1. Introduction
Bioenergy is a sustainable solution to reduce dependence on fossil-based fuels. Rising energy demand and problems associated with conventional feedstocks has driven the quest for novel feedstocks for bioenergy generation. Great amount of interest has been shown by researchers, policy makers and energy industry in identifying and developing novel feedstocks in recent past. A considerable amount of research is currently being conducted in this area mainly focused on identifying suitable feedstocks, developing efficient conversion techniques and reducing the overall production cost. Much emphasis is given on lignocellulosic waste, municipal waste, microalgae, fungi and other biomass in recent past as biofuel feedstocks (Vasić et al., 2021). These feedstocks has shown promising potential for biofuels production (Raslavičius et al., 2018). Energy is commodity product and therefore huge amount of feedstock is required. Aquatic weeds are unwanted growth and present in such a great quantity that lot of efforts goes in managing these weeds. These unutilized weeds can be directed towards production of bioenergy and other value added products. This abundant potential feedstock is scarcely studied for its biofuel production potential and needs attention from researchers and policy makers.
Aquatic weeds are unwanted plants which grow in water bodies and cause problems to aquatic fauna and water quality(Ganguly et al., 2012). However, recent studies have shown its potential as phytoremediator, feedstock for biofuels and as a resource to develop fertilizer. Managing aquatic weeds through its potential application gained wide attention throughout the world mainly due to their alarming reproductive capacity, which can result in serious ecological damage to water sources (Feng et al., 2017). The damage caused due to aquatic weeds can be prevented by using them as raw material not only for the fuel but also for the production of chemicals and materials, i.e. the development of aquatic weed based biorefineries (Farrell et al., 2006). Aquatic weed biomass as biofuel feedstock provides many advantages over terrestrial energy crops. Aquatic weed biomass does not necessarily require scarce resources such as arable land and fresh water. Due to its fast growth rate aquatic weed biomass productivity could be higher than many terrestrial energy crops. Global distribution of aquatics weeds advocates its use as biofuel feedstock to provide energy security.
Aquatic weeds have been studied for several other applications such as fertilizer production, medicinal properties, enzymes and industrially important chemicals production. Development of sustainable biorefinery could be envisaged considering these applications and biofuel production potential of aquatic weeds. This review critically evaluates the potential of aquatic weed as a feedstock for various biofuels. The review also emphasizes on distribution and problems caused by aquatic weed. Beneficiation of wastewater, aquatic weed based biorefinery, techno-economic challenges and future prospects are also discussed for production of biofuels using aquatic weed biomass.
2. Problems associated with aquatic weed and its management
The aquatic weeds include widespread vegetation in different aquatic ecosystems. Aquatic weeds are widespread throughout the world from tropics to every temperate zone and are generally termed as nuisance throughout even in sub-arctic regions. Aquatic weeds are found in various types of aquatic bodies such as lakes, dams, ponds, tanks, canals. The major aquatic weeds found in different part of the world are listed in Table S1.
Aquatic weeds or macrophytes reproduce at rapid rates and start creating various socio-economic problems. Aquatic weed invasion have become obstacle for ecologists and people dependent on water body as they degrade the water quality and also affect the biodiversity. Aquatic weed restrain the growth and population of planktons and fishes, and negatively impact the net productivity of infested water body (Zhang et al., 2007). Overgrowth of these aquatic weeds stimulates the deposition of sediments causing siltation ultimately decreasing the depth of water body which affects the habitat of the fauna living in that water body (Varshney and Babu, 2008). Aquatic weed occupy the living space of the fauna thereby limiting their movement which increases the competition among the population, adversely affecting the population size(Mehta et al., 1973). It also reduces the light penetration inside the water body by forming mats, resulting in lower primary production and also hampers the nutrient cycling (Aloo et al., 2013). They also restrict activities like aquaculture, fishing, irrigation canals and agricultural practices (Abbas et al., 2015).They create stagnant water conditions which may provide nesting and propagating conditions for harmful insects like mosquitoes. The leaves and roots of these weeds protect them from their predator. These insects are responsible for several diseases like malaria, dengue, yellow fever, chikungunya etc. (Mailu, 2001). In addition to these problems, aquatic weed is also a cause of concern over their allelopathic effect on crops, nutrient availability in the water body and other problems in agro-ecosystem.
To control and manage invasion of water bodies by aquatic weeds, huge amount of efforts and resources are used worldwide. Various mechanical, chemical and biological control measures are used to remove aquatic weeds from infested water bodies. Manual removal of aquatic weeds is also practiced. These control methods are cost intensive and most the time are ineffective in the long term. Jayan and Sathyanathan (2012) reported that annual approximate cost for manual control is $490 per hectare and for mechanical control is $910 per hectare. Once removed from water bodies, aquatic weed as a waste needs proper management for its disposal. Transport and disposal of aquatic weed also puts extra pressure on waste management systems. Various applications of this aquatic weed biomass will lead to waste to value generation. Researchers worldwide are investigating aquatic weeds for their potential application in the production of biofuel, fertilizer, medicines, etc(Jayan and Sathyanathan, 2012).
3. Aquatic weeds as biofuel feedstock
Aquatic weeds due to their high compositional richness in carbohydrates, proteins and lipids can be utilized for the production of biofuels (Table 1).Various types of biofuel can potentially be produced from aquatic weed (Khan, S., & Sarvvar, 2002). Aquatic weeds are composed of significant amount of cellulose, hemicellulose and lignin which could be converted to biofuels. Lignin and sugars are major component of aquatic weed biomass. Lignin could be used to produce bio-oil, combustible gases and heat energy by thermochemical techniques. The sugar part can undergo direct fermentation to produce bio-ethanol. There is also a vast possibility to produce other alcohol compounds such as bio-methanol and bio-butanol (Bhattacharya et al., 2010). Aquatic weeds also contain lipids and a waxy coating on its surface which is essentially made up of modified fatty acids. Biodiesel can be produced from these lipids by transesterification process (Naik et al., 2010).Aquatic weeds can also be used for biomethane production using anaerobic digestion and biohydrogen production through biological processes (Nong et al., 2020). Aquatic weeds are fast propagating plants and thus it can give comparatively higher biomass productivities compared to many terrestrial energy crops (Table 2).
Table 1. Biochemical composition of aquatic weed and other feedstocks.
| Feedstocks | Carbohydrate (%) | Lipid (%) | Protein (%) | Ash (%) | References |
|---|---|---|---|---|---|
| Aquatic weed | |||||
| Eichhornia cressipes | 38.7 | 0.36 | 12.5 | 19.4 | Sunwoo et al. (2019) |
| Azollafiliculuoides | 41 | – | 20 | 7.3 | Miranda et al. (2016) |
| Lemna minor | 310 mg g−1 | 16.79 | – | 18.7 | Gusain and Suthar (2017) |
| Pistiastratiotes | 49.45 | 3.56 | – | 23.75 | Pantawong et al. (2015) |
| Ceratophylumdemursum | – | 5.97 | 21.7 | 20.6 | Varshney and Babu (2008) |
| Nymphaeaenouchali | 13.92 | 0.10 | 17.47 | 1.6 | Parimala and Shoba (2013) |
| Other feedstocks | |||||
| Sugarcane | 33.4 | 1.3 | 2.9 | 7.4 | Brehmer (2008) |
| Maize | 32.3 | 1.7 | 10.6 | 7.1 | Brehmer (2008) |
| Chlorella sp. | – | 19 | 22 | 3 | Hossain et al. (2019) |
| Sunflower seed | 27.76 | 36.85 | 25.08 | 4.8 | Ingale and Shrivastava (2011) |
| Jatropha seed | 18.35 | – | 27.65 | 6 | Thomas Odey et al. (2018) |
| Switchgrass | – | 7.3 | 7.3 | 4.8 | Bals et al. (2007) |
‘-’ Not Reported.
Table 2. Biomass productivity of aquatic weed and other bioenergy feedstocks.
| Feedstocks | Productivity (ton/ha/year) | References |
|---|---|---|
| Aquatic weeds | ||
| Eichhornia crassipes | 60–100 | Mishima et al. (2008) |
| Pistiastatiotes | 60–110 | Mishima et al. (2008) |
| Typha spp. | 40 | (Zhang et al., 2011) |
| Duckweed | 33 | Xu et al. (2011) |
| Phragmitesaustralis | 30 | Köbbing et al. (2013) |
| Salviniamolesta | 53 | Abbasi et al. (1995) |
| Other feedstocks | ||
| Poplar | 15 | Wang et al. (2012) |
| Eucalyptus | 15–25 | Lima et al. (2013) |
| Switchgrass | 10.9 | (Wang et al., 2010) |
| Sugarcane | 60–74 | Jayus et al. (2016) |
| Grain sorghum | 5.57 | Williams et al. (2016) |
| Corn | 6.61 | Williams et al. (2016) |
| Corn stover | 40 | McAloon et al. (2000) |
3.1. Bioethanol
Over recent years there have been several advancements in conversion technologies to produce bioethanol. As a common methodology, aquatic weed biomass like any other bioethanol feedstock is pretreated using simple hydrolysis processes and subsequently sugars obtained are fermented to produce bioethanol (Kityo et al., 2020). High cellulose and hemicellulose and low lignin content of aquatic weed makes it promising feedstock for bioethanol production (Table 3). Aquatic weed as a raw material for producing bioethanol is relatively new area of research where only scattered research reports are available for aquatic weeds like duckweed, water lettuce, water hyacinth and so forth. Mishima et al. (2008) compared the bioethanol production from Eichhornia crassipes and Pistia stratiotes biomass with common agricultural raw materials particularly wheat straw and cotton gin waste and found comparable yields. The production of bioethanol may vary depending upon the composition of fermentable sugars like glucose and xylose present in aquatic weeds. There is a need to thoroughly investigate the potential of various aquatic weed as bioethanol feedstock and determine its sugar composition for employing effective conversion technologies.
Table 3. Chemical composition of lignocellulosic feedstocks.
| Feedstock | Lignin % | Cellulose % | Hemicellulose % | References |
|---|---|---|---|---|
| Aquatic weeds | ||||
| Eichhornia crassipes | 2.01 | 30.65 | 20.82 | Poomsawat et al. (2019) |
| Pistiastatiotes | 1.14 | 37.83 | 5.26 | Mthethwa et al. (2019) |
| Lemna minor | 1.5 | 30.4 | 23.6 | Li et al. (2019) |
| Landoltiapunctata | 1.8 | 26.64 | – | Su et al. (2015) |
| Elodea nuttalli | 3.2 | 35.9 | – | Koyama et al. (2014) |
| Egeriadensa | 4.4 | 36.2 | 1.9 | Koyama et al. (2014) |
| Azollamicrophylla | 10.13 | 27.4 | 15.67 | Kaur et al. (2019) |
| Azollafiliculoides | 14.7 | 29.0 | 16.5 | Pirbazari et al. (2019) |
| Salviniamolesta | 13.96 | 29.71 | 9.90 | Syaichurrozi et al. (2019) |
| Typha spp. | 12.5 | 36.7 | 16.9 | Froese et al. (2020) |
| Other feedstocks | ||||
| Corn stover | 19 | 38 | 26 | Kumar and Sharma (2017) |
| Rice straw | 12 | 38 | 32 | Baruah et al. (2018) |
| Barley straw | 14–19 | 31–45 | 27–38 | Saini et al. (2015) |
| Wheat straw | 17–19 | 33–38 | 26–32 | Saini et al. (2015) |
| Switchgrass | 12–20 | 45 | 32 | Ussiri and Lal (2014) |
| Peanut | 41 | 47 | 10 | Fang et al. (2014) |
| Poplar | 21–29 | 42–49 | 16–23 | Sannigrahi et al. (2010) |
| Coconut | 27 | 34 | 21 | Bledzki et al. (2010) |
| Municipal Solid Waste | 10–14 | 33–49 | 9–16 | Andresen and Li (2012) |
| Pine (Softwood) | 25–35 | 45–50 | 25–35 | Bajpai (2016) |
| Miscanthus giganteus(Silvergrass) | 17–21 | 37–45 | 19–25 | Haffner et al. (2013) |
‘-’ Not Reported.
The choice of pre-treatment and microorganism for saccharification and fermentation dictates the yield of ethanol production from aquatic weeds. Aswathy et al. (2010) observed 4.4 gL-1 of ethanol production from E. crassipes upon fermenting the hydrolysate with Saccharomyces cerevisiae. When E. crassipes biomass was pretreated with acid-alkali followed by fermentation 0.21 g g−1 of ethanol production was observed (Mukhopadhyay and Chatterjee, 2010). Similarly water hyacinth biomass for the production of bioethanol was studied by Magdum, (2012) using yeast strain Pichia stipitis NCIM 3497 to obtain a bioethanol yield of 19.2 gL-1.These studies showed that the same Eichhornia biomass showed varying yields by applying different pre-treatment methods and microorganisms. Thus, there is need to identify an efficient conversion technology and consortia of microorganisms for bioethanol production from aquatic weeds as the results are very promising when compared with the ethanol yield of other agricultural lignocellulosic feedstock such as that of 7.0 gL-1 ethanol yield of corn stover (Jin et al., 2012)and 28.6 gL-1 ethanol yield of rice straw (Suriyachai et al., 2013). Manivannan and Narendhirakannan (2015) in their study tried finding most suitable yeast strain to produce ethanol from Eichhornia crassipes biomass. They employed four potential yeast strain namely Pichia stipitis, Pachysolen tannophilus, Saccharomyces cerevisiae and Candida intermedia. Their results showed that the yeast strain P. tannophilus gave maximum bioethanol yield of 0.043 g g−1 from E.crassipes biomass, followed by P. stipitis and C. intermedia with the bioethanol yield of 0.037 g.g−1and 0.021 g.g−1respectively. S cerevisiae showed least bioethanol yield of 0.015 g g−1 and the reason was attributed to be the inability of the strain to assimilate pentose sugar.
Other aquatic weeds have also been investigated to produce bioethanol. Ge et al. (2012) demonstrated bioethanol production using Lemnagibba (duck weed) employing two strains of yeast (conventional yeast ATCC 24859 and self-flocculating yeast SPSC01) for fermentation. Their results showed mean bioethanol yield of 0.485 g g−1. Pandey et al. (2014) used aquatic weed azolla to estimate bioethanol production using Saccharomyces cerevisiae and Klebisellaoxytoca. They reported bioethanol production using Saccharomyces cerevisiae and Klebisellaoxytoca to be 5.20% and 4.04% respectively. Sunil et al. (2015) investigated the potential of six aquatic weeds (Typhalatifolia, Eichhornia crassipes, Alternantherasessilis, Baccopamonnieri, Pistiastratiotes and Ipomoea aquatica) for production of bioethanol. They employed different yeast strain from fruits of Ananascomosus, Musa paradisiaca, Citrulluslanatus, Manilkarazapota, CucumismeloandPunicagranatum. The results showed that Typhalatifolia and Alternantherasessilis showed highest alcohol yield of 115.4 and 160.5 μg ml−1 respectively. Bioethanol is most widely studied among biofuels produced from aquatic weeds. Still there is a need for studies to make overall process efficient and cost efficient.
3.2. Biodiesel
Aquatic weeds are predominantly researched for the production of fuels viz. bioethanol, biogas, bio-oil, etc. rather than biodiesel as lipid content of biomass is comparativelylow. Due to lower lipid content, the biodiesel yield is much lower in comparison to other biodiesel feedstock such as vegetable oil. Production of biodiesel from aquatic weed is still a new area of research and a field which demands attention. Brouwer et al. (2016) estimated lipid content of Azolla sp. to be 7.92% and similarly Gusain and Suthar (2017) estimated lipid content of Lemna sp. as 1.26% and stated that these aquatic weeds could be suitable for the synthesis of biodiesel. There is very limited literature available on using aquatic weed as biodiesel feedstock. Venu et al. (2019) investigated the efficiency of water hyacinth as a potential biodiesel feedstock and its compatibility in compression ignition engines. In their work, Water Hyacinth Biodiesel (WHB) was blended with petroleum diesel at different proportions (10%, 20%, 30%, 40%, and 100%) to analyze their efficiency and performance as per ASTM set standards for its possible utilization as biodiesel mix. The results showed that 20% WHB blend outperformed other blending proportions in term of performance and tailpipe emission status. The blend was also stated as to be as prominent as neat biodiesel fuel in terms of smoke, HC and CO emission. However, on the downside CO2 and NOx emission of blends were considerably higher in comparison to neat diesel fuel. Aquatic weed has an obvious advantage that make it a sustainable biofuel feedstock, however research based on production of only a single fuel, i.e. biodiesel is still at infancy and significant development is still required in the future.
3.3. Bio-oil
The bio-oil is generally a blend composed of several organic compounds. These components are used as a raw material for synthesis of some pure chemicals like phenol, alcohol, organic acids, aldehyde, etc. (Asadullah et al., 2007). Bio-oil has the potential to be upgraded to refined fuels (No, 2014).It also contains chemicals in economically recoverable concentrations which can be further utilized for other applications (Şensöz and Angın, 2008). Bio-oil has great advantages in terms of combustion, storage, transport, flexibility and retrofitting in production and marketing.
There is a paucity of data in the literature on bio-oil production using aquatic weed. Some researchers have tried producing bio-oil and other co-products from aquatic weed using thermochemical processes. Muradov et al. (2010) tried determining main products of Lemna minor (duckweed) through pyrolysis process. The work identified major gaseous and liquid products of duckweed obtained through the process of pyrolysis. Their result showed that even at relatively lower intensity of pyrolysis(Temp: 500 °C), the bio-oil obtained amounted to 40 wt% of dried biomass of Lemna minor. The authors concluded that the amount of bio-oil can increase depending on the pyrolysis temperature. Similarly, Bhattacharjee and Biswas (2018) evaluated Alternantheraphiloxeroides (alligator weed) for its potential to produce bio-oil and its physico-chemical characteristics. The whole experiment was conducted inside a semi batch quartz glass reactor at a temperature ranging from 350 to 550 °C with constant heating temperature rate of 25 °C.min−1 and constant nitrogen flow at a rate of 0.1 L min−1. The results showed bio-oil yield of 40.10 wt% of dried biomass at 450 °C. Apart from this, the authors also studied the effect of different pyrolysis process parameters on the yield of bio-oil. Four sets of temperature rate of 25, 50, 75 and 100 °C.min−1 and different nitrogen flow rates of 0.2, 0.3, 0.4 & 0.5 L min−1 were selected for the second experiment. The result for this experiment showed maximum bio-oil production (43.15 wt%) at heating rate of 50 °C.min−1 along with 0.2 L min−1 nitrogen gas flow rate.
Wauton and Ogbeide (2019) characterized the bio-oil produced from fixed bed pyrolysis reactor using Eichhornia crassipes biomass at temperature of 450 °C and nitrogen flow rate of 100 cm3 min−1.The GC-MS analysis showed the bio-oil composition with major 21 compounds. Authors characterized the bio-oil and found the heating value of 28.35 MJ kg−1. The bio-oil obtained was found to have insignificant nitrogen content and no sulfur content and thus the authors concluded this to be an environmentally friendly fuel. In another study, Promdee et al.,(2012) conducted a comparative study for the bio-oil produced from Manila grass (Zoysia Matrella) and Water hyacinth (Eichhornia crassipes) at 450–600 °C. They studied the physical and chemical properties of the fuel and found it to be suitable.
3.4. Biohydrogen
Biohydrogen is considered as a promising green energy and an alternative to fossil fuels because of its higher energy content (122–142 kJ g−1) in comparison to other fuels such as biomethane (approx. 56 kJ g−1) and biodiesel (37 kJ g−1) (Vi et al., 2017). On the advantageous side biohydrogen can be produced under normal temperature and pressure, requires low energy input and releases only water as bioproduct on combustion (Mishra and Maiti, 2017; Mitsushima and Hacker, 2018). Biohydrogen is used in fuel cells, petroleum engines, vehicle engine etc. (Łukajtis et al., 2018). Currently 96% of the H2 production is obtained from fossil fuels which are very costly and non-efficient (Mohanty et al., 2015). Therefore, biological alternatives are currently being explored with considerable achievements and aquatic weeds seem as promising biological option for cleaner and cost efficient biohydrogen production.
Several aquatic weeds species are currently being studied for biohydrogen production. Mthethwa et al. (2018) studied Pistia stratiotes for the production of fermentative biohydrogen using dark fermenting method. The experiment was conducted at optimum pH of 5.5 and temperature 25 °C. The result showed 2.46 ± 0.14 mol-H2.mol-glucose of hydrogen production upon acid-hydrolysis using H2SO4. The result was significantly high when compared to biohydrogen production from other feedstocks in studies performed by Pattra et al. (2008) where biohydrogen production of sugarcane baggase was found to be 1.73 mol-H2/mol-glucose as well as rice straw with yield of 0.76 mol-H2/mol-glucose (Lo et al., 2010). Hydrogen productivity was confirmed using microbial analyses including FISH (fluorescence in-situ hybridization), PCR and qPCR. Reliability of the fermentation was further confirmed by electron equivalent balance of 92–98%. The final cost of fermentation system was established to be 0.08 $.kg−1dry mass of Pistia stratiotes. Similarly, biohydrogen production through dark fermentation method for Eichhornia crassipes using Pseudomonas aeroginosawas conducted by Mechery and Sylas (2016). The study included the estimation of hydrogen production through different acid and alkali pretreatment concentrations (viz. 2%, 4% and 8%) using H2SO4 and NaOH. The result showed that hydrogen production was maximum for 2% acid concentration (19.55%) in comparison to 4% and 8% acid concentration (4.28% and 4.20% respectively) and similarly, hydrogen production was highest at 2% alkali concentration (33.51%) in comparison to 4% and 8% alkali treatment (4.35% and 4.21% respectively). The author also stated that methane was not observed in any of the treated reactors.
Pattra and Sittijunda (2015) conducted a study for the optimization of factors that affect hydrolysis process for improving the production of biohydrogen using water hyacinth. The study was conducted in two consecutive steps first, reaction time (h), acid concentration (v/v) and rotating speed (rpm) was investigated. After this, the produce of acid hydrolysis was treated with anaerobic sludge for biohydrogen production. The results showed that for the first part (Water hyacinth Stem hydrolysis) optimum reaction time was established at 7.73 h. Similarly, 1.31% H2SO4 (v/v) and 264.41 rpm was shown to be the optimum for the maximum sugar production of 13gL-1. The final result showed maximum biohydrogen synthesis of 127.7 mmol H2.L−1 under optimized conditions.
3.5. Biomethane
Biomethane is one of the most versatile and clean burning biofuel obtained through anaerobic digestion process from a wide variety of raw material. The advantages of biomethane include its easy storage upon liquefaction, easy transport and supply through same pipeline which is used to supply natural gas (Urban, 2013). The byproduct of the production process can additionally be used as fertilizer in farmland. Biomethane can be produced from all kinds of biomass which also has advantages over other feedstocks not only from renewability term but also from waste disposal perspective (Koupaie et al., 2019). Therefore, aquatic weeds in particular offer great potential as feedstock as it can be directly applied for the production of biomethane. Potential of aquatic weed (Eichhornia crassipes) within a slurry mixture for the production of biomethane was explored by Clementson et al. (2017). The authors conducted an experiment in which chopped biomass of water hyacinth was mixed with different combinations of manure (100%, 75%, 50%, 25% and 0%) with 250 mL of water in each bioreactor. Biomethanation was conducted with retention time of 6 weeks between mesophilic temperature ranges (20–45 °C) for five concentrations of fermentation slurries. The results showed high biomethane production from the co-digested water hyacinth and manure in comparison to the instances where water hyacinth or manure was digested alone. Particularly 25:75 ratio of weed and manure mix resulted in highest biogas and biomethane production. The study concludes that the manure in slurry functioned as inoculum of microorganisms and the microorganisms successfully digested the organic lignocellulosic biomass of aquatic weed together resulting in high yield of biomethane. Güngören Madenoğlu et al. (2019) investigated biomethane production potential of water lettuce using waste sludge as inoculum in batch system experiments. The findings showed that highest biogas yield of 321 mL g−1 which comprises of 72.5% methane. Experimental biomethane yield was reported to be 81.5–232.7 mL CH4/g volatile solid which was 27.7–79.0% of the theoretical maximum value and energy conversion efficiency of 78.4%was observed.
Kaur et al. (2019) investigated a biorefinery approach for the production of bioethanol and biomethane using Lemna minor, A. microphylla and E. crassipes. The whole experiment was conducted following four different conditions, where each condition comprised of three consecutive processing steps: Condition (A): Hydrothermal Processing, Anaerobic Digestion and Ethanol Fermentation (HT-AD-EF). Condition (B): Thermochemical Pretreatment, Anaerobic Digestion and Ethanol Fermentation (TC-AD-EF). Condition (C): Hydrothermal Treatment, Ethanol Fermentation and Anaerobic Digestion (HT-EF-AD). Condition (D): Thermochemical Treatment, Ethanol Fermentation and Anaerobic Digestion (TC-EF-AD)). The results showed that anaerobic digestion of aquatic weed biomass as per condition(A)produced higher methane with yield of 32.9–52.5 m3ton−1 which was attributed to the less inhibitor generation during hydrothermal production as compared to other treatment methods. The study concluded that HT + AD combination can prove to be efficient, eco-friendly and cost-effective method and can be used over other options such as chemical pretreatment alone for improving the utilization efficiency of cellulose and hemicellulose component of aquatic weed or any biomass which has relatively low lignin content (lower than 10%). This ultimately resulted in enhancement of bioenergetic potential of biomass. Table 4 depicts the biofuels produced using various aquatic weed as feedstock.
Table 4. Various biofuels produced from aquatic weeds.
| Aquatic weed | Pretreatment method | Product yield | Reference |
|---|---|---|---|
| Bioethanol | |||
| Salvinia molesta | Sonication | 15.9 gL-1 | Kityo et al. (2020) |
| Eichhornia crassipes | Alkali/Dilute acid | 13.6 mgml−1 | Das et al. (2016) |
| Lemna gibba L. | Alkali/Dilute acid | 20% | Dhruba et al. (2010) |
| Landoltia punctata | Enzymatic | 30.8 gL-1 | Chen et al. (2012) |
| Eichhornia crassipes | Alkali/Acid | 4.1 gm L−1 | Ganguly et al. (2018) |
| Wolffia globosa | Enzymatic | 170 g kg−1 | Soda et al. (2015) |
| Biodiesel | |||
| Eichhornia crassipes | Sonication | 3.22–6.36% of lipid extract | Shanab et al. (2018) |
| Eichhornia crassipes | Alkali/Dilute acid | – | Venu et al. (2019) |
| Eichhornia crassipes | Alkali/Dilute acid | – | Alagu et al. (2019) |
| Bio-oil | |||
| Eichhornia crassipes | – | 30.41% dwt | Gulab et al. (2016) |
| Eichhornia crassipes | Mechanical | 31.6% | Gulab et al. (2019) |
| Alternanthera philoxeroides | Mechanical | 42.3% | Liu et al. (2011) |
| Duckweed | – | 30% | Xiu et al. (2010) |
| Ipomoea carnea | Mechanical | 41.17% | Saikia et al. (2015) |
| Biohydrogen | |||
| Spirodelapolyrrhiza | Acid | 75.3 mL g−1 | Xu and Deshusses (2015) |
| Duckweed (not specified) | Acid hydrolysis | 169.30 mL g−1 | Mu et al. (2020) |
| Eichhornia crassipes | Microwave assisted acid and enzymatic hydrolysis | 112.3 mL g−1 TVS | Cheng et al. (2013) |
| Alternantheraphiloxeroides | Enzymatic and steam heated acid pretreatment | 89.8 mL g−1VS | Song et al. (2020) |
| Biomethane | |||
| Cabomba Caroliniana | Physical | 49.32% VS | O'Sullivan et al. (2010) |
| Ceratophyllum demersum | Mechanical | 57.1% COD | Koyama et al. (2014) |
| Salvinia molesta | – | 29.09% VS | Syaichurrozi (2018) |
‘-’ Not Reported.
4. Techno-economic challenges of aquatic weed as biofuel feedstock
Aquatic weeds are fast growing and invasive in nature. These characteristics of aquatic weeds need to be given proper attention when grown for their potential application for production of biofuel and other products (Bayrakci and Koçar, 2014). To check the invasion in natural water bodies, aquatic weeds should be grown in quarantine facility and periodically harvested for its application. Harvesting of aquatic weed from infested water body or cultivation facility is a tedious process. Harvesting of aquatic weed can be achieved by manual harvesting at small scale. However, mechanical harvesting is needed for large scale operations. Aquatic weed biomass may contain up to 90% water content in their tissue which can affect the biofuel conversion process. Efficient and cost-effective dewatering processes needs to be evaluated for easing the downstream process of biofuel generation from aquatic weed. Transport of aquatic weed biomass and maintaining continual supply to the downstream processing could add to production cost. It is recommended to build the processing facility near the harvesting sites.
Extraction of carbohydrates and lipids from the aquatic weeds needs to be studied for maximizing the biofuels yield. The aquatic weed biomass has a complex composition which arise challenges for effective extraction and conversion of biofuels. Pre-treatment methods and process intensification by various chemical, physical and biological methods can alleviate this challenge; however, there are very limited studies in this area. There are limited studies on fuel properties and actual engine performance of biofuels produced from aquatic weeds. Venu et al. (2019) showed that 20% blend of aquatic weed biodiesel with petroleum diesel was at par with the conventional diesel performance; however, their result also showed considerably high percentage release of CO2 and NOx compared to neat diesel. Some Aquatic weeds like water hyacinth can have higher sulfur content which can cause result or produce some corrosive substances reducing fuel efficiency (Mullen and Boateng, 2008).
There is extensive literature available on phytoremediation potential of aquatic weed. Aquatic weed has been studied for removal of nutrients and pollutants from various types of wastewater. However, very few studies report the application of aquatic weed biomass after wastewater treatment. The heavy metals and other pollutants accumulated in aquatic weed biomass could be problematic for certain applications of aquatic weed biomass also to the extraction and conversion steps of biofuels production processes. Evaluating the biomass for presence of pollutants and designing the processing steps is important to alleviate this challenge. Wastewater grown aquatic weed biomass could be harmful for its application as fertilizer, medicinal uses and so forth. Application and/or disposal of such wastewater grown aquatic weed biomass may lead to health and environmental problems. Therefore, extensive risk assessment studies are needed for application of wastewater grown aquatic weeds.
Many applications of aquatic weeds such as biodiesel, biohydrogen production and value-added products are in early developmental stages. Even though other applications such as bioethanol, biomethane and fertilizer production have been studied in recent past, there are still research gaps such as process optimization, identifying suitable feedstock species and conversion technology, enhancing the efficacy of the final product and making the overall process cost effective. The challenges at large scale production of aquatic weed biofuels may include harvesting, drying, transportation and cost-effective conversion technique. These challenges need attention. There are very less studies on techno-economics and life cycle assessment of biofuel production from aquatic weed biomass. Aquatic weed has shown tremendous potential for production of biofuel and other applications, however, there are still challenges which need to be addressed before its successful implementation to benefit environment and human kind. Recent studies are focused on addressing some of the above-mentioned challenges.
5. Recent advancements and novel technologies to mitigate challenges
5.1. Pre-treatment of aquatic weed biomass using microorganisms
Biological pretreatment is one of the safest and an environmentally friendly treatment method. This mostly involves the use of microorganisms and biological enzymes that possess the ability to degrade various compounds like lignin, hemicellulose and other polyphenols for the purpose to extract energy easily (Zabed et al., 2019). Fungal pretreatment of lignocellulosic biomass like that of aquatic weed is a relatively new process to improve the digestibility of the biomass (Sinegani et al., 2005). White-, soft- and brown-rot fungi are the most used fungi for degrading lignocellulosic compounds from aquatic weeds. Out of these, brown-rot mainly degrades cellulosic compounds whereas, white rot and soft rot fungi degrade both lignin and cellulosic compounds by production of several enzymes (Madadi and Abbas, 2017). When compared, white-rot is most attractive biological pretreatment of lignocellulosic biomass attributed to its eco-friendly aspect (Fang et al., 2020). Fungi species such as Trichoderma sp. (Pérez et al., 2002), Pichiastipis (Pothiraj et al., 2014), Aspergillus terreus (Emtiazi et al., 2001), Penicillium camemberti (Taseli, 2008) etc. have also been studied for treatment of aquatic weed biomass. Barua et al. (2018) studied biological pretreatment using three lignocellulose breaking bacterial strains- Bordetellamuralis, Citrobacterwerkmanii and Paenibacillus sp. which were isolated from soil, gut of silverfish and millipede respectivelyto enhance the production of biogas from water hyacinth. The study concluded that microbial pretreatment efficiently enhanced the biodegradability of water hyacinth biomass and Citrobacter werkmanii was more effective in increasing biogas production. Alvi et al. (2014) reported the bioethanol yield of 16.7 g.100 g−1 from water hyacinth using Aspergillus sp. for hydrolysis.
Biological pretreatment for lignocellulosic biomass is a very promising technique with various advantages such as low energy input, minimal or no chemical requirement and environment friendly working procedures (Salvachúa et al., 2011). However, there are few disadvantages of biological pretreatment as well which make them less attractive for large scale and commercial production of bioenergy (Eggeman and Elander, 2005). This includes slow processing time making it non-feasible for industrial purpose, requirement of large area and controlled conditions. Apart from this, biological pretreatment may produce some inhibitory enzymes and some part of the carbohydrate is consumed by microorganisms resulting in low biofuel yield (Agbor et al., 2011). Due to all these reasons, biological pretreatment faces barriers to be used at commercial scale. To overcome these difficulties, biological pretreatment can be used in combination with other efficient pretreatment methods. Ma et al. (2010) evaluated the effect of combinations of biological pretreatment with mild acid pretreatment. The result concluded that white-rot fungus Echinodontiumtaxodiiin combination with sulfuric acid was more effective in boosting the enzymatic hydrolysis process and production of ethanol from water hyacinth biomass. The combination increased the bioethanol production 1.13–2.11 times than that with the chemical or biological pretreatment alone. The conversion of the feedstock to bioenergy using the best microorganism for pretreatment could increase productivity in a cost-effective way. The production might be enhanced by having proper knowledge of the microorganism characteristics, optimum incubation temperature and incubation time which may vary based on each microbial consortium. Therefore, identification of suitable microorganism for each process and development of integrated approaches such as combined strategies can improve the overall production economics.
5.2. Process intensification by microwave and sonication technique
The main problem faced in digestion of weed biomass is low biodegradability as it has complex lignocellulosic material which results in slow hydrolysis (Boudet et al., 2003). Therefore, production of biofuel from lignocellulosic compound like that of aquatic weed needs process intensification through pretreatment methods which can improve the overall yield without having any adverse effect on economics. Process intensification using microwave and sonication technique help in solubilizing and separation of compounds in biomass. Both these techniques help improve the sugar recovery and reduce the loss of important compounds. Microwave is a direct and rapid heating method which involves direct interaction between the material and the radiated energy. Rapid heating at such rate causes explosive effect on the target molecules which effectively degrades more recalcitrant compounds like lignin. Apart from successful degradation of hemicellulose and lignin, microwave irradiance also alters the cellulosic structure on nanoscale level which greatly effects on the biofuel production. Compared to conventional thermal pretreatment methods, microwave irradiance technique has significant advantages as it forms no residual compounds, reduces reaction time and the heating is selective and uniform (Dai et al., 2017). On the other hand, ultrasound pretreatment alters the surface structure using ultrasonic waves. Additionally, sonication produces oxidizing radicals that attacks and disrupts the lignocellulosic matrix such as alpha and beta linkages in the lignin resulting in the rupturing of lignin fractions and some structural polysaccharides (Kumar and Sharma, 2017; Shirkavand et al., 2016). Currently, ultrasound pretreatment can be classified into two main groups (a) Ultrasound assisted pretreatment under low frequency (40 kHz) that enhances the delignification and (b) Ultrasound assisted pretreatment under higher frequency range (~1000 kHz) that improves carbohydrate solubilization (Bussemaker et al., 2013).
Liang et al. (2019) analyzed the effect of microwave technique on the water hyacinth structure for the production of pyrolysis product. They concluded that microwave pretreatment was very effective in changing and destroying the smooth surface structure of water hyacinth which positively affected the pyrolysis behavior of the weed biomass, thereby increasing the pyrolysis product yield. On the other hand, Kist et al. (2020) studied the performance of sonication pretreatment and its efficacy on lag time removal along with methane production increase for Eichhornia crassipes, Pistiastratiotes and Salviniamolesta. In their study, Biomethane potential test was conducted to analyze the decomposition of aquatic weeds and the results showed that ultrasonication successfully reduced the lag time and increased the methane production at the same time. Both, microwave and ultrasound can enhance the hydrolysis and degradation of a compound. They have also been used as a combined pretreatment method which helps in rapidly degrading and hydrolyzing the lignin and cellulosic compounds (North, 2014).
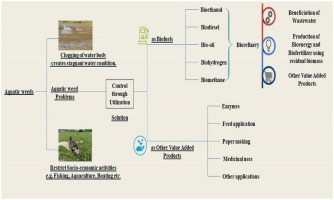
6. Aquatic weed based biorefinery
Biorefinery concept has emerged as a sustainable and economical way of exploiting biological resources. In biorefinery, biological resource is exploited to its full potential through its multiple applications in an integrated approach (Suganya et al., 2016). Aquatic weeds are known for its phytoremediation potential which can be used for simultaneous treatment of wastewater and generation of biomass. The generated biomass can be utilized for various applications such as biofuel, fertilizer and other industrially important chemicals in systematic pattern (Awasthi et al., 2020). The systematic approach of biorefinery is adopted to develop zero waste generating circular process. In following sections, applications of aquatic weed are discussed which can be integrated along with biofuel production.
6.1. Beneficiation of wastewater using aquatic weed
Secondary and tertiary water treatment processes are costly and complex processes requiring high input of energy, chemicals and technological inputs (Tchnobanoglous, 1990). These complex treatment processes, therefore, is needed to be replaced by some lesser complex, economical and eco-friendly replacement process which can be equally effective and require less energy inputs. This can be achieved through employing aquatic plants which has been proven to possess ability to remove nutrients and harmful compounds from the wastewater (Prabhakar et al., 2017).
The literature shows that aquatic weeds are very efficient in reducing nutrient load as well as other compounds from water thereby bringing down the pollution load and therefore these aquatic plants are considered as one of the best suitable eco-friendly way of waste water treatment (Table 5) (Boyd, 1970; Okia, 1993). Sustainable, efficient and cost-effective treatment of wastewater can be achieved by designing appropriate technology based on aquatic weed.
Table 5. Application of aquatic weed for treatment of various types of wastewater.
| Species name | Wastewater type | Nutrient | Removal (in%) | References |
|---|---|---|---|---|
| Pistiastratiotes | Industrial effluent | Pb | 70.7 | Aurangzeb et al. (2014) |
| Cu | 66.5 | |||
| Eichhornia crassipes | Industrial effluent | Pb | 73 | Aurangzeb et al. (2014) |
| Cu | 78.6 | |||
| As | 74 | |||
| Al | 73 | |||
| Cd | 82.8 | |||
| E. crassipes and P. stratiotes | Wastewater | COD | 82.45 | Victor et al. (2016) |
| BOD | 82.45 | |||
| PO43− | 50.04 | |||
| Rapa natansL. | Pulp and paper mill effluent | BOD | 38.89 | Kumar et al. (2016) |
| COD | 32.03 | |||
| TKN | 49.89 | |||
| PO43- | 47.14 | |||
| Eichhornia crassipes | Pulp and paper mill effluent | BOD | 42 | Kumar et al. (2016) |
| COD | 35.22 | |||
| TKN | 57.18 | |||
| PO43- | 55.66 | |||
| P.stratiotes | Rice Mill wastewater | COD | 65 | Mukherjee et al. (2015) |
| NH4–N | 98 | |||
| NO3–N | 70 | |||
| P | 65 |
One of the most widely distributed aquatic weed in the world is Eichhornia crassipes commonly known as water hyacinth (Holm et al., 1977). The tendency of Eichhornia crassipes to accumulate heavy metal contamination present in the water bodies has made it one of the most widely studied plants in this field. A study conducted in Brazil on the uptake of heavy metal by Eichhornia crassipes observed that its threshold for tolerance is immense and it has high uptake of nutrient as well as heavy metals (Salati, 1987). Similarly, Elankumaran et al. (2003) studied the removal efficiency of Hydrillaverticillata and Salvinia sp. The comparative study revealed that Hydrillaverticillata had greater nutrient removal efficiency under low strength water of 5 ppm whereas Salvinia sp. showed greater removal capacity in high strength wastewater.
The aquatic weed employed for wastewater treatment generates substantial amount of biomass. This biomass can be periodically removed from the treatment system and utilized for various biofuel production processes. The energy generated from aquatic biomass can be used to cater the energy demand of the wastewater treatment plant. Surplus energy can be supplied to local communities. The heavy metal and other contaminants accumulated in aquatic weed biomass could pose challenge for the processing or even to its end application. Therefore, a thorough risk assessment studies are needed for biofuel and other applications of wastewater generated aquatic weed biomass.
6.2. Fertilizer production from aquatic weed
According to USDA (2012) aquatic weeds produce abundant amount of biomass in any water body. These aquatic weeds are source of food and shelter for all kinds of organisms in the aquatic environment as it is rich in essential minerals like potassium, nitrogen, phosphorus etc. Therefore, Eichhornia crassipes, Hydrilaverticillata, Pistiastratiolesandsalvinia spp. which are considered as problematic weeds creating concerns of biomass disposal throughout the world can be effectively used to recycle the nutrients present in them to beneficiate the growth of plants by using them as fertilizers (Gusain et al., 2018). Fertilizer production through these weeds can be an answer to multiple problems since the problematic weeds can be converted into nutrient rich fertilizer which will also help in the nourishment of degraded soil.
Several scientists have investigated utilization of these weeds for soil beneficiations in an efficient way. Bernal et al. (2016) studied the alternative methods for the management of Eichhornia crassipes; the main aim of the study was to use this weed to produce a stable and good quality vermicompost. In their study, three treatments were established with 100% E. crassipes, 100% cow manure and a mixture of Eichhornia crassipes and cowdung at 1:1 respectively, using earthworm specie Eisiniafetida. The vermicompost was analyzed physically, chemically and biologically and was evaluated by different indexes of quality and maturity. Similarly, Kannadasan et al. (2013) in the study of vermicomposting of E. crassipes with earthworm species E. eugeniaeand E. fetidafound that the compost was rich in macro- and micro plant essential nutrients. Microbial analysis of the gut of the earthworms also showed presence of Bacillus cereus and Micrococcus luteus. The authors estimated micro- and macro nutrients and even carried out microbial studies, but, the plant growth estimation was done using only two parameters, which may have given the required result but inclusions of few more parameters would have been more advantageous.
Although vermicomposting is arguably the best method to produce fertilizer from aquatic weed, other processes like composting and direct application has also been researched extensively. Dorahy et al. (2009) evaluated the quality of compost prepared using alligator weed, Egeriadensa and Salviniamolesta along with environmental risk assessment. The authors concluded that there are particular risks of using weeds like alligator weed as they might survive and therefore, they suggested additional composting measures to improve the whole process. There are ongoing efforts to improve the fertilizer efficiency. Gajalakshmi and Abbasi (2002) tried improving the quality of product by high-rate composting procedure prior to vermicomposting of the aquatic weed biomass. The authors concluded that the combined method increased the productivity and quality of the final vermicast.
The residual biomass of aquatic weed after its application for biofuel production can also be used for fertilizer production. Biofuels such as ethanol and biodiesel only use carbohydrate and lipid component of biomass respectively. The residual biomass still contains nutrients after extraction of carbohydrate and lipids. Similarly, anaerobic digestion of aquatic weed biomass also produces residual biomass as co-product. The thermal conversion processes also produce biochar as co-product (Liu et al., 2018). These residual biomass and co-products are effectively being used for fertilizer application through various processing technologies.
6.3. Other value-added products from aquatic weed
Apart from biofuels and fertilizers several other value-added products can be produced using aquatic weed biomass. Aquatic weed have been potentially used for production of enzymes, polymers, precursors of nanoparticles, paper pulp, fish and animal feed, medicinal compounds, herbicides, bricks and others.There could be numerous other possible uses of aquatic weeds which has been reported to be used by indigenous people and is yet to be documented. Following sections discuss the various value-added products which can be produced from aquatic weed.
6.3.1. Enzymes
Aquatic weeds can be used to produce a number of enzymes like cellulase and xylanase. Cellulase in particular is a group of hydrolytic enzymes that hydrolyzes the cellulose into useful sugar components like glucose. It has a very high cost of production and therefore several researches are ongoing to make the production of cellulase cost efficient by using easily available lignocellulosic substrate like that of aquatic weed. Cellulase production by using water hyacinth as carbon substrate was conducted by Amriani et al. (2016). Cellulase producing fungi strains of Aspergillus niger and Trichoderma reesei were used in their study. The authors concluded that Aspergillus niger showed better yield of cellulase. Similarly, Rathnan et al. (2012) evaluated cellulase production using Salviniamolesta as substrate using wild type of Pseudomonas strain. The results showed that highest crude cellulase production was obtained at temperature 50 °C and at pH 7. The authors concluded that Salviniamolesta has great potential to be effectively used for the commercial production of cellulase enzyme.
Xylanase similar to cellulase are enzymes that hydrolyze xylan to produce compounds like xylose and xylobiose. These are among the largest produced commercial enzymes, commonly used in paper industry, food additive in baking industry, detergent industry, etc. Xylanase production using aquatic weed has been studied by several researchers and successful xylanase production through Solid State Fermentation (SSF) using water hyacinth as substrate was observed by Deshpande et al. (2008). The authors reported that SSF provided more space and greater surface area for the activation of substrate as well as better control over moisture, temperature, aeration etc. The authors concluded maximum xylanase production was observed on the 7thday of experiment and stated that addition of Toyoma Ogawa nutrient medium increased the production by 8–9 times than original. However, more studies are required for screening the aquatic weed for production of several other industrially important enzymes.
6.3.2. Feed application
Due to high nutritive values of the aquatic macrophytes, they can be used as food source for animals, fish or even for humans. Particularly fish food is much expensive and therefore several studies are being conducted for identifying a cost-effective supplementary feed. Different parts of aquatic weeds such as Lemna minor, Pistiastratiotes, Eichhornia crassipes and some other aquatic weeds may containupto 25–29% protein. These aquatic weeds can directly be utilized to feed a number fish species as live diet including common carp, golden carp, silver dollar fish, Nilem fish, Brazillian fish, Milk fish, Channel Catfish etc. (Mandal et al., 2010). Other method is formulated fish food which is prepared using aquatic weeds as a component or protein source like duck weed, Spirodella sp., Pistia sp. and combinations are commonly used in fish feed formulations. Evaluation and optimization of the prepared feed ingredient using Ipomoea aquatica for Rohuand Hamilton fish was conducted by Ali and Kaviraj (2018). The aquatic weed was fermented using bacteria Stenotrophomonasmaltophiliawhich produces enzyme phytase. Four types of feed with four different concentrations (0%, 25%, 50%, 75%) with market available fish feed were used for the growth of fingerlings of Rohu and Hamilton fish for the period of 8 weeks. The result showed that feed with 25% inclusion showed best growth results among the fish. Similarly, other aquatic weed species have also been proven to make effective fish food including Salviniacuculata(Ray and Das, 1992), Duck weed (El-Shafai et al., 2004), Azolla sp. (Gangadhar et al., 2014), Wolffia sp. (Chareontesprasit and Jiwyam, 2001) etc. At the same time, aquatic weed like water hyacinth has also been used as animal feed for animals such as cows, pigs, goats, ducks etc. due to their higher growth and higher protein content (Tham, 2016).
6.3.3. Paper making
The major source of fiber for the production of pulp and paper is wood and therefore there is growing concern about the future fiber supplies. Therefore, the interest is gradually moving towards the use of alternate sources such as lignocellulosic wastes. Aquatic weeds with low lignin content are very impressive potential fiber source for paper making industry. Stability of the aquatic weed fiber as potential raw material for handmade papermaking was conducted by Bidin et al. (2015). Five aquatic weed specie Scirpusgrossus, Cyperusdigitatus, Cyperushalpan, Cyperusrotundus and Typhaangustifolia; were used for the fiber and chemical analysis to evaluate best compatibility of the aquatic weed for the production of paper and the results were compared with wood fibers. All five aquatic weeds showed short fiber length (0.71–0.83 mm), thin diameter (ranging from 9.13 to 12.11 μm) and thin cell wall (thickness ranging from 2.25 to 2.83 μm). Analysis of physical composition, chemical and fiber characteristics showed that Scirpusgrossus, CyperusrotundusandTyphaangustifoliaare the three aquatic weed species which are best suited for paper making. Similarly, Emmclan et al. (2018) evaluated the physical and chemical characteristics for handmade papermaking using three species of aquatic weed (Cyperusiria, Cyperusdigitatus, and Scirpusgrossus). The result concluded that handmade paper produced using Cyperusdigitatus was better in comparison to Cyperusiria and Scirpusgrossus. Application of aquatic weed in paper industry has the potential to alleviate the environmental concern of deforestation and thus should be given emphasis for its successful implementation at commercial level.
6.3.4. Medicinal uses
Some aquatic weeds possess very unique biological features and are considered suitable for its potential medicinal use. Various aquatic environment particularly macrophytes may contain phytochemicals such as saponin, lipophilic compounds, flavanoids, alkaloides, steroids, tannin, phenols etc. (Al-Amin Sarker et al., 2016). These phytochemicals have been proved to have pharmacological properties such as antimicrobial, anti-inflammatory, anti-diabetic, antioxidant etc. These phytochemicals can be extracted from the aquatic weeds and used in medicinal formulations. However, studies are limited in this area. More studies are required to explore the potential of aquatic weeds for their medicinal applications. Efficient extraction processes need to be developed for extraction of targeted phytochemicals. Several aquatic weeds and their medicinal properties are given in Table 6.
Table 6. Medicinal uses of aquatic weeds.
| Aquatic weeds | Medicinal uses | References |
|---|---|---|
| Ceratophyllumdemursum (Coon tail) | Fever, dysentery, ulcer, piles | Shankar and Mishra (2012) |
| Eichhornia crassipes (Water hyacinth) | Antitumor/Anticancer (against melanoma and liver cancer cell line) |
Ali et al. (2010) Aboul-Enein et al. (2011) |
| Larvicidal | Thorat and Nath (2010) | |
| Antimicrobial | Vadlapudi (2010) | |
| Nelumbonucifera (Lotus) | In curing urinary problem, diarhhoea, piles | Panda and Misra (2011) |
| In curing blood vomiting, epistaxis | Sridhar and Bhat (2007) | |
| Anticancer | Arjun et al. (2012) | |
| Nymphaeanouchali (Water lily) | In curing heart, liver and kidney disorder | Lakshmi et al. (2014) |
| Anti-inflammatory | Jahan et al. (2012) | |
| Antidiabetic | Rajagopal and Sasikala (2008) | |
| Pistiastratiotes | In curing anemia, cronic skin disorder | Kirtikar and Basu (2001) |
| Antimicrobial | Ziada et al. (2008) | |
| Antifungal | Natarajan et al. (2003) | |
| Antidiabetic, Antitubercular, Antidyssentric | Tripathi et al. (2010) | |
| Vallisneriaspiralis (Tape grass) | Stomach ache | Swapna et al. (2011) |
6.3.5. Other applications
In addition to the products mentioned in above sections aquatic weeds are being studied for their application in other sectors such as mushroom cultivation, soap manufacturing, for polymer production etc. Due to their availability and fast growth they can be very beneficial for any mainstream manufacturing process when used as raw material. Development of high-performance composites of water hyacinth and polyester resin was studied by (Ramirez et al., 2015)where they used water hyacinth as raw material. The result showed that forming the composite of polyester resin using water hyacinth had no adverse effect on mechanical and thermal properties and had an overall reinforcing effect on the final product when compared to the other natural fibers such as rice straw, abaca and jute. Furthermore, suitability of the same aquatic weed water hyacinth for mushroom cultivation was studied by Murugesan et al. (1995) The study revealed that water hyacinth when used as substrate for oyster mushroom production resulted in good quality mushrooms which were mainly attributed to the low lignin content and ideal C/N ratio of the aquatic weed biomass. There are many other applications of aquatic weeds such as handicrafts, which have been practiced by local people from ancient times. Bioprospecting studies are required for aquatic weeds to identify ideal species and potential applications of industrial and environmental importance.
7. Conclusion and future prospects
Aquatic weed is gaining interest as a valuable resource for production of biofuels and other value added products. Fast growth rate, ideal composition and minimal land requirement gives aquatic weed edge over many conventionally used terrestrial biofuel feedstocks. Aquatic weed has shown promising potential for production of bioethanol, bio-oil and biomethane. Biodiesel and biohydrogen production from aquatic weed need attention from the scientific community. Aquatic weed based biorefinery is a sustainable approach for biofuels and other industrially important chemicals, however there are following points which needs attention of scientific community.
-
•
Studies are needed to develop efficient pre-treatment and conversion technologies.
-
•
There are challenges such as harvesting of biomass, inefficient hydrolysis and conversion, scaling up and high processing cost which needs to be addressed
-
•
Biological and other hybrid pretreatment methods, process intensification can be applied for achieving higher biofuel yields.
-
•
To attain economic viability aquatic weed biomass needs to be utilized with its full potential. Residual biomass after biofuel production could be used for other industrial applications under integrated biorefinery approach.
-
•
A thorough life cycle analysis and risk assessment studies are required to develop and scale up the aquatic weed based biorefineries for sustainable future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Mr. Shahrukh Nawaj Alam is grateful to Central University of Jharkhand, Ranchi, Jharkhand, India for UGC university fellowship. Author Dr. Abhishek Guldhe is thankful to Department of Biotechnology, Govt. of India for the award of Ramalingaswami