Introduction
Mitral regurgitation (MR) is frequent in patients with severe aortic stenosis (AS), and present in 20% – 33% of patients undergoing transcatheter aortic valve replacement (TAVR), in which case it remains untreated.1,2 The presence of moderate-to-severe MR at baseline has a negative impact on both in-hospital and late mortality in the TAVR population,1,3 and persistent MR after TAVR has been reported to be a predictor of late mortality.4 In addition, post-procedural deterioration of MR has been shown to be associated with significantly higher mortality, compared to improved or unchanged MR severity one month after TAVR.5 Less is known however, on the immediate changes in MR severity following TAVR, with conflicting results reported in the literature in terms of magnitude and direction.6, 7, 8
Aortic-mitral coupling via a fibrous continuity and synchronized reciprocal behavior suggest that structural and hemodynamic changes to the left ventricle (LV)-valve complex can occur during the TAVR procedure, which may influence MR severity.9 In fact, while some imaging clinical studies have found no significant changes in MV geometric parameters before and after TAVR,10,11others have reported a structural impact on the mitral apparatus, including decreased mitral annulus (MA) height, area and motion.12 These studies, however, have been based on echocardiography (echo) data; which can be limited by poor acoustic windows, geometric assumptions, low spatiotemporal resolution and bias related to operator experience.13 The design of the two TAV devices currently used, balloon-expandable (BEV) and self-expandable (SEV) valves, might also contribute to the observed differences and add complexity to the interpretation of the results. The SEV consist of a Nitinol stent with a longer frame length and self-expansion that has less radial force to surpass the resistance of the calcified oval-shaped aortic annulus (AA), compared to the BEV stent.14 Some observational studies have pointed toward a higher degree of MR improvement in patients treated with a BEV as compared to a SEV,2,15,16 while others have found no differences.17
A thorough evaluation of aortic-mitral coupling and MR severity response after TAVR is of particular importance in the current era, where operable patients at low surgical risk are increasingly referred for TAVR. The presence of significant MR with low likelihood of MR improvement may influence the selection of combined surgical aortic valve (AV) replacement with MV repair/replacement therapies in such patients. Patient-specific computer simulations for transcatheter interventions can be helpful in quantifying the interaction of a broad spectrum of devices with the human tissue/organs, based on the integration of detailed anatomic and biomechanical parameters in an objective and reproducible manner.18 In the TAVR population, however, a quantitative mechanistic evaluation of the effect of TAVR on aortic-mitral coupling and MR severity by computer modeling has only been recently studied when implanting a BEV.19
Thus, the aim of this study is to investigate the complex biomechanical interaction between a SEV and the left heart (LH) structures during the TAVR procedure. Specifically, we will 1) simulate the TAVR procedure using a SEV in a previously validated patient-specific LH model with severe AS and concomitant functional MR, 2) evaluate the influence of stent implantation depth on aortic-mitral structural coupling and function, and 3) model post-TAVR LH dynamics throughout the cardiac cycle with the goal to quantify the immediate tissue and hemodynamic changes, identify the determinants of MR improvement, and compare SEV and BEV performance.
Material and methods
Patient-specific LH model with AS and concomitant MR
De-identified pre-operative cardiac multi-slice computed tomography (MSCT) images of a 71-year-old male patient were retrospectively collected from Hartford Hospital (Hartford, CT) under approval of the Institutional Review Board. Details of patient’s clinical information, image segmentation and LH model reconstruction were described in a recent publication.19 As this was a retrospective study of routinely collected clinical data, the ethics committee stated that written consents were not required by patients. Briefly, the patient was diagnosed with classical low-flow low-gradient severe AS, mild AV regurgitation, and a bicuspid AV (BAV) with fused left and right coronary leaflets, with no raphe between them. This corresponds to a BAV type 0 L/R, using the classification described by Sievers and Schmidtke.20 Moderate-to-severe functional MR was observed with restricted posterior mitral leaflet (PML) motion, resulting in a posteriorly directed regurgitant jet. The LV wall thickness was normal, but the chamber was mildly dilated. The measured AA was 23 mm. During the TAVR procedure, a 26 mm first generation BEV Edwards SAPIEN device (Edwards Lifesciences, Irvine, CA, USA) was successfully deployed. Post-operative echo examination showed a correct TAV deployment, absence of paravalvular leakage (PVL), while moderate-to-severe MR remained.
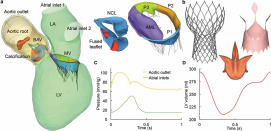
MSCT images were imported into Amira-Avizo (Thermo Fisher Scientific, MA) and 3D Slicer (www.slicer.org) software to segment the cardiac structures, which included the proximal ascending aorta, aortic root, BAV, MV, aortic-mitral curtain, calcification, myocardium and LV/left atrial (LA) endocardial walls. HyperMesh software (Altair Engineering, Inc., MI) was then used to generate a computer model of the LH, as seen in Figure 1a. 3D solid elements were used to model the aorta, aortic root, valves, aortic-mitral curtain, calcification and myocardium, truss elements were used for the mitral chordae, while shell elements were used for the endocardial wall. A uniform thickness of 2 mm and 0.7 mm was set for the aortic wall and AV leaflets, respectively. For the MV, varying leaflet thickness ranging from 1.26 mm to 2.09 mm was used. Four layers of elements were used across the aortic wall and AV leaflets, while two layers were used for the MV leaflets.19
 Figure 1. (a) Patient-specific LH model reconstructed from the MSCT images, (b) SEV TAV model, (c) Aortic and LA pressure waveforms, (d) LV volume waveform. BAV: bicuspid aortic valve, LV: left ventricle, LA: left atrium, MV: mitral valve, NCL: non-coronary leaflet, AML: anterior mitral leaflet, PML: posterior mitral leaflet is divided into lateral P1 scallop, central P2 scallop and medial P3 scallop.
Figure 1. (a) Patient-specific LH model reconstructed from the MSCT images, (b) SEV TAV model, (c) Aortic and LA pressure waveforms, (d) LV volume waveform. BAV: bicuspid aortic valve, LV: left ventricle, LA: left atrium, MV: mitral valve, NCL: non-coronary leaflet, AML: anterior mitral leaflet, PML: posterior mitral leaflet is divided into lateral P1 scallop, central P2 scallop and medial P3 scallop.LH soft tissues were assumed to be homogeneous, non-linear and elastic. An anisotropic hyperelastic material model (MHGO)21,22 was adopted to characterize the mechanical response of most cardiac tissues, while the isotropic hyperelastic Ogden material model23 was used to characterize the mechanical behavior of the chordae and the aortic-mitral curtain. Human material parameters used in this study, obtained in-house using an age- and gender-matched approach, are listed in Supplementary Table 1. Calcification deposits, found in the fused leaflet and right coronary ostia were assumed to be a linear-elastic homogeneous material with a Young’s modulus of 12.6 MPa and a Poisson ratio of 0.324 Further details on the LH model reconstruction and constitutive modeling can be found in the Supplementary Material.
Self-expandable TAV model
A SEV model, based on a 26 mm first-generation Medtronic CoreValve device (Medtronic, Minneapolis, MN, USA) was used in this study.25 As seen in Figure 1b, the SEV consists of a Nitinol stent, skirt and porcine pericardium leaflets. Two layers of 3D solid elements were used to model the TAV leaflets and the stent, while shell elements were used for the skirt. Abaqus (3DS, Dassault Systéms, Paris, France) built-in superelasticity material library was used to model the Nitinol stent, with austenite elasticity of 50 GPa, austenite Poisson’s ratio of 0.3, martensite elasticity of 25 GPa, and martensite Poisson’s ratio of 0.326 TAV leaflets had a thickness of 0.28 mm and porcine pericardium properties obtained from our previous studies that characterized the mechanical properties of chemically treated pericardial tissues.27,28
FE modeling of TAVR procedure
The TAVR procedure was computer simulated in the patient-specific LH model in three major steps:
Step 1 – Stent crimping: The nominal SEV stent was positioned coaxially within the aortic root and centered into the AA, defined as the virtual plane formed by joining the points of basal attachment of the leaflets. Next, the stent was crimped to an exterior diameter of 6 mm (18 Fr catheter) by applying a radial displacement field to a cylindrical-surface sheath, as seen in Figure 2a. Finally, the AV leaflets were pre-opened with a cone-shaped catheter to simulate the effect of the insertion of the delivery system across the valve. Pre-dilatation of the AV with a balloon was not simulated during the virtual procedure.
 Figure 2. (a) Crimped SEV stent at three deployment heights, (b) LH models with myocardium after TAVR deployment.
Figure 2. (a) Crimped SEV stent at three deployment heights, (b) LH models with myocardium after TAVR deployment.To quantify the impact of stent implantation depth on aortic-mitral coupling and MR severity, the axial positioning of the stent with respect to the AA was parametrized to replicate three possible clinical scenarios: 1) Optimal, the conventional manufacture’s recommendation for the CoreValve system is to position the lowest point of the crimped stent ~5 mm below the AA,29 2) High, defined as 3.6 mm higher than the optimal stent position, and 3) Low, defined as 3.6 mm lower than the optimal stent position.30
Step 2 – Stent release: The deployment of the crimped stent was simulated by axially displacing the cylindrical sheath away from the aortic root, followed by a stent-tissue interaction phase to stabilize the dynamic component of the system and its contacts. The aorta and myocardium were constrained at their distal ends allowing only rotational degrees of freedom. The friction coefficient between the stent and cardiac tissues was assumed to be 0.1,25 a frictionless contact was defined between the outer stent surface and the inner sheath surface, while a self-contact formulation was used for the stent. In order to reach a quasi-static state, viscous-elastic damping was added. This value was monitored to ensure that it did not exceed 0.8% of the total system energy. The kinetic energy was also monitored to ensure that the ratio of kinetic energy to internal energy remained under 10%.
Step 3 – TAV leaflet and skirt positioning: The TAV leaflets and sealing skirt were not included during steps 1 and 2, but were added after stent expansion. It has been demonstrated that they have a negligible impact during the TAVR procedure.31 The TAV leaflets were mapped into the deformed stent through a set of non-uniform imposed displacements calculated with reference to the original configuration.32 The pressure gradient was neglected during the TAVR procedure, since in the clinical setting rapid pacing is applied during device deployment. The deformed LH geometries, as shown in Figure 2b, were extracted from the FE simulations and used to assess the post-TAVR LH dynamics using FSI.
FSI modeling of pre- and post-TAVR LH dynamics
The FSI modeling framework used in this study has been previously developed, validated and implemented to evaluate LH dynamics under a variety of physiologic, pathological and repaired states.19,33, 34, 35, 36, 37 Briefly, the FSI approach combines smoothed particle hydrodynamics (SPH) for the blood flow and non-linear FE analysis for the heart valves. As seen in Figure 1c, time-dependent pressure boundary conditions were applied at the two atrial inlets (pulmonary veins) and at the aortic outlet of the pre- and post-TAVR LH models. A time-dependent atrial pressure waveform with an elevated systolic V-wave due to MR was applied at the inlets,38 while a physiologic aortic pressure waveform was employed at the outlet. These waveforms were fitted to match this particular patient’s pressure values measured clinically.19
Endocardial wall motion and chordae origins motion were imposed as a time-dependent nodal displacement boundary condition based on the MSCT images.33,34Figure 1d shows the time-varying LV volume waveform obtained for the pre-TAVR model. This cardiac wall motion was assumed to remain unchanged in all post-TAVR LH models, simulating an immediate post-operative state without considering the LV remodeling that occurs over time. SPH particles were uniformly distributed in the LH domain with a spatial resolution of 0.8 mm and given Newtonian blood properties with a density of ρ = 1056kg/m3 and a dynamic viscosity of μ = 0.0035Pa · s. SPH particle sensitivity33,39 and FE mesh sensitivity40 studies were previously performed. The patient’s heart rate was approximately 60 bpm, corresponding to a cardiac cycle of 1 s. Two cardiac cycles were conducted and the results from the second cycle were analyzed in this study. Abaqus/Explicit 6.17 was used for all FE and FSI simulations presented in this study.
Validation studies
Two previous computer studies have been conducted for the validation of the patient-specific LH dynamics before and after TAVR. The first study evaluated the accuracy of the structural MV model with functional MR by quantitatively comparing the closed MV geometry obtained from the simulation with the in vivo MV geometry obtained from the clinical MSCT images at systole. MV model optimization, including chordae tethering adjustment and pretension were performed until correct valve morphology and leaflet deformation were obtained.41 The second study focused on the accuracy of the FSI modeling framework in simulating patient-specific pre- and post-TAVR hemodynamics using a BEV.19 As shown in Table 1, comparison of computer FSI results with the patient’s available pre- and post-operative echo data revealed a close quantitative agreement in terms of the major hemodynamic variables such as transvalvular pressure gradients, blood velocities, stroke and regurgitant volumes, valve orifice area, etc. Overall, these studies demonstrated the robustness and the predictive capabilities of our computer FSI modeling framework in simulating the pathophysiological features of the patient’s LH anatomy and the biomechanical outcomes of the TAVR procedure.
Table 1. Validation of the pre- and post-TAVR FSI hemodynamics using a BEV.19.
| Pre-TAVR | Post-TAVR | |||
|---|---|---|---|---|
| FSI | Echo | FSI | Echo | |
| SVAV (ml) | 46.28 | 43 | 52.24 | 48 |
| RVMV (ml) | 37.59 | ̶ | 33.84 | 32 |
| MR severity (RFMV) | Moderate-to-severe | Moderate-to-severe | Moderate-to-severe | Moderate-to-severe |
| LVEF (%) | 28.55 | 25 | 29.30 | 30 |
| AV peak gradient (mmHg) | 34.82 | 34 | 22.64 | 16.6 |
| AV mean gradient (mmHg) | 23.97 | 20 | 11.98 | 8.4 |
| AV peak velocity (m/s) | 2.82 | 2.9 | 1.74 | 2.0 |
| EOAAV (cm2) | 0.77 | 0.67 | 1.20 | 1.4 |
| E wave (m/s) | 0.79 | 0.9 | 0.80 | 0.87 |
| A wave (m/s) | 0.54 | 0.6 | 0.54 | 0.54 |
| E/A | 1.47 | 1.5 | 1.48 | 1.6 |
| MR peak gradient (mmHg) | 118.59 | ̶ | 114.09 | 108 |
Parameters analyzed
Aortic-mitral geometrical parameters
Aortic-mitral structural coupling during the TAVR procedure was evaluated in terms of the geometrical parameters shown in Figure 4a. The following measurements were obtained during peak systole: a) AA and MA area, b) antero-posterior (AP) distance, defined as the distance between mid-anterior and mid-posterior MA points, c) anterolateral-posteromedial (AL-PM) distance, d) inter-commissural (CC) distance, e) MA height, defined as the maximum vertical distance between the highest and lowest points of the saddle-shaped MA, f) aortic-mitral angle, defined as the angle between the planes of the MA and the AA, g) aortic-mitral distance, defined as the centroid distance between the AA and MA, h) AA motion, defined as the longitudinal excursion of the anulus during TAVR, and i) MA motion, defined as the posterior displacement of the anterior annular segment during TAVR.
 Figure 4. (a) Aortic-mitral geometrical parameters, (b) Aortic-mitral coupling at peak systole.
Figure 4. (a) Aortic-mitral geometrical parameters, (b) Aortic-mitral coupling at peak systole.Fluid parameters
The regurgitant volume in the MV (RVMV) and the AV (RVAV) were obtained from the pre- and post-TAVR FSI models by integrating the negative MV systolic flow rate curve and negative AV diastolic flow rate curve over time, respectively. The RV was defined as the sum of the valve closing and the leakage volumes. Similarly, the stroke volume in the MV (SVMV) and in the AV (SVAV) were obtained by integrating the positive MV diastolic flow rate curve and positive AV systolic flow rate curve over time, respectively. MR severity was graded using the regurgitant fraction criterion,42 RFMV = RVMV/ LVSV, where LVSV is the total SV of the LV (SVAV + RVMV). AV effective orifice area was calculated as

Structural parameters
The three stent deployment height models were analyzed and compared during the TAVR procedure in terms of the peak (SɪMAX) and average (SɪAVRG) maximum principal stress values in the AV leaflets, sinus, calcification and MA; contact radial force between the stent and aortic root, and stent deformation. To facilitate comparison between different models and avoid the bias caused by local high stress concentration, only the 99-percentile values of the peak stress were evaluated.44 Moreover, leaflet annular regions were not included in the average stress calculation in order to avoid boundary effects. The contact radial force between the SEV stent and surrounding cardiac tissues was calculated as

Additionally, post-TAVR tissue mechanics were evaluated by the average maximum principal stress values calculated in the AV/TAV and MV leaflets during peak diastole and systole, respectively. Chordae forces (Fchordae) at peak systole were also reported. The force experienced by a particular chordae group was calculated as the sum of vectors representing the tension in each individual chorda attached to that chordae group.
Results
TAVR biomechanics
Figure 2 shows the crimped and deployed stent geometries at the three implantation depths. No potential risk for coronary obstruction was observed after TAVR. The shortest distance between the coronaries and the AV leaflets was found to be 7.6 mm for the high deployment model, which was between the left coronary ostia and fused leaflet. No evident gaps between the stent and aortic root were observed following TAVR, which was later confirmed in the FSI simulations by the absence of PVL.
Figure 3 presents the stress distribution on the AV leaflets after stent deployment. Similar stress patterns were observed for the three implantation configurations, with high stress values found in the fused leaflet, especially in the upper half region. For the high implantation model, the non-coronary leaflet (NCL) also presented high stress values in the upper region. As shown by the red circles, peak stress values were located in the leaflet-root attachment line close to the commissures, in the leaflet free edge in contact with the stent, and in the fused leaflet belly region in contact with the large calcification deposit.
 Figure 3. Stress distribution (MPa) in the AV leaflets after deployment. Red circles denote regions of peak stress values. A maximum stress value threshold of 3.5 MPa was applied such that higher stress values were displayed in gray, facilitating comparison between models.
Figure 3. Stress distribution (MPa) in the AV leaflets after deployment. Red circles denote regions of peak stress values. A maximum stress value threshold of 3.5 MPa was applied such that higher stress values were displayed in gray, facilitating comparison between models.Table 2 summarizes the peak and average stress values in the aortic tissues, the stent-tissue contact force, and stent eccentricity. The high deployment model gave the highest peak and average stress values in the AV leaflets, the lowest peak stress values in the calcification and MA, and the lowest stent-tissue contact force. All three SEV stents showed a similar deformation, with an eccentricity value of ~1.08.
Table 2. Intra-operative TAVR biomechanical parameters.
| Low | Optimal | High | |
|---|---|---|---|
| SɪMAX (MPa) | |||
| AV leaflets | 1.16 | 1.31 | 1.88 |
| Sinus | 0.80 | 0.89 | 0.90 |
| Calcification | 0.82 | 0.97 | 0.57 |
| Anterior MA | 0.250 | 0.141 | 0.024 |
| Posterior MA | 0.046 | 0.048 | 0.020 |
| SɪAVRG (MPa) | |||
| AV leaflets | 0.22 | 0.27 | 0.40 |
| Sinus | 0.10 | 0.12 | 0.09 |
| Contact radial force (N) | 31.70 | 39.55 | 28.79 |
| Stent eccentricity | 1.09 | 1.08 | 1.08 |
TAVR impact on MR: structural changes
Figure 4b and Table 3 present the aortic-mitral structural coupling before and after TAVR during peak systole. From Figure 4b it is evident that although the regurgitant orifice appeared to decrease in most post-TAVR models, MR was still present. As presented in Table 3, we quantified a marked reduction (>20%) in the MA height from pre- to post-TAVR states, with the highest reduction found for the optimal implantation model (35%). Similarly, the aortic-mitral angle decreased (~10%) after low and optimal configurations. Although the aortic-mitral distance (~4.2%), MA area (~2.5%), AP distance (<1%), and CC distance (<1%) decreased in all post-TAVR models, there were no significant changes when comparing these before and after TAVR. Similarly, both AL-PM distance and AA area had a slight tendency to increase after TAVR. Regarding the displacement of the valve annuli, it was found that the anterior MA was displaced posteriorly ~1.6 mm during the TAVR procedure, while the AA was displaced ~2.6 mm toward the left ventricular outflow tract (LVOT). Importantly, this annuli motion seemed to increase as the stent implantation height increased.
Table 3. Aortic-mitral geometrical parameters during peak systole.
| Pre-TAVR | Low | Optimal | High | |
|---|---|---|---|---|
| AA area (cm2) | 4.93 | 4.96 | 5.01 | 4.94 |
| MA area (cm2) | 11.40 | 11.19 | 11.15 | 10.99 |
| AP distance (mm) | 35.53 | 35.90 | 35.20 | 34.62 |
| AL-PM distance (mm) | 39.54 | 39.58 | 40.21 | 39.78 |
| CC distance (mm) | 31.14 | 30.82 | 31.02 | 30.74 |
| MA height (mm) | 6.00 | 4.43 (−26) | 3.93 (−35) | 4.66 (−22) |
| Aortic-mitral angle (⁰) | 123.05 | 111.26 (−10) | 110.10 (−11) | 121.26 |
| Aortic-mitral distance (mm) | 28.46 | 27.48 | 27.14 | 27.17 |
| AA motion (mm) | ̶ | 1.61 | 2.93 | 3.14 |
| MA motion (mm) | ̶ | 1.17 | 1.67 | 1.88 |
Note. Percentage variations with respect to the pre-TAVR model are reported in parentheses.
TAVR impact on MR: hemodynamic changes
Figure 5 shows the flow rate waveforms across the valves throughout the cardiac cycle for the pre- and post-TAVR models. The positive flow indicates forward flow toward the aorta (Figure 5a) and the LV (Figure 5b) during systole and diastole, respectively. On the other hand, the negative flow indicates the backward blood flow due to valve closure and regurgitation. Table 4summarizes the main hemodynamic parameters quantified for the pre- and post-TAVR models from the FSI simulations. Note that the diastolic flow across the MV was approximately the same for all models, since LV/LA size and motion were assumed to remain unchanged immediately post-TAVR.
 Figure 5. Flow rate (ml/s) across the (a) AV and (b) MV throughout the cardiac cycle.
Figure 5. Flow rate (ml/s) across the (a) AV and (b) MV throughout the cardiac cycle.Table 4. Pre- and post-TAVR FSI hemodynamics.
| Pre-TAVR | Low | Optimal | High | BEV Optimal19 | |
|---|---|---|---|---|---|
| SVAV (ml) | 46.28 | 47.89 | 43.97 | 51.01 | 52.24 |
| RVAV (ml) | 9.34 | 7.91 | 6.87 | 15.19 | 11.43 |
| SVMV (ml) | 74.64 | 76.12 | 77.60 | 69.54 | 74.54 |
| RVMV (ml) | 37.59 | 35.96 | 40.49 | 33.65 | 33.84 |
| RFMV (%) | 44.82 | 42.89 | 47.94 | 39.75 | 39.32 |
| MR severity (RFMV) | Moderate-to-severe | Moderate-to-severe | Moderate-to-severe | Moderate | Moderate |
| LVEF (%) | 28.55 | 28.54 | 28.75 | 28.82 | 29.30 |
| LV mean systolic pressure (mmHg) | 95.15 | 91.16 | 89.38 | 91.59 | 91.77 |
| AV peak gradient (mmHg) | 34.82 | 27.32 | 27.78 | 28.13 | 22.64 |
| AV mean gradient (mmHg) | 23.97 | 15.66 | 15.69 | 16.24 | 11.98 |
| AV peak velocity (m/s) | 2.82 | 1.76 | 1.66 | 1.70 | 1.74 |
| EOAAV (cm2) | 0.77 | 1.00 | 0.93 | 1.04 | 1.20 |
| E wave (m/s) | 0.79 | 0.80 | 0.78 | 0.67 | 0.80 |
| A wave (m/s) | 0.54 | 0.56 | 0.56 | 0.49 | 0.54 |
| E/A | 1.47 | 1.44 | 1.40 | 1.36 | 1.48 |
| MR mean gradient (mmHg) | 57.25 | 51.83 | 52.85 | 50.90 | 50.79 |
| MR peak velocity (m/s) | 5.42 | 5.18 | 5.13 | 4.89 | 4.92 |
Several findings can be described from Figure 5 and Table 4. First, improved systolic function and hemodynamic success of the procedure was found in most post-TAVR models, given by lower AV peak/mean pressure gradients, AV peak velocity, MR mean pressure gradient, and MR peak velocity; and higher EOAAVand SVAV. Second, the greater degree of MR improvement (~10%) was for the high deployment model; which based on the RFMV criterion can now be classified as moderate MR. The degree of MR severity remained unchanged for the low and optimal implantation models. Third, the optimal deployment model gave a higher RVMV than the pre-TAVR model (40.49 vs 37.59 ml). Due to the coupled valve dynamics and mass conservation, this model also gave a lower SVAV than the pre-TAVR model (43.97 vs 46.28 ml).
Fourth, central AV regurgitation was resolved following TAVR for the low and optimal implantation models, while the SEV of the high deployment model did not fully close until mid-diastole (Figure 5a). No PVL was quantified in any of the post-TAVR models, as found clinically. Fifth, when comparing the pre- and post-TAVR AV flow waveforms shown in Figure 5a, a noticeable change in valve closure dynamics was found. The TAV leaflets had a distinct slower closure and higher closing volume than the native AV leaflets.
Finally, Figure 6 shows the intraventricular velocity streamlines colored by velocity magnitude during peak systole. Due to the restricted PML motion, the pre-TAVR model displayed a posteriorly directed regurgitant jet, which qualitatively and quantitatively matched the regurgitant jet measured clinically.19 The regurgitant jet structure was similar between pre- and post-TAVR states, with an eccentric “wall-hugging” jet that impinged the postero-lateral LA wall. The strength and velocity of the jet, however, decreased following TAVR (Table 4).
 Figure 6. Pre- and post-TAVR velocity streamlines showing the regurgitant jet structures at peak systole. Note the different velocity scales between pre- and post-TAVR models.
Figure 6. Pre- and post-TAVR velocity streamlines showing the regurgitant jet structures at peak systole. Note the different velocity scales between pre- and post-TAVR models.Post-TAVR biomechanics
Table 5 shows the average stress values in the AV/TAV leaflets and MV leaflets during peak diastole and systole, respectively. Following TAVR, AML stress decreased between 5% – 21%, while PML stress decreased between 14% – 42%, potentially due to the reduction in the MR driving force. In general, the AML was subjected to a higher stress than the PML before and after TAVR. An imbalanced chordae force distribution was quantified for the pre- and post-TAVR models; a result of the restricted PML motion and postero-lateral chordae tethering. A higher tension was found for the PML chordae than for the AML chordae. Following TAVR, there was a force redistribution between the different chordae groups. AML and PML basal chordae tension decreased at least 36% and 22%, respectively, while AML marginal/strut and PML marginal chordae tension either increased or decreased depending on the stent implantation depth. More importantly, the total chordae tension decreased following TAVR (~20 N vs ~15 N) for the low and high implantation models, which suggests an improvement of mitral leaflet tethering. This phenomenon was not quantified for the optimal deployment model.
Table 5. Pre- and post-TAVR tissue mechanics.
| Pre-TAVR | Low | Optimal | High | |
|---|---|---|---|---|
| SɪAVRG (MPa) | ||||
| AV/TAV | 0.076 | 0.113 | 0.106 | 0.108 |
| AML | 0.126 | 0.120 (−5) | 0.109 (−14) | 0.099 (−21) |
| PML | 0.082 | 0.048 (−42) | 0.071 (−14) | 0.056 (−32) |
| Fchordae (N) | ||||
| AML marginal | 1.14 | 0.90 (−21) | 1.14 | 1.28 (13) |
| AML strut | 2.04 | 2.67 (31) | 3.07 (51) | 1.82 (−11) |
| AML basal | 3.84 | 2.42 (−37) | 2.48 (−36) | 2.31 (−40) |
| PML marginal | 6.13 | 4.25 (−31) | 6.91 (13) | 5.41 (−12) |
| PML basal | 7.33 | 5.19 (−29) | 5.72 (−22) | 4.99 (−32) |
Note. Percentage variations with respect to the pre-TAVR model are reported in parentheses.
Discussion
The main findings of this study can be summarized as follows: 1) Although improved systolic function was quantified for most post-TAVR models when using a SEV, no marked differences (<10%) in early MR improvement/worsening were noted at the three implantation depths, 2) For this patient case, the high implantation model gave the highest reduction in the RVMV and MR severity, and the lowest stent-tissue contact force. On the contrary, the optimal implantation model gave a higher RVMV (~8%) than the pre-TAVR model, the highest stent-tissue contact force, and no mitral leaflet tethering improvement, 3) Acute changes in MR severity following TAVR were predominantly related to: i) structural changes in the aortic-mitral complex caused by the grade of stent apposition and device anchoring, and ii) hemodynamic changes in global LV afterload/impedance caused by relief of the AV obstruction.
Effect of TAVR on MR severity
While previous imaging studies evaluated the geometrical changes on aortic-mitral anatomy when using the BEV Edwards Sapien device,10,11 to the authors’ knowledge, only one clinical study quantified the structural impact of the SEV Medtronic device.12 In agreement with the latter study, we found that the MA height had a marked reduction following TAVR, while the MA area, AP distance, CC distance and aortic-mitral distance showed no significant changes before and after TAVR. Besides confirming these structural changes clinically observed, three additional findings can be described from our engineering analysis: First, we quantified a mechanical compression of the AV and MV annuli during the TAVR procedure, with increasing magnitude as the SEV implantation height increased. The anterior MA was displaced ~1.6 mm posteriorly, whereas the AA was displaced longitudinally ~2.6 mm toward the LVOT (Table 3). Second, the post-TAVR model that demonstrated the highest MR reduction (high implantation model) presented the lowest stent-tissue contact force, and the largest mitral and aortic annuli compression (Tables 2 and 3). Third, the post-TAVR model that gave a worse MR than the pre-TAVR model (optimal implantation model) presented the highest stent-tissue contact force, and no mitral leaflet tethering improvement (Tables 2 and 5). Overall, these results give a plausible mechanistic proposition for early MR improvement following TAVR. Geometric perturbation of the aortic-mitral fibrous continuity during TAVR can contribute to improved MV leaflet coaptation and decreased leaflet tethering; therefore reducing MR. These mechanistic parameters may suggest early avenues of MR improvement, such as contact force between the TAVR stent and the surrounding tissue, with potential compression of the aortic-mitral continuity.
From a hemodynamics perspective, after relief of the AV obstruction by TAVR, acute reduction of the LV afterload accounted for an improvement in systolic function and better hemodynamic balance. In fact, LV pressure immediately decreased following TAVR, and consequently, the MR pressure gradient decreased, which contributed to a reduction in the pathological retrograde flow through the MV (Table 4). Although AV peak and mean pressure gradients were similarly reduced among the three post-TAVR models, there was a trend toward a higher reduction of the MR pressure gradient among those models with improved MR (Table 4). Assessment of MR severity by Doppler echo under severe AS can be challenging and relies on several mathematical assumptions.45For example, MR jet velocity may increase due to a high LV pressure. A large color Doppler jet with a high driving velocity but small effective regurgitant orifice area is probably not severe MR, and is likely to appear less severe after TAVR.46 On the other hand, moderate-to-severe MR may lead to underestimation of the severity of the AS, since the decreased SVAV due to MR lowers the flow across the valve and, hence, the AV velocity and gradient.47 To the best of our knowledge, this is the first in-silico study to directly quantify the changes in the intraventricular hemodynamics pre- and post-TAVR under different SEV deployment configurations, and to accurately measure the RVMVirrespective of MR jet geometry and geometric assumptions.
SEV vs. BEV performance
Procedural characteristics, including the type of TAV, may also influence the post-operative degree of MR. When comparing the results of this study with our previous work that evaluated the impact of TAVR on MR severity when using a BEV device,19 we found no significant changes (<10%) in the RVMV following TAVR, independent of the TAV type. It should be emphasized that in both computer studies, the same patient-specific LH model was used, and post-TAVR dynamics were simulated under the same loading and boundary conditions. Although no marked changes occurred in the degree of MR, some important differences were identified when comparing SEV and BEV device performance. First, there was a trend toward a more consistent MR reduction with the BEV, whereas the likelihood of improvement was lower and less predictable when using a SEV; with mixed MR improvement/deterioration results depending on the deployment height. As shown in Table 4, when comparing SEV vs. BEV performance at an optimal implantation depth, while the SEV model gave a higher RVMV than the pre-TAVR model (40.49 vs 37.59 ml), the BEV model gave the lowest RVMV (33.84 vs 37.59 ml). Moreover, while the optimal SEV model was the only post-TAVR model that resulted in worsening of MR, the optimal BEV model led to the highest decrease in the RVMV between the three BEV implantation models.19 These results are in agreement with clinical studies that point toward a greater degree of MR reduction in patients treated with a BEV as compared to a SEV.2,15,16,48,49
Several valve type-related factors have been postulated to help explain these differences in MR outcome: i) it has been suggested that the longer frame of the SEV may anatomically or functionally interfere with the AML, especially in the presence of a low implantation.50,51 This was not confirmed in a large CoreValve series,52 or found in this computer study. In line with this hypothesis is the observation that a deep positioning of the SEV could be correlated with MR worsening,50 although this has not been observed in all clinical studies.53 Our data also rule out this phenomenon for this patient-specific model, since MR worsening only occurred in the post-TAVR model with an optimal implantation height, ii) the first generation CoreValve device has been associated with a higher degree of PVL, which may maintain volume overload and contribute to a lower MR improvement.54,55 PVL however, was not detected in any of our post-TAVR computer models. Moreover, the first-generation SEV has now been replaced by newer and enhanced versions with low PVL rates which are usually implanted at a shallower depth than the older generation CoreValve device, and iii) the need for pacemaker implantation and a higher rate of left bundle branch block seem higher after SEV implantation, which can lead to LV asynchrony and adversely affect MR.56,57 These clinical factors, however, were not taken into consideration in this study.