between 2.85 and 15 GPa. These results suggest that both the softer organic matrix and the structure of aragonite tiles have an effect on the overall properties of nacre [9], [25]. Water also has an effect on the nanomechanical properties of the aragonite tiles evidenced by the presence of bigger and more blunted pile-ups, indicating a hardness reduction compared to dry tiles [9], [25].
2.2.3. Inter-tile toughening mechanism
Nacre is well known for its high toughness and as such, in order to synthesise nacre-like materials, it is important to understand the mechanisms that provide nacre with this outstanding feature. Earlier research focused mainly in the mechanisms mentioned by Currey, which were plastic deformation ahead of the crack tip, crack deflection, aragonite platelet pull-out and organic matrix bridging [26]. Sarikaya proposed tortuosity as the only toughening mechanisms present in nacre [27]. However, tortuosity by itself cannot provide nacre toughness in the magnitude order that it possesses. One of the most currently accepted models explaining nacre's high toughness proposes the combination of four mechanisms acting in a synergetic way [9]. This include aragonite asperities contact (Fig. 1 C - (i)), stiffening of biopolymer after elongation (Fig. 1C - (ii)), mineral bridge relocking after fracture (Fig. 1 C - (iii)) [18], and locking generated by the microscale waviness and dovetail of tiles (Fig. 1 C – (v)) [17].
While the aragonite asperities and mineral bridges are responsible for shear resistance, the elongation and consequent stiffening of biopolymer fibrils is responsible for load transfer across nacre's structure. When tensile loads are applied to nacre, inelastic deformation is about 1%, which represents aragonite tablets (diameter around 9 μm) sliding for about 100 nm in a homogenous fashion. After the initial sliding, strain hardening occurs at a local level. The enhanced stress is then transferred along the initial tablet to another one, which consequently slides generating another local strain hardening. These cause-effect occurrences are cumulative and spread parallel to the tablets until the material fails under platelet pull-out mode. This stiffening effect occurs in the biopolymer fraction connecting different tablets, but also in the biopolymers that are embedded in those same tablets, meaning that energy dissipation in nacre can be attributed to both inter and intra-platelet deformations working in a synergistic way [6], [9].
It is important to refer that nacre's brick-and-mortar microstructure has also evolved in order to maximize its toughness by minimizing the shear stress felt at the ends of overlapping areas between two platelets. In fact, considering the overall thickness of the platelets and the thickness of the organic layer between them, as well as the values of E for platelets and the shear modulus (G) of the organic layer, shear stress felt at the ends is theoretically minimum for an overlapping length of approximately 2.5 μm, a value that is reasonably close to values found in nature (approximately 1.6 μm) [28].
3. Techniques for the development of nacre-inspired nanocomposites
Due to its hierarchical structure, mimicking nacre is a more complex task when compared to other biologic structures. Overall, nacre-based composites lie in one of three categories [9]: (i) mimicking the organic-inorganic layered structure; (ii) mimicking its mineralization process; (iii) mimicking its 95 wt% inorganic and 5 wt% organic composition.
Focusing on the first category, research tends to follow on composite materials produced mostly by one of five techniques [6]: (i) conventional method for bulk ceramics; (ii) freezing casting; (iii) layer-by-layer (LbL) deposition; (iv) electrophoretic deposition; (v) mechanical assembly. Each one of these techniques has been successfully used to produce nacre-like structures. This review will focus specifically on nacre-like structures produced by LbL deposition. LbL deposition consists in the alternate dipping of a substrate in at least two different solutions, resulting in a layered deposition of the solutions components. This technique allows for a precise control of the obtained structure's thickness and interfaces and can be used to combine both organic and inorganic materials, which permits to obtain nacre-like structures (6, 11, 29).
This technique, schematized in Fig. 2, relies on the negative charges that many surfaces present when immersed in solutions due to surface oxidation and hydrolysis. As such, when immersed in solutions containing a positively charged polyelectrolyte (PE), that same polyelectrolyte will be adsorbed to the immersed substrate, turning its surface from negatively to positively charged. The same process can be repeated with a solution containing a polyelectrolyte with a negative charge, resulting on the deposition of a double polyelectrolyte layer. Repeating this bi-layer formation method one can produce multilayer films with the desirable structure and thickness controlled at the nanoscale [11], [12], [30].
 Fig. 2. Schematics for the LbL deposition technique: A - Steps 1 and 3 represent the immersion of the substrate in opposite charged polyelectrolytes. Steps 2 and 4 represent the washing steps. This 4 step cycle can be repeated as many times as intended, with each cycle representing the formation of one bilayer; B - Simplified representation of the process at a molecular level; C – Simplified representation of the process, when containing charged particles. Adapted from [30].
Fig. 2. Schematics for the LbL deposition technique: A - Steps 1 and 3 represent the immersion of the substrate in opposite charged polyelectrolytes. Steps 2 and 4 represent the washing steps. This 4 step cycle can be repeated as many times as intended, with each cycle representing the formation of one bilayer; B - Simplified representation of the process at a molecular level; C – Simplified representation of the process, when containing charged particles. Adapted from [30].The LbL processing method can use almost any types of charged species, namely biological macromolecules, nanoparticles (NPs), nanotubes, nanowires, nanoplates, inorganic molecular clusters, organic dyes, dendrimers, porphyrins, polypeptides, nucleic acids, proteins and virus. Fig. 2 C shows the particular case of intercalating a polymeric component and nanoparticles, one of the methods adopted to reproduce the layered morphology of nacre. LbL assembly is not limited to electrostatics interactions, as assemblies based on hydrogen-bonding, charge transfer, covalent bonding, biological recognition and hydrophobic interactions have been investigated with promising results [31]. As a result of this wide range of potential materials and forces that can be explored, the LbL technique has been used not only to produce films, but several different types of stable structures, with different functional properties [11], [29].
Due to the aforementioned advantages the use of LbL-produced structures, namely coatings and 3D devices, has been deeply studied for biomedical applications [32]. Not only is it possible to produce structures using biocompatible materials, but one can also easily integrate proteins, growth factors and drugs. These applications include fabrication of scaffolds, medical adhesives, stents and wound healing systems, and coatings for implants with complex geometries [11], [29], [32].
4. Nacre-inspired layer-by-layer nanocomposites
Due to the abundance of materials that can be processed through LbL deposition, different approaches were investigated in order to produce new materials and structures that are inspired by nacre, most of them resulting from the combination of polymeric materials (both biologic or synthetic), which assume the role of the organic matrix, and inorganic materials, used as a substitute for the aragonite tablets [6], [9], [11], [29]. Table 1 summarises some of the most recent developments in this area, which will be explored in higher detail in the next sub-chapters.
Table 1. Recent developments on nacre-inspired LbL nanocomposites.
| Strategy | Ref | Key aspects | Chalenges/Further investigation |
|---|---|---|---|
| Artificial nacre | [33] |
|
|
| [34] |
|
|
|
| [35] |
|
|
|
| [36] |
|
|
|
| Nano-clays | [37] |
|
|
| [40] |
|
|
|
| [41] |
|
[N/A] | |
| Metallic oxides and particles | [42] |
|
|
| [43] |
|
|
|
| [46] |
|
|
|
| Bioactive glass nanoparticles | [52] |
|
[N/A] |
| [53] |
|
|
4.1. Artificial nacre
As the inorganic phase of nacre is composed by aragonite, which is a crystallographic form of CaCO3, several different strategies were developed using this material, mainly for the production of artificial nacre.
In 2012, Finnemore et al. [33] developed a 5-step methodology for the development of artificial nacre, being as they claim, the first successful attempt to replicate nacre using CaCO3. These steps include: [1] LbL deposition of poly(acrylic acid) (PAA) and poly(4-vinyl pyridine) (PVP) onto a glass surface, [2] dissolution of PAA, leading to the formation of nanoporosity in the PVP layer, [3] stabilization of the PVP layer by UV-cross linking, followed by functionalization with COO− groups by immersion in PAA, as to promote mineral deposition, [4] deposition of amorphous CaCO3 (ACC) precursor phase by using the ammonium carbonate diffusion technique with a PAA-containing 1:5 Ca2 +:Mg2 + solution in a gas-permeable beaker to provide uniform distribution of CO2 in the solution , [5] CaCO3 crystallization by exposure to high humidity. These five steps can then be repeated several times in order to achieve the number of desired bi-layers. The result, as seen in Fig. 3, is the formation of a structure at the substrate's surface, which mimics the macro and micro morphologies of nacre [33].
 Fig. 3. Comparison of biogenic and artificial nacre: A - Macroscopic view of biogenic nacre (scale bar 5 mm); B - SEM image of a stack of nacre tablets (scale bar 2 μm); C - Organic inter-crystalline film which allows for vertical crystal continuity between tiles (scale bar 500 nm); D - Artificial nacre (scale bar 5 mm); E - SEM image of CaCO3 tablet separated by organic film (scale bar 1 μm); F - SEM image of PVP film on calcite showing pore distribution similar as in C (scale bar 300 nm); G - AFM height image of porous film (scale bar 300 nm); H - Cube corner indentation (imaged by AFM) caused plastic deformation in a 7-layer artificial nacre sample (scale bar 1 μm). Adapted from [33].
Fig. 3. Comparison of biogenic and artificial nacre: A - Macroscopic view of biogenic nacre (scale bar 5 mm); B - SEM image of a stack of nacre tablets (scale bar 2 μm); C - Organic inter-crystalline film which allows for vertical crystal continuity between tiles (scale bar 500 nm); D - Artificial nacre (scale bar 5 mm); E - SEM image of CaCO3 tablet separated by organic film (scale bar 1 μm); F - SEM image of PVP film on calcite showing pore distribution similar as in C (scale bar 300 nm); G - AFM height image of porous film (scale bar 300 nm); H - Cube corner indentation (imaged by AFM) caused plastic deformation in a 7-layer artificial nacre sample (scale bar 1 μm). Adapted from [33].Although artificial nacre did not possess an average plain strain modulus as high as natural nacre (38 GPa compared to 69 GPa, respectively), it was possible to reproduce nacre's high toughness. After cube corner indentation (Fig. 3 H), there was the appearance of pile-ups surrounding the residual indent, similar to those present in nacre and contrasting with pure crystalline CaCO3 [33].
Contrary to the work of Finnemore and co-workers, which allowed CaCO3 to crystallize, Huang et al. [34] used ACC to produce composite LbL films that mimic nacre structure. They used PAA to produce stabilized ACC particles with an average size of 180 ± 40 nm. The obtained particles were then used together with poly(diallydimethylammonium chloride) (PDDA) to produce several nacre-inspired LbL films with thicknesses ranging from 230 to 710 nm onto a glass substrate.
Another strategy for the production of composite films with CaCO3 was developed by Patel et al. [35] in 2015. Contrary to the previous works, in which CaCO3 was directly used during the formation of the composite films, Patel and co-workers produced fully polymeric LbL films (using poly(sodium 4-styrenesulfonate) (PSS), poly(allylamine hydrochloride) (PAH) and poly(diallyldimethylammonium chloride) (PDADMAC)), which were then chemically infiltrated with CaCO3 through alternately dipping the membranes in CaCO3 and Na2CO3 solutions. This results on the formation of CaCO3 particles inside the membranes, as it is observed in Fig. 4.
 Fig. 4. Cross-sectional SEM images of (PAH/PSS)20 films before (A) and after (B) 20 cycles of infiltration with calcium carbonate [35].
Fig. 4. Cross-sectional SEM images of (PAH/PSS)20 films before (A) and after (B) 20 cycles of infiltration with calcium carbonate [35].The mechanical properties of the produced films were measured through nanoindentation. It was found an increase in both elastic modulus (from 3.8 ± 0.3 GPa to 10.0 ± 0.3 GPa) and hardness (from 0.16 ± 0.01 GPa to 6.3 ± 0.3 GPa) after 20 cycles of CaCO3 infiltration [35].
The LbL technique was also used before to produce polymeric multilayers as templates for the local precipitation of calcium phosphate. This was achieved by repeated dipping organic coatings in Ca2 + and PO43 − solutions [36]. Such formulation could be particularly interesting to be used in the orthopaedic field.
4.2. Nacre-inspired structure
4.2.1. Nano-clays
The production of nacre-like structure is not limited to the use of different forms of CaCO3. As an alternative, nanoscaled clays can be used as an analogue to the inorganic phase of nacre.
In 2011, Han et al. [37] combined layered double hydroxides (LDH) tiles with poly(vinyl alcohol) (PVA) to produce free-standing LbL films with enhanced strength and ductility. LDH are a group of layered anionic clays which have already been used as additives to improve the mechanical, barrier and thermal properties of polymers and rubbers [38], [39]. The produced films presented a clearly layered structure, as shown in Fig. 5 A, similar to that of nacre. The addition of the LDH nanotiles had a mixed effect on the mechanical properties of PVA films. While it was observed a general increase on elastic modulus and yield stress values with the increase of LDH content on the membranes, there was also a decrease of elongation at break, elastic and inelastic deformations. However, all membranes presented a ductile behaviour similar to nacre, evidencing the success of the authors in creating materials which mimicked nacre's structure and properties [37].
 Fig. 5. A - Cross-sectional SEM image of the (LDH/PVA)300 film. Adapted from [37]; B - Stress-strain curves of the LbL nanocomposite films, composed by PVA(P), laponite (L) and HAPI [40]; C - Cross-sectional SEM images of a (PVA/MMT)130 film. Adapted from [41]; D - Cross-sectional TEM image of the PE/TiO2 film, where PE = (PEI/PSS)(PAH/PSS)3. Adapted from [42].
Fig. 5. A - Cross-sectional SEM image of the (LDH/PVA)300 film. Adapted from [37]; B - Stress-strain curves of the LbL nanocomposite films, composed by PVA(P), laponite (L) and HAPI [40]; C - Cross-sectional SEM images of a (PVA/MMT)130 film. Adapted from [41]; D - Cross-sectional TEM image of the PE/TiO2 film, where PE = (PEI/PSS)(PAH/PSS)3. Adapted from [42].
Multilayer nanocomposite films were developed using hydrophilic aliphatic polyisocyanate (HAPI), PVA and laponite, a clay that exfoliates easily in water [40]. One main difference between this strategy and all the previously mentioned is the use of covalent bonding forces between  NCO groups on HAPI chains and
NCO groups on HAPI chains and  OH groups on PVA and laponite, instead of electrostatic interactions. Again, similar to the work of Han et al., the produced films presented an increase on elastic modulus and yield stress when compared to pure PVA/laponite films and a decrease on maximum strain – see Fig. 5 B. However, compared to PVA/laponite films which were thermally treated, the films containing HAPI are shown to be more ductile, while maintaining maximum strain values of the same order [40].
OH groups on PVA and laponite, instead of electrostatic interactions. Again, similar to the work of Han et al., the produced films presented an increase on elastic modulus and yield stress when compared to pure PVA/laponite films and a decrease on maximum strain – see Fig. 5 B. However, compared to PVA/laponite films which were thermally treated, the films containing HAPI are shown to be more ductile, while maintaining maximum strain values of the same order [40].
Montmorillonite (MMT) nanotiles can also be used in the construction of multilayer films [41]. Fig. 5 C shows the resulting film obtained by the assembly of 130 bi-layers combining PVA and MMT. The presence of MMT was shown to have a positive effect in the mechanical properties, when compared to pure PVA films, increasing both elastic modulus (150.2 MPa compared to 85.1 MPa, respectively) and maximum load (11.2 GPa compared to 1.9 GPa) [41].
4.2.2. Metallic oxides and particles
The use of metallic oxides and magnetic nanoparticles as the inorganic phase analogue has also been studied for the production of nacre-like nanocomposites. Zhang et al. [42] developed a method combining TiO2nanoparticles and three different polyelectrolytes: poly(ethyleneimine) (PEI), poly(sodium 4-styrenesulfonate) (PSS) and poly(allylamine hydrochloride) (PAH). The researchers first developed through LbL a PE film with the formulation PE = (PEI/PSS)(PAH/PSS)3, followed by the deposition of TiO2. The final layered PE/TiO2 composite exhibited a similar structure to nacre - see Fig. 5D.
The mechanical properties of the produced films were measured through nanoindentation. The elastic modulus was of 17.6 ± 1.4 GPa, being lower than that of nacre. However, these results may be due to the larger volume of polymeric material (50% against nacre's 5%). By contrast, the mean strength of the film was determined as 245 MPa, which is of the same order of magnitude to that of nacre (100 to 300 MPa) [42].
In 2014, Tan et al. [43] developed a similar strategy, now using a PE formulation of PE = (PEI/PSS)(PAH/PSS)2. The result was a film with a lower polymeric content. Again, the mechanical properties were measured through nanoindentation. They obtained an elastic modulus of 35.77 ± 7.45 GPa for these films. The mean strength of the film also increased to 346.67 MPa, a value that is above the normal range for nacre [43].
The combination of magnetic nanoparticles (MNPs) in polymeric structures can also be a strategy to obtain devices with other functional properties that can have applicability in the biomedical area [44], [45].
Using superparamagnetic iron-oxide nanoparticles (SPIONS), freestanding membranes were produced by the deposition of 100 chitosan-alginate (CHI-ALG) bi-layers onto polypropylene substrates, followed by the deposition of 5 CHI-ALG-CHI-MNPs tetra-layers [46]. The effect of genipin as a crosslinking agent was also studied. Usually, LbL is used to obtain thin coatings, but in this case the procedure allowed the production of freestanding films. Fig. 6 show the obtained composite membranes, which present magnetic properties and shape memory effect triggered by hydration. In particular, regarding the magnetic properties of the produced membranes, the authors showed that only the membranes containing MNPs are attracted to a magnet when one is placed in their proximity [46].
 Fig. 6. A - Photographs of the produced membranes: (i) CHI-ALG, (ii) CHI-ALG with genipin cross-linking, (iii) CHI-ALG-MNPs, (iv) CHI-ALG-MNPs cross-linked with genipin. The insert shows a TEM image of the used MNPs (scale bar is 50 nm); B - Magnetic response of CHI-ALG-MNPs membranes to external magnetic field; C - Magnetic response of cross-linked CHI-ALG-MNPs membranes to external magnetic field [46].
Fig. 6. A - Photographs of the produced membranes: (i) CHI-ALG, (ii) CHI-ALG with genipin cross-linking, (iii) CHI-ALG-MNPs, (iv) CHI-ALG-MNPs cross-linked with genipin. The insert shows a TEM image of the used MNPs (scale bar is 50 nm); B - Magnetic response of CHI-ALG-MNPs membranes to external magnetic field; C - Magnetic response of cross-linked CHI-ALG-MNPs membranes to external magnetic field [46].The authors found that the presence of MNPs did not affect significantly the mechanical properties of the membranes. This could have been due to the deposition of MNP only in the last five tetra-layers and not across the whole membrane, which result in the deposition of a MNPs quantity high enough to provide the membranes with magnetic properties, but not high enough to alter significantly their mechanical properties. However, the crosslinking of the membranes with genipin significantly alters their mechanical behaviour, increasing the membranes' elastic modulus and ultimate tensile strength [46].
4.2.3. Bioactive glass nanoparticles
Since their discovery in 1969, bioactive glasses have been used to improve the bond between bone and orthopaedic implants [47], [48], [49]. In particular, bioactive glass nanoparticles (BGNPs), obtained from sol-gel routes, have been used to produce composites with not only an increased ability to induce apatitedeposition in vitro [50], [51], but also with improved mechanical properties.
CHI and BGNPs were used to produce bioactive coatings inspired by nacre [52]. The coatings were shown to have bioactive properties, leading to the precipitation of hydroxycarbonated apatite (HCA) onto the substrates, after immersion in simulated body fluid (SBF) for 14 days. Analysis through quartz crystal microbalance with dissipation monitoring (QCM-D) also showed that the produced coatings were viscoelastic in nature, similar to nacre.
Nacre-like bioactive coatings were also produced using CHI, BGNP and hyaluronic acid (HA) modified with catechol groups [53]. This modification of HA was inspired by the presence of catechol groups present in marine mussels' adhesive proteins (MAPS) and aims to improve the membranes' adhesive properties [54]. The resulting coatings were shown to have bioactive properties, as seen in Fig. 7. In fact, the presence of the cauliflower-like structures after immersion in SBF for 7 days indicates the precipitation of a HCA layer. The coatings also presented a viscoelastic nature, similar to nacre. Moreover, the presence of modified HA was able to considerably improve the adhesive properties of the coatings, when compared with controls produced with unmodified HA.
 Fig. 7. SEM pictures and EDS analysis for one of the developed coatings before (A) and after (B) 7 days of immersion in SBF. Adapted from [53].
Fig. 7. SEM pictures and EDS analysis for one of the developed coatings before (A) and after (B) 7 days of immersion in SBF. Adapted from [53].