1. Introduction
After the dilemmas regarding the costly methods of producing graphene, difficulties of transferring methods, and small scale of production, graphene oxide (GO) was introduced as a low-cost two-dimensional alternative that could fit well into the different application demands via variable tuning techniques. Easy and cost-effective producing methods of GO and its hydrophilic characteristics introduced it as a relevant material that can efficiently be utilized as a coating or thin film on an industrial scale via the conventional water-based deposition techniques. Gilje et al. [1] utilized a spray coating approach for depositing a large-scale film of GO for use in electronic devices. Robinson et al. [2] used spin coating as a simple solution-based method for depositing GO on silicon oxide substrate as an active material for molecular sensors. Naghdi et al. [3] deposited GO on an aluminum surface via electrophoretic deposition to take advantage of the chemical stability of the coating and its corrosion barrier characteristics in saline solutions.
Although GO can compete with graphene in the cost of production and applicability, its electrical characteristic is not comparable with graphene. While the surface of GO sheets is covered with oxygen functional groups, which make it an ideal candidate for water-based deposition techniques, the presence of such groups affects the electrical characteristics of GO drastically. Therefore, in the electrical and optoelectronic application of GO, reduction of its oxygen functional groups (reduced-GO or rGO) is mandatory in order to improve its electrical properties.
Among the different techniques of the oxygen functional group reduction, the thermal reduction has been attracted attention since there is no need for toxic chemicals or any advanced facilities [4]. The effectiveness of thermal reduction on the oxygen functional groups removal and improving the electrical properties of GO via thermal reduction was already investigated via the different research groups [5]. Wang et al. [6] investigated the thermally reduced GO in solid-state dye-sensitized solar cells as window electrodes, which they could achieve a high conductivity (550 S/cm). Becerril et al. [7] reduced a GO thin film via different reduction techniques, which among them the thermal reduction, was more promising and resulted in the rGO thin film with the sheet resistance of 102–103 Ω/sq. They claimed that the solution-processed rGO films had the potential to be used as transparent electrodes. Chen et al. [8] reported a highly conductive, free-standing rGO film reduced via thermal reduction of GO film, which, based on their observations, the size of the sp2 domains were decreased due to the thermal reduction. Vallés et al. [9] reported a gentle and direct thermal treatment of GO under argon or H2 atmosphere for producing conductive rGO. Based on their experience, thermal annealing was successfully reduced the different oxygen functional groups of GO, resulted in recovering the sp2 network structures properly, and enhanced the conductivity of rGO (8100 S/m). Therefore, thermal annealing was introduced as a favorable approach for enhancing the electrical properties of GO.
The oxygen functional group reduction of GO is not the only approach for altering the electrical properties of this material. Naghdi et al. [10] reviewed the different approaches to modify the electrical characteristics of GO, which among the different modification techniques, one method is doping the GO layers with chemical agents. Chemical doping was approved to be a significant factor to affect the electrical properties of the graphene family [11,12]. The chemical dopants, such as n-doping agents (electron donors), were proven to increase the electron concentration in both graphene and GO structure and reduce its work function [13]. While the p-dopants (electron acceptors) were presented as a factor for decreasing the charge carrier density, increasing its work function [14], and prepare it for application as anode or hole injection electrodes in applications such as organic thin film transistors [7].
Hwang et al. [15] reported the successful sheet resistance reduction of nitrogen-doped GO (N-doped GO) to as low as 300 Ω/sq for using as a cathode for high-performance polymer light-emitting diodes. Furthermore, they observed the work function reduction of the N-doped GO from 4.6 eV (for GO) to 4.4 eV (for N-doped GO). Liu et al. [16] functionalized GO via Cs atoms to reduce GO work function (4.7 eV) to 4.0 eV for GO-Cs, which facilitated its application as a cathode in bulk heterojunction solar cells. Cheng et al. [17] used CsF and Cs2CO3as the n-doping agents for lowering the work function of graphene from 4.2 eV to 3.2 eV (for CsF/graphene) and 3.3 eV (for Cs2CO3/graphene). They showed that the n-doped graphene was a great candidate as cathode for organic light-emitting diodes. Yao et al. [18] used Bphen:Cs2CO3 as the n-doping agent and reduced the work function of graphene from 4.5 eV to 4.3 eV for its application as cathode for molecular organic light-emitting diodes. Therefore, it can be concluded that both oxygen functional group reduction and chemical doping of GO layers must be taken into account for producing low sheet resistance and low work function materials for application as a cathode in the electrical and optoelectronic applications.
In this work, the combination of these methods on the electrical and optical properties of GO is investigated. First, the GO layers were doped with the alkali metal dopants, and later, the doped samples were thermally reduced. The chemical composition of the samples was investigated using EDX and XPStechniques. The effect of the dopants and thermal reduction on the morphological characteristics of the samples was investigated using the HR-SEM and OM images. XPS and Raman spectroscopy clarified the type of dopants. The effect of the dopants and thermal reduction on the optical and electrical properties of the samples were investigated thoroughly in the last section of this work.
2. Experimental details
In this work, all chemicals were purchased from Alfa Aesar and used as received. The alkali metal carbonates (Li2CO3, Na2CO3, K2CO3, Rb2CO3, and Cs2CO3) were used as the doping agents. For doping GO, the first 4 ml of the GO solution (0.5 g/L concentration) was dispersed in 20 ml deionized water in an ultrasonic bath for 60 min. Then 1 ml of the alkali metal carbonate solutions (1 mol) was added into the GO solution and stirred the solution for 1 h. The samples were washed several times by deionized water to purify the GO/Li, GO/Na, GO/K, GO/Rb, and GO/Cs solutions, and filtered through the polyvinylidene fluoride (PVDF) membrane (0.5 μm). By investigating the pH of the solutions after washing and filtration process and comparing it with the GO solution, the neutralization of the GO/Li, GO/Na, GO/K, GO/Rb, and GO/Cs sample was confirmed. In this process, alkali metal carbonates were easily dissolved in a deionized water solution, and the resulting M+ (alkali metal cations) were attracted to the negatively charged GO layers. The final samples were dried at 50 °C overnight. In order to investigate the influence of thermal annealing on the doped samples, they were thermally treated in an oven at 200 °C in ambient conditions for over 2 h.
In order to identify the elements and compositions of the materials energy-dispersive X-ray spectroscopy (EDX, EDAX GENESIS 2000 (EDAX Inc., USA)), X-ray photoelectron spectroscopy (XPS, using a K-alpha System spectrometer (Thermo Electron, Waltham, MA, USA) equipped with an Al Kα monochromatic X-ray source (1486.7 eV) and a micro-focused monochromator), and Raman spectroscopy (RFS 100/S, Bruker; λ = 532 nm) was used. To investigate the morphological characteristics of the samples, optical microscopy (OM, ZEISS Axio Zoom.V16 in the reflectance mode), and high-resolution scanning electron microscopy (HR-SEM, HITACHI, S-4800) was utilized. The optical characteristics of the samples were investigated using ultraviolet–visible (UV–Vis) spectroscopy (Varian CARY 300 Bio). The effect of doping and thermal annealing on the electrical properties of the samples was investigated by measuring the sheet resistance of the samples using a four-point probe system (ST-2258A). The samples’ work function determined by using ultraviolet photoelectron spectroscopy (UPS) technique using the He I irradiation (hν = 21.2 eV). (UPS, R4000 spectrometer, VG scienta, UK).
3. Results and discussion
3.1. EDX
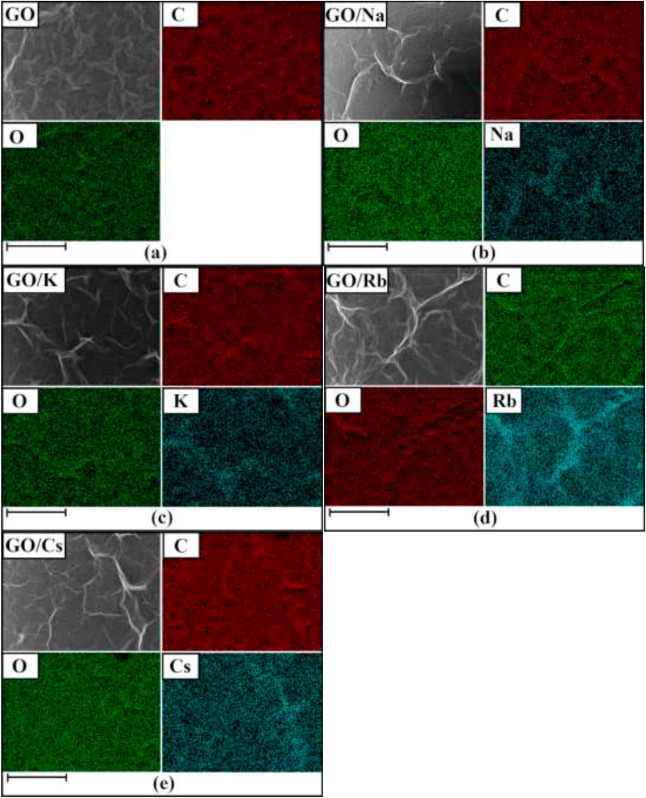
The EDX analysis (Table 1) was performed to investigate the chemical composition of the samples and to investigate the presence of alkali metal elements in the doped samples. From the EDX data, it can be seen that C and O accompany the alkali metal atoms presented in the doped-GO samples. The atomic percentage of oxygen decreased after doping that suggested replacing the oxygen functional groups with the dopant elements that resulted in a partial reduction of the GO layers during the doping process. EDX elemental maps revealed that the doping process of the GO layers resulted in an even distribution of elements (Na, K, Rb, and Cs) on the GO surface (Fig. 1), and there was no tendency to form agglomerated alkali metals on the GO sheets. After the doping process, alkali metal elements and O were homogeneously distributed throughout the basal plane of GO layers [19]. In the previous reports on doping graphene with alkali metal carbonates, alkali metals tend to form particles on the graphene layers, which could be due to the lack of active site on the graphene sheets to interact with the alkali metal elements [20]. While on the GO surface, the presence of the oxygen functional groups provides homogenous active sites for interaction with alkali metal cations [21]. As can be seen in Table 1, the C/O ratio of GO had been increased after the alkali metal doping, that it was indicating reducing the oxygen functional groups via the alkali metal doping process.
Table 1. EDX analysis of GO and doped-GO samples (Note: Since the intensity of the X-ray signal generated by Li elements are low and the possibility of absorbing the X-ray is high, the emitted intensity of these materials is small. Therefore, detecting the Li K in an EDX device is challenging and we have not presented here).
| Sample | C (At %) | O (At %) | C/O | Alkali metal (At %) |
|---|---|---|---|---|
| GO | 59.06 | 40.94 | 1.44 | – |
| GO/Li | – | – | – | – |
| GO/Na | 59.57 | 37.73 | 1.58 | Na: 2.70 |
| GO/K | 62.60 | 35.54 | 1.76 | K: 1.86 |
| GO/Rb | 60.60 | 36.58 | 1.66 | Rb: 2.82 |
| GO/Cs | 58.90 | 38.41 | 1.53 | Cs: 2.69 |
 Fig. 1. Elemental mapping images of (a) GO, (b) GO/Na, (c) GO/K, (d) GO/Rb, and (e) GO/Cs (the scale bar is 50 μm).
Fig. 1. Elemental mapping images of (a) GO, (b) GO/Na, (c) GO/K, (d) GO/Rb, and (e) GO/Cs (the scale bar is 50 μm).3.2. HR-SEM and OM
HR-SEM and OM images have been obtained to study the state of the GO sheet before and after alkali metal doping. Furthermore, the OM images utilized to observe the visual changes in the GO after doping and thermal annealing. Fig. 2present the HR-SEM images of the samples. As shown in Fig. 2b–f (doped samples), there was no sign of particles in the doped samples, thus revealing that the alkali metal atoms did not agglomerate on the GO sheets, and they did not form particles, in consistence with the EDX elemental maps. Therefore, it can be concluded that the GO sheets uniformly doped with the alkali elements.
 Fig. 2. HR-SEM images of (a) GO, (b) GO/Li, (c) GO/Na, (d) GO/K, (e) GO/Rb, and (f) GO/Cs, there is no sign of particles in the images.
Fig. 2. HR-SEM images of (a) GO, (b) GO/Li, (c) GO/Na, (d) GO/K, (e) GO/Rb, and (f) GO/Cs, there is no sign of particles in the images.Fig. 3 shows the OM images of the GO (Fig. 3a) and the doped samples (Fig. 3b–f) before and after thermal annealing. When the doped samples inspected under an optical microscope, they appeared to be yellow (GO) to brown color (doped-GO), which could easily recognize by the naked eye (Fig. 3, left images). A comparison between the GO and the doped samples revealed that the yellow color of the GO sample turned to brownish color after the doping process. Pei et al. [22] suggested that the change in the color of GO from yellow to dark brown indicates a reduction of GO structure, which is due to a partial restoration of the p-networks (a partial re-graphitization of the graphene oxide) within the carbon structures [23]. Since the alkali metal carbonates were all colorless, this phenomenon was a significant sign of reducing the oxygen functional groups of the GO by the dopants. After thermal annealing, the samples appeared to be dark brown to black color when recorded on a digital camera (Fig. 3, right images). The darker color of all samples after thermal annealing showed the successful oxygen functional group reduction of the samples after the thermal annealing process.

 Fig. 3. OM images before and after thermal annealing (a) GO and rGO, (b) GO/Li and rGO/Li, (c) GO/Na and rGO/Na, (d) GO/K and rGO/K, (e) GO/Rb and rGO/Rb, (f) GO/Cs and rGO/Cs.
Fig. 3. OM images before and after thermal annealing (a) GO and rGO, (b) GO/Li and rGO/Li, (c) GO/Na and rGO/Na, (d) GO/K and rGO/K, (e) GO/Rb and rGO/Rb, (f) GO/Cs and rGO/Cs.3.3. X-ray photoelectron spectroscopy (XPS)
To further investigate the doped-GO samples and analyze their elemental composition, XPS was utilized before and after thermal annealing. The XPS survey spectra of the samples before and after thermal annealing is presented in Fig. S1 and the detail information are presented in Table 2, which provides useful evidence about the atomic composition and carbon to oxygen (C/O) ratios of the samples. The survey spectra of the doped samples have pronounced alkali metal peaks, indicating successful doping of GO. From the atomic composition results presented in Table 2, it can be concluded that the ratio of carbon to oxygen (C/O) of the GO was increased after alkali metal doping that was in accordance with the results presented for EDX (Table 1). The suggested results were due to partial reduction of the GO and also the functional groups of GO were replaced via the alkali metal elements [21].
Table 2. The atomic composition of the samples measured by XPS and C/O ration of the samples before and after thermal reduction.
| Sample | C1s | O1s | C/O | Alkali metal element | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BE (eV) | At (%) | At (%) | At (%) | |||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | |
| GO | 284.5 | 284.8 | 66.10 | 72.54 | 33.90 | 27.46 | 1.95 | 2.64 | – | – |
| GO/Li | 284.9 | 285.2 | 64.88 | 70.57 | 29.67 | 24.17 | 2.19 | 2.92 | 5.45 | 5.26 |
| GO/Na | 284.9 | 285.3 | 64.59 | 70.52 | 30.51 | 25.10 | 2.12 | 2.81 | 4.90 | 4.38 |
| GO/K | 285.5 | 285.7 | 64.20 | 68.89 | 30.06 | 24.26 | 2.14 | 2.84 | 5.74 | 6.85 |
| GO/Rb | 285.6 | 285.7 | 64.86 | 69.49 | 29.70 | 23.80 | 2.18 | 2.92 | 5.44 | 6.71 |
| GO/Cs | 285.8 | 285.9 | 64.72 | 70.86 | 30.44 | 24.43 | 2.13 | 2.90 | 4.84 | 4.71 |
After thermal reduction of the samples at 200 °C (Table 2), the C/O ratio exhibited further increase that was due to reducing the remnant oxygen functional groups of the samples at high temperatures. There was an interesting observation that showed after thermal reduction of GO, the C/O ratio changed from 1.95 (GO) to 2.64 (rGO), while for the doped samples, the C/O ratio was increased less than GO itself. These results suggested that on the GO surface, there were more oxygen functional groups to remove via thermal reduction, while the alkali metal elements already replaced some of the oxygen functional groups of the doped samples. Therefore, after thermal reduction, the rGO presented a higher increase in the C/O ratio compare to the doped samples. From the alkali metal composition provided in Table 2, it can be seen that the atomic percentage of these elements was about 4–6% in the doped samples, in which the amount of these elements stayed intact even after thermal reduction. It can be suggested that these elements were chemisorbed on the GO basal plane, and the bonding was strong enough and stable at 200 °C.
Another information from XPS was the binding energy (BE) of the chemical components. The BE of C (C1s) of the pristine GO was about 284.5 eV (Table 2) that, after the doping process, the C1s position shifted to higher binding energies. The peak shift was attributed to the lifting of the Fermi level of graphene that supports the assumption of transferring the electron from alkali metal dopants to the GO, which leads to the core levels to appear at higher binding energies and n-type doping of the doped-GO samples [24]. As the shift is bigger, the n-doping effect of the dopant is stronger. As can be seen from Table 2, the GO/Cs presented the bigger shift compared to the other doped samples, which can be due to the lower electron affinity of Cs, compare to the other alkali metals. After thermal reduction, C1s peak of all samples was shifted to higher BE, which from these shifts, it can be concluded that since the oxygen functional groups have a p-doping effect on the samples [25], removing such functional groups strengthened the n-doping effect.
Fig. 4 presents the deconvoluted C1s spectra of pristine GO and doped-GO with peaks assigned to different functional groups. Generally, the C1s peak of GO contains different carbon bond such as C C bond (sp2 hybridized carbon) at about 284.5 eV, C–C bond (sp3 hybridized carbon) at 285.4 eV, C–OH bond (hydroxyl group) at 286.6 eV, C
C bond (sp2 hybridized carbon) at about 284.5 eV, C–C bond (sp3 hybridized carbon) at 285.4 eV, C–OH bond (hydroxyl group) at 286.6 eV, C O bond at 287.8 eV, and peak at around 288.8 eV was assigned to COOH bond [26]. A comparison of C1s XPS spectra of GO and doped-GO samples exhibited a decreased peak intensity for oxygen functional groups of the doped-GO samples after the doping process. Which these results were in agreement with the elemental composition of the samples tabulated in Table 2 and the C/O ratio. After thermal annealing, there was a decrease in the hydroxyl and epoxy groups for all samples, which was due to the thermal reduction procedure (Fig. 5).
O bond at 287.8 eV, and peak at around 288.8 eV was assigned to COOH bond [26]. A comparison of C1s XPS spectra of GO and doped-GO samples exhibited a decreased peak intensity for oxygen functional groups of the doped-GO samples after the doping process. Which these results were in agreement with the elemental composition of the samples tabulated in Table 2 and the C/O ratio. After thermal annealing, there was a decrease in the hydroxyl and epoxy groups for all samples, which was due to the thermal reduction procedure (Fig. 5).
 Fig. 4. C1s diagram of (a) GO, (b) GO/Li, (c) GO/Na, (d) GO/K, (e) GO/Rb, and (f) GO/Cs (before thermal annealing).
Fig. 4. C1s diagram of (a) GO, (b) GO/Li, (c) GO/Na, (d) GO/K, (e) GO/Rb, and (f) GO/Cs (before thermal annealing). Fig. 5. XPS C1s spectra of (a) rGO, (b) rGO/Li, (c) rGO/Na, (d) rGO/K, (e) rGO/Rb, and (f) rGO/Cs (after thermal annealing).
Fig. 5. XPS C1s spectra of (a) rGO, (b) rGO/Li, (c) rGO/Na, (d) rGO/K, (e) rGO/Rb, and (f) rGO/Cs (after thermal annealing).3.4. Raman
To recognize the number of graphene layers, presence of defects and disorders, and the effect of doping and thermal reduction on the graphene structure, Raman spectroscopy as a non-destructive method can be useful. To identify GO via Raman technique, two prominent Raman features of GO, D band at 1350 cm−1 (the defects or edges) and G band at about 1584 cm−1 (the first order scattering of the E2g mode) are beneficial. Here the GO layers were doped with alkali metals and later were thermally reduced. Therefore, by studying the Raman pattern of the samples, first, we identify the success of the doping process and its effect on the structure of GO regarding the defects. Moreover, we investigate the effect of thermal reduction on the doped samples.
There are different reports on the effect of doping on the Raman pattern of graphene. Any doping (electron doping or hole doping) has been reported to shift the G band, and as the doping is strong, the shift is more prominent [27]. Based on the Raman pattern of GO and doped-GO samples (Fig. 6a), the G band was shifted to the higher wavenumbers (blue shift), which was in agreement with the literature that showed n-doping increased the G peak position [28] and led to a blue shift of G band [29]. In Fig. 6a, the G band of GO is at about 1581 cm−1 that after the doping process, the G band was shifted to higher wavenumbers. The shifts of the G band were all the evidence of successful n-doping of the GO that were in agreement with the XPS results. From Fig. 6a it can be observed that the shift of G band for Cs dopant was higher than the shift for the other alkali metal elements and indicated a slightly higher n-type doping effect, which can be explained by the lower electron affinity of Cs in comparison with the other alkali metals.
 Fig. 6. Raman patterns of the GO and alkali metals doped-GO (a) before and (b) after thermal reduction.
Fig. 6. Raman patterns of the GO and alkali metals doped-GO (a) before and (b) after thermal reduction.Fig. 6b showed the Raman pattern of GO and doped-GO samples after thermal reduction. As can be concluded from the G band position of all samples, it had been blue shifted after thermal annealing (Table 3). Based on Fig. 6b, there was a shift for G band of GO after the doping process, and again there was another shift after thermal annealing. The reason for this new shift was due to the oxygen functional group reduction and isolating carbon double bonds that resonate at higher frequencies in comparison with the G band of pristine GO. Since the oxygen functional groups have a p-doping effect on graphene, the reduction of such groups shifts the G band [25].
Table 3. The G band position of the samples (after doping and after thermal reduction) and ID/IG ratios.
| G band (cm−1) | ID/IG | |||
|---|---|---|---|---|
| Before reduction | After reduction | Before reduction | After reduction | |
| GO | 1581 | 1598 | 0.93 | 1.00 |
| GO/Li | 1586 | 1594 | 0.97 | 0.98 |
| GO/Na | 1589 | 1597 | 0.95 | 0.99 |
| GO/K | 1588 | 1598 | 0.95 | 0.95 |
| GO/Rb | 1589 | 1599 | 0.94 | 0.95 |
| GO/Cs | 1590 | 1600 | 0.94 | 0.95 |
Doping and the thermal reduction processes not only affect the peak positions, but they also alter the structure and quality of the GO layers. The D band that is related to the disorders of the GO structure can be shifted, or its intensity changes based on factors that affect GO. By investigating the intensity of D band to G band (ID/IG), we can investigate the structural changes in the GO layers after doping and thermal reduction procedure [30]. Furthermore, changes in the electrical conductivity of the GO after doping and thermal reduction (Section 3.5) can be explained based on the changes in the defects and disorders in the structure of the samples concluding here. As can be seen from Table 3, the ID/IGratio of the doped samples was higher than the pristine GO that suggested the destructive effect of the doping procedure on the structure of the GO sheets. After thermal reduction of the pristine GO and doped-GO, the ID/IG ratio increased further that is due to the oxygen functional group reduction. Based on the previous reports [8], the loss of carbons from the GO lattice during oxygen functional group reduction led to introducing the disorders, i.e., vacancies and distortions, which divided an sp2 GO domain into the smaller domains. Therefore, increasing the ID/IG ratio of all samples after thermal reduction suggests the high efficiency of restoring the sp2 carbon networks. Therefore, thermal energy rearranged the carbon atoms during the thermal annealing and assisted the formation of sp2 carbon domains and led to the recovery of the hexagonal network of carbon atoms. These effects will be discussed further in the next sections.
3.5. Sheet resistance
The sheet resistance of GO and alkali metal doped-GO samples was measured from three different points on the samples’ surface in ambient conditions utilizing a four-point probe system. Based on the different researches, doping graphene with alkali metals (that are n-type dopants) slightly increase the sheet resistance of the graphene layers [20] in comparison with the p-dopants that decrease the sheet resistance [31], while in the case of GO, n-doping reduces the sheet resistance of the GO layers [15]. Additionally, the oxygen functional groups cover the GO layers, which by eliminating these groups, the sheet resistance of the rGO decreases dramatically.
Based on the results provided via XPS, doping GO with alkali metals increased the C/O ratio (compare to pristine GO). Therefore, it can be a sign of replacing the oxygen functional groups with the alkali metal elements. The reports showed that the functionalization of GO with alkali metals leads to charge neutralization of oxygen functional groups on the GO sheets that can tune the electrical properties of the GO [21]. Therefore, based on the results provided in this work (Table 4), in comparison with pristine GO, doping GO with alkali metals has decreased the sheet resistance of the samples from 311.0 kΩ/sq to 33.0 kΩ/sq for GO and GO/Cs respectively that it was due to the partial reduction of GO due to the doping.
Table 4. Sheet resistance and work function of the samples before and after thermal reduction.
| Sheet resistance (kΩ/sq) | Work function (eV) | |||
|---|---|---|---|---|
| Before reduction | After reduction | Before reduction | After reduction | |
| GO | 311.0 | 37.1 | 4.76 | 4.55 |
| GO/Li | 54.5 | 47.3 | 4.12 | 3.86 |
| GO/Na | 46.9 | 38.4 | 3.95 | 3.78 |
| GO/K | 41.3 | 39.4 | 3.78 | 3.53 |
| GO/Rb | 33.2 | 34.6 | 3.48 | 3.22 |
| GO/Cs | 33.0 | 32.1 | 3.18 | 3.02 |
Thermal reduction and reducing the oxygen functional groups of the samples further affected their sheet resistance. Increasing the ID/IG ratio after thermal reduction can be considered as a factor for increasing the number of sp2domains, which increases the charge carrier transport in individual graphene sheets [9]. Therefore, reducing the sheet resistance of the samples after thermal reduction can be easily understood from the changes in the ID/IG ratio. Therefore, decreasing the sheet resistance (or increasing electrical conductivity) showed an approximately linear rise with increasing the ID/IG ratio. Based on the results presented in Table 4, the GO/Cs showed the lower sheet resistance before and after thermal reduction.
In the case of rGO, its ID/IG is the highest among the other samples (Table 3), while its sheet resistance is not the lowest one. Before thermal reduction, due to the presence of oxygen functional groups on GO, it considers an insulator. After thermal reduction, removal of such groups enhances the electrical conductivity drastically, while the residual oxygen functional groups and lowest C/O ratio of the rGO sample (Table 2) resulted in higher sheet resistance of this sample compare to some of the reduced doped samples.
3.6. Ultraviolet–visible optical spectroscopy (UV–Vis spectroscopy)
The optical transmittance and optical absorption were investigated at 550 nm wavelength, using a UV–Vis spectrophotometer. In order to make a comparison among the optical transmittance and optical absorption of the GO and doped-GO samples, the solution of the samples with the same concentration was prepared to see the effect of doping on the optical characteristics of the GO.
3.6.1. Optical transmittance
It is undeniable that the optical transmittance of graphene reduces due to doping (regardless of the type of dopant) that was already reported by different groups. Decreasing the optical transmittance of multi-layer graphene due to hole doping [32], decreasing the optical transmittance of graphene from 96.7% to 94% via electron doping [20], and decreasing from 96.7% to 93% via hole doping [33] all showed that doping reduces the optical transparency of graphene. In this research, a decrease in the optical transmittance of the GO was observed in agreement with the previous studies (Fig. 7a). The optical transmittance of the doped samples at 550 nm was all lower than the pristine GO, with 56.2% at 550 nm. Among the different doped samples, GO/Li had the lowest transmittance (13.7%), and the GO/Cs had the highest one (20.0%). Besides, from the sheet resistance measurements, it was found out that the GO/Cs possess the lowest sheet resistance among the other doped samples. These results suggest the superiority of Cs dopant for optoelectronic applicationcompare to the other alkali metal dopants.
 Fig. 7. (a) Transmittance diagram, and (b) UV–Vis absorption spectra of pristine GO and the alkali doped-GO samples in solution form.
Fig. 7. (a) Transmittance diagram, and (b) UV–Vis absorption spectra of pristine GO and the alkali doped-GO samples in solution form.3.6.2. Optical absorption
Fig. 7b presents the absorption diagram of the samples in the UV–Vis region. The absorption of the samples increased gradually in the spectral region of 250–800 nm, and all the doped samples presented higher absorption in comparison with GO. Increasing the absorption of material, especially in the visible range, may increase their potential application in the sensing devices. Among the different doped samples, the GO/Li showed the highest absorption, and GO/Cs exhibited the lowest absorption, which was in agreement with the transmittance results.
On the whole, the high absorption of the doped samples in the UV region, in comparison with the pristine GO, suggests the application of doped-GO samples in the UV detector devices. Moreover, the absorption peak of GO was observed at about 250 nm in the UV–Vis spectrum that corresponded to the π-π∗ transitions of the remaining sp2 C C bonds. Due to the doping process the absorption peak of GO was increased and red-shifted (to the longer wavelength at about 260 nm). The partial reduction of the doped-GO samples was exhibited in the different characterizations above (EDX, OM, and XPS), which resulted in increasing the π-electron concentration (or, in other words, restored the π-conjugation network and sp2 carbon) that were the reason of the red-shift in absorption peak [34]. By increasing the π-conjugation, less energy is required for the transition, which corresponds to the red-shift of the absorption peak [34].
C bonds. Due to the doping process the absorption peak of GO was increased and red-shifted (to the longer wavelength at about 260 nm). The partial reduction of the doped-GO samples was exhibited in the different characterizations above (EDX, OM, and XPS), which resulted in increasing the π-electron concentration (or, in other words, restored the π-conjugation network and sp2 carbon) that were the reason of the red-shift in absorption peak [34]. By increasing the π-conjugation, less energy is required for the transition, which corresponds to the red-shift of the absorption peak [34].
3.7. Ultraviolet photoelectron spectroscopy (UPS)
UPS was utilized to determine the changes in the GO work function first by introducing alkali metals among its structure and after thermal reduction. During the UPS measurements, the samples were biased at −10 V to avoid interference of the spectrometer threshold in the UPS spectra and observe the secondary electron edge. The work function of the sample W is computed as:(1)W = hν−(EF−Ecutoff)which hν is the photon energy of the excitation light (21.2 eV), EF is the Fermi edge, and Ecutoff is the inelastic high binding energy cutoff. Fig. 8 shows the results of the UPS measurement (cutoff region) of GO and doped-GO samples before and after thermal reduction. As it is shown in Fig. 8a, the cutoff binding energy of GO is 16.44 eV. According to Eq. (1) and the average measured values for a work function of 3 different GO samples was about 4.76 eV, which was in agreement with previously reported values for GO (4.7–4.9 eV) [35]. The work function of GO obtained here was higher than the work function measured for graphene, which was due to the large electronegativity and p-dopant effect of the oxygen functional groups.