1. Introduction
Water is the greatest gift from nature because of its indispensable importance for the survival of different biota on our planet (Mikosch et al., 2020). As it is one of the finite natural resources, maintaining its quality is a global demand that extensively increases with each passing day. Regrettably, in the new millennium, many crises, conflicts, and wars on limited water resources have emerged. Also, different water pollutants are related to innumerable human activities being synchronously emitted into water bodies causing severe declination in water specifications (Richa and Roy Choudhury, 2020). Agricultural, commercial, domestic and industrial sectors will suffer from clean water scarcity. The current and next generations will pay a huge price as a result of an acute contradiction between limited resources and economic development. Many types of industries such as machinery manufacturing, printing, textile (Yao et al., 2020), chemicals, electronics, and pharmaceutical industries are implicated in water pollution (Özdemir et al., 2019). They are responsible for the seepage of different pollutants such as dyes (Tonato et al., 2019), heavy metals, phenols (Mohammed et al., 2018), pesticides (Behloul et al., 2017), insecticides, and drugs (de Lima et al., 2018) into the aquatic environment as byproducts without proper manipulation. The presence of pollutants in water bodies above their allowable levels set by the World Health Organization (WHO) and Environmental Agencies may have tremendous effects (Nishikawa et al., 2018). These effects can impair human and animal health (neural toxicity, carcinogenicity, and reproduction capabilities) (Fontana et al., 2018). It also may result in eutrophication and photosynthesis inhibition (Aboelfetoh et al., 2018). This was due to its poor degradability and/or highly accumulating effects (Novais et al., 2018).
Both dyes and heavy metals are conceived to be some of the most dangerous pollutants in water systems as they exhibit mutagenic, immunogenic, carcinogenic, and teratogenic characteristics (Azari et al., 2019). Discharge of wastewater into aquatic bodies without proper purification causes numerous significant environmental and health problems for aquatic flora, fauna, and finally human health (Yin et al., 2019).
Dyes can be categorized into three main groups: (a) anionic (acid, reactive, and direct dyes) with negative charge mainly due to (SO3−) group, (b) cationic (basic dyes) due to the protonated amine group, and (c) nonionic (disperse dyes) according to their dissociation behavior in aqueous solutions (Sakin Omer et al., 2018). Azo dyes either cationic or anionic bear one or more azoic bonds (N N). Besides its stability against light, heat, and aerobic digestion, it may cause severe threats to human health such as vomiting, cyanosis, allergic problems, and genetic mutation. The dye industry gives emblematic examples of the potential impact of uncontrolled management of contaminated flows (Pellicer et al., 2018). Dyes are used in a great diversity of sectors: textile, paper, leather, dyestuff, printing, plastic, cosmetics, and coatings which are widely disseminated because of low-cost production, brightness, and high resistance against environmental conditions (Reck et al., 2018). Even at low concentrations, dyes are optically active and detectable (Bello et al., 2018); their impact on water bodies is then very acute (dos Santos et al., 2018). Indeed, the dispersion of dyes in water assets disturbs gas solubility, which, in turn, affects the gills of aquatic organisms and disrupting their spawning sites and refuges (Senthil Kumar et al., 2018). While hindering light penetration in water, the dyes also limit photosynthesis. Apart from these secondary effects, the dyes have frequently specific toxicity due to carcinogenic and mutagenic effects (associated with the presence of benzidines, and naphthalenes derivatives) (Pleşa Chicinaş et al., 2018).
N). Besides its stability against light, heat, and aerobic digestion, it may cause severe threats to human health such as vomiting, cyanosis, allergic problems, and genetic mutation. The dye industry gives emblematic examples of the potential impact of uncontrolled management of contaminated flows (Pellicer et al., 2018). Dyes are used in a great diversity of sectors: textile, paper, leather, dyestuff, printing, plastic, cosmetics, and coatings which are widely disseminated because of low-cost production, brightness, and high resistance against environmental conditions (Reck et al., 2018). Even at low concentrations, dyes are optically active and detectable (Bello et al., 2018); their impact on water bodies is then very acute (dos Santos et al., 2018). Indeed, the dispersion of dyes in water assets disturbs gas solubility, which, in turn, affects the gills of aquatic organisms and disrupting their spawning sites and refuges (Senthil Kumar et al., 2018). While hindering light penetration in water, the dyes also limit photosynthesis. Apart from these secondary effects, the dyes have frequently specific toxicity due to carcinogenic and mutagenic effects (associated with the presence of benzidines, and naphthalenes derivatives) (Pleşa Chicinaş et al., 2018).
Heavy metals are inorganic notorious contaminants that are defined as chemical elements with relatively high atomic weight and density. It is poisonous even at low concentrations (Ali et al., 2019). Massive amounts of toxic metals like As(III), Cd(II), Cu(II), Cr(VI), Ni(II), Pb(II), and Zn(II) are dumped into water assets without proper treatment (Dobrowolski et al., 2019). These pollutants in waterways represent a serious threat (Que et al., 2019).
Owing to the mentioned discredited impacts on different biota, a strong demand for water purifying from dyes and heavy metals becomes a paramount need. It is an important issue for the industry, the environment, and health. Physical, chemical, and biological approaches are implemented to capture it from water systems. Physical and chemical processes include coagulation/flocculation (Hou et al., 2019), precipitation (Chen et al., 2018), ion exchange (Feng et al., 2019), photo-oxidation (Du and Chen, 2018), electrocoagulation (Doggaz et al., 2019), electrodialysis (Nemati et al., 2017), membrane separation (Hosseini et al., 2018), ultrafiltration (Kavitha et al., 2019), forward osmosis (Qiu and He, 2019) and irradiation (Ghobashy and Elhady, 2017), and adsorption (Wei et al., 2021). Whilst the biological processes include phytoextraction (Napoli et al., 2019) and biological degradation (Jacob et al., 2018). Figure S1 (see supplementary material) presents the conventional wastewater treatment processes in more detail.
However, most of these sophisticated processes have many certain limitations. They are high costs, inefficiency in low pollutant concentrations, huge time consumption, reagents and energy, and chemical sewage sludge formation (Fernando et al., 2018). It is time to come up with an alternative, greener, and cheaper technology for the efficient elimination of different contaminants from water (Hadiani et al., 2019). Consequently, exploring an efficient route for dyes and heavy metals elimination remains a great challenge. It is a bottleneck for preserving water resources. Recently, Researchers have assessed several biomaterials for the remediation of contaminated effluents in the quest for environmental protection and sustainability. For example, Ubando et al. conducted a bibliometric analysis for heavy metal removal via microalgae (Ubando et al., 2021), The study summarized the application of various microalgae to remove heavy metals by identifying the different factors that affect the efficiency of biosorption. More examples are the use of fish scales (Ighalo and Eletta, 2020), Banana peel (Akpomie and Conradie, 2020), extremophile microorganisms (Giovanella et al., 2020), cyanobacterial biomass (Shetty and Krishnakumar, 2020), lignocelluloses (Tran et al., 2019), chitosan (Crini et al., 2019), Tannin (Santos et al., 2019) and plant leaves (Adeniyi and Ighalo, 2019) as a biosorbents for the decontamination of water pollutants. It was observed that most recent review articles have focused on the factors that affect the biosorption process such as biosorption equilibrium and kinetics in a typical one biosorbent. The goal of this review is to provide a broader view of the current research trend, to identify various available biosorbents, to identify potential knowledge gaps, and to explain future research perspectives.
2. Pollution
2.1. Water pollution
Water is the artery of life on earth. It is one of the main primitive requirements necessary for livelihood on our planet. It covers more than 71% of the earth's surface and represents about 70% of its mass. It is the only substance on the earth naturally existing in three physical states. The paramount need for pure water cannot be ignored. Regrettably, over the last decades, the unchecked population expansion and industrial invasion have led to huge pressure on limited water resources (Calero et al., 2018). What is worse, innumerable water pollutants are synchronously released into aquatic bodies. It causes severe and accelerated dramatic deterioration in water quality. Water pollution as a crucial problem has considerably become a matter of worldwide concern. Firstly, knowing its nature, main sources, and discredited effects on human health and the ecosphere is important. Generally, pollution is environmental contamination, causing acute threats to different biota. It is mainly classified into five categories including; water, air, soil, biological and nuclear pollution.
Recently, the overutilization of water resources and water contamination simultaneously occur as a consequence of uncontrolled greed associated with human civilization. Unfortunately, where people live and work, wastewater is always found. Several industries are strong producers of wastewater laden with a great diversity of pollutants. These pollutants include dyes, heavy metals, phenols, pesticides, insecticides, and drugs. Discharge of these contaminated effluents into the aquatic environment has extremely tremendous impacts on our biosphere. It exhibits immunogenic, teratogenic, mutagenic, and carcinogenic characteristics (Azari et al., 2019). It is well known that about 70–80% of all illnesses cases in developing countries are related to water pollution (Qadri and Faiq, 2020).
2.1.1. Sources of water pollution
Two foremost categories that contribute to water pollution can be classified into two main groups; point and non-point sources.
Point sources: It originates from a single source such as effluent outfalls from different sewage systems. Other sources are oil wells, factories, power plants, and refineries. Annually, about 300–400 MT of different harmful pollutants is directly discharged into water systems.
(ii) Non-point sources: This type of pollution originates from multiple sources. Its release is related to the movement of originated water from the natural process. According to (NORMAN Network) report, more than 700 listed environmental contaminants exist in the European aquatic bodies (Kar et al., 2019). These include heavy metals, dyes, agrochemicals, oil products, and pharmaceuticals. Additionally, the European Union reported that other pollutants are found in water bodies (Barbosa et al., 2016). They include natural hormone (17-b-estradiol (E2)), synthetic hormone 17-a-ethinylestradiol (EE2)), pesticides (oxadiazon, imidacloprid, methiocarb, thiamethoxam, and triallate), pain killer diclofenac, major macrolide antibiotics (clarithromycin, azithromycin, and erythromycin), triallate), UV filter and dyes additives.
2.2. Petroleum pollution
Natural petroleum production processes are the main sources of petroleum or crude oil and its derivatives (Jain et al., 2019). It includes extraction, storage, transportation, ships, marine terminals, and parking lots. Petroleum accidents related to oil tankers, pipelines, and oil rigs also participate in water pollution. Approximately, 5 million tons of petroleum products enter aqueous systems from oil spills (Bo et al., 2017). Petroleum produced water is known to be one of the most destructive types of water pollution. It has carcinogenic and mutagenic features because of its complex nature. It is laden with a mixture of naphthenic, paraffinic, polycyclic aromatic hydrocarbons (PAHS), trace amounts of sulfur, oxygen, and nitrogen compounds (Gao et al., 2019). Accumulation of these persistent compounds in the aquatic environment negatively impacts the health of different environmental compartments.
2.3. Sewage pollution
Sewage contamination has become a growing global issue resulting from rapid industrialization. It is largely unrecognized with >50% of the world's population census living with coastal cities (Abaya et al., 2018a). Untreated sewage is introduced into water assets from accidental spills of treatment plant failures. It includes sewer overflow, broken sewer pipes, clogged lift stations, and effluents discharged from onsite sewage disposal systems. Environmentally, it represents a complex problem because it contains a cocktail of elevated hazardous levels of the pollutant. It contains pathogens (bacteria and viruses), inorganic/organic compounds, endocrine disruptors, and hydrocarbons (Ahmed et al., 2018). It causes detrimental effects on human health as well as coastal ecosystems. Human exposure to contaminated sewage results in skin and urinary tract infection, hepatitis, and gastroenteritis. Furthermore, it also negatively influences coral reefs that are considered one of the most economically valuable and biologically diverse ecosystems on the earth. Increased coral disease prevalence in addition to severity has been linked to it (Abaya et al., 2018b).
3. Major pollutants
Owing to different municipal, agricultural, and industrial activities, numerous industries particularly implicated in water pollution (Özdemir et al., 2019). Currently, the purification of aqueous effluents contaminated with toxic chemicals has become a serious environmental burden (Dallel et al., 2018). Their existence in water bodies beyond guidelines is unsolicited. They simultaneously provoke serious threats for both the ecosphere and human health. These pollutants have non-biodegradable, highly toxic, and carcinogenic characteristics. Even at low concentrations, it can cause rigorous damage to different ecosystem compartments (Nguyen et al., 2016). Among the multitude of pollutants, toxic heavy metals and synthetic organic dyes have been ecologically causing more worldwide concerns.
3.1. Dyes
Colorants are substances having the capability of transmitting their color to the other substrates. Mainly, it can be used in a single case or in combination with other ingredients. They are greatly consumed in innumerable industries such as plastics, prints, photographs, clothes, paints, and ceramics. Generally, colorants are divided into two main classes namely, dyes and pigments (Pavithra et al., 2019). Dyes are organic compounds, used to impart color to various substrates like paper. They are capable to bind themself to the external surfaces of different subjects or fabrics. Whereas pigments require another substance, called a binder, to help them adhere to the substrates (Har Bhajan and Bharati, 2014). Commercial dyes are broadly categorized in several ways in terms of the source of origin, chemical structure, color, and applications as shown in Figure S2 (see supplementary material). Based on origin, dyes are classified into natural and synthetic dyes. Natural dyes are derived or extracted from natural resources including animals, minerals, plants, and invertebrates. Majorly, they are obtained from vegetable and archeological sources. They are characterized by numerous merits such as harmonized nature, cost-effective, biodegradability, and easy disposal. However, the mentioned advantages, the rampant industrial activities in addition to uncontrolled civilization growth move people away from natural dyes usage. Because of its disabilities to meet the rapid industrial progress demand, it is mostly consumed in food industries (Li et al., 2019). Contrarily, synthetic dyes are fabricated from organic or inorganic compounds and used as alternates to natural dyes. The term synthetic is related to the chemical structure of the chromophore group. It is primarily made from aniline or chrome. Each type has a different behavior based upon light fastness and brightness degree. One exposed to sunlight; aniline (coal tar) dyes fade easily, while chrome dyes are colorfast and non-corrosive in nature (Fontana et al., 2018). Dealing with synthetic dyes requires taking into account numerous critical parameters. They include fading, boiling, machine washability, dry cleaning, gas fume fading, saltwater, steam pressing, and perspiration. Also, they can be classified based on their dissolution behavior in an aqueous medium. Dyes can be divided into three categories: (a) cationic (basic dyes), (b) anionic (acid, direct, and reactive dyes), and (c) nonionic (disperse dyes) (Sakin Omer et al., 2018). Several industries such as carpet and rubber largely used them to provide different products with attractive colors (Reck et al., 2018). All these dyes are naturally soluble in water except disperse and vat dyes. Dyes also contain traces of elements like lead, zinc, copper, chromium and cobalt. Due to their complex chemical structures, synthetic dyes are mostly stable against oxidation agents. It leads to increase the chemical oxygen demand and hence being harmful to aquatic biota and human health. They adversely affecting on the metabolic processes of microalgae as well as aquatic plants. Table 1 shows various classes of dyes (Kausar et al., 2018), chemical properties and its undesirable effects (Pavithra et al., 2019).
Table 1. Various classes of dyes and their serious health effects.
| Dye name | Examples | Usage method | Solubility | Nature | Applications | Health effects | References |
|---|---|---|---|---|---|---|---|
| Direct dyes | Direct black, Direct blue. | Applied directly to the substrate. | Soluble in water. | anionic | Cellulose, Cotton, and blended fibers. | Bladder cancer carcinogen | Kausar et al. (2018) |
| Reactive dyes | Reactive red, Reactive black 5. | The reactive group reacts with fiber polymer. | Soluble in water. | anionic | Cellulosic fiber and fabric. | Allergic respiratory problem | Dawood and Sen (2014) |
| Acid dyes | Acid red 57, Congo red. | Neutral as well as acidic dye baths are used. | Soluble in water. | anion | Leather, silk, wool, paper, and synthetic fibers. | Burns and skin, mucous membrane irritation | Abusaif et al. (2021) |
| Basic dyes | Methylene blue, malachite green | – | Soluble in water. | – | Cotton, wool, and silk. | Carcinogens, allergic skin reactions, allergic dermatitis, skin Irritation, mutations, cancer. | McYotto et al. (2021) |
| Vat dyes | Vat Green 6, Indigo. | Fabric is padded in stable dispersion often microdispersion to the dye | Insoluble in water. | – | Blended Fibers, cellulose, and cotton. | Severe burns, skin and mucous membrane irritation | Yang et al. (2021) |
| Disperse dyes | Disperse blue, Disperse yellow. | Successive treatments with solutions of two chemical components that react to form dye on the fibre surface. | Insoluble in water. | Cation | PVC industry, Vilene, velvets, cellulose acetate, nylon, and synthetic fibers. | DNA damage, induction of bladder cancer in humans, splenic sarcomas | Vendemiatti et al. (2021) |
| Sulfur dyes | – | Converted to water-soluble substantive form before application. | Insoluble in water. | Alkaline | Cellulose fiber and cotton. | – | Zheng et al. (2021) |
3.1.1. Chemical properties of some common biosorbed dyes
3.1.1.1. Methylene blue
Methylene blue dye (MB), known as methyl thioninium chloride, is a heterocyclic aromatic chemical compound with the molecular formula [C16H18N3SCl]. It is classified as a cationic dye (Peres et al., 2018). It is consumed in various fields like drugs, dyestuff industries, paper, textile, and pesticide industries (Elwakeel et al., 2021b). Some of the undesirable negative effects on human health are heart palpitations increase, shock, vomiting, jaundice, cyanosis, quadriplegia, and tissue necrosis (El-Moselhy and Kamal, 2018). A huge amount of MB (>7.0 mg kg−1) can lead to high blood pressure, mental disorder, nausea, and abdominal pain (Lyu et al., 2018).
3.1.1.2. Crystal violet
Crystal violet (CV) (or gentian violet) is a triaminoarylmethane dye with the chemical structure C25H30N3Cl. It is classified as cationic dyes. It is extensively used in different industries such as pharmaceutical, paper, textile, and printing ink (Abbasi et al., 2017) and (Elwakeel, K. Z. et al., 2017). It is harmful, depending on concentration, by different ways of exposure (inhalation, ingestion, and skin contact). It is carcinogenic and can cause digestive tract, skin, and eye irritation in humans (Muthukumaran et al., 2016).
3.1.1.3. Reactive black 5
Reactive black 5 (RB5), C26 H21N5Na4O19S6, is a type of diazo acidic reactive dyes. It has two sulfonate groups and two sulfato-ethylsulfon groups in its molecular structure with a negative charge in aqueous solutions. It characterizes by a simple dyeing procedure and covalent binding with cellulose fibers (Jóźwiak et al., 2017). Commonly, it is one of the most reactive dyes used to a large extent of different fixtures such as cotton. The presence of RB5 in aqueous solutions is of great concern because of its toxic, carcinogenic, and mutagenic characteristics (Nematollahzadeh et al., 2015). It adversely affects the biotic communities' aquatic ecosystem (fauna and flora) and has direct toxicity to humans. It can cause severe damage to the liver, digestive, and central nervous systems of human beings (Elwakeel et al., 2016).
3.1.1.4. Congo red
Congo red (CR) is the sodium salt of 3,3′-([1,1′-biphenyl]-4,4′-diyl)bis(4-aminonaphtalene-1-sulfonic acid). Its chemical structure is [C32H22N6Na2O6S2] (Thanh Tu et al., 2018). It is classified as an anionic dye. Once, it solubilizes in water, it yields a red colloidal solution. It is used in many fields such as biology, biochemistry, and the textile industry (Aliabadi and Mahmoodi, 2018). It has many negative effects on human public health including vomiting, nausea, diarrhea, allergic problems, and difficulty in breathing. Also, it is easily metabolized to benzidine, which is identified as having possible carcinogenic effects for humans (Deng et al., 2018). Chemical structures of different mentioned dyes are shown in Figure S3 (see supplementary material).
3.2. Heavy metals
Surface and groundwater contamination by toxic heavy metals were globally reported as a serious threat to different water resources. They are inorganic notorious contaminants with a relatively high atomic weight between 63.5 and 200.6, and a density greater than 5 g cm−3. Different types of heavy metals, anthropogenic sources, toxicity, and their permissible limits are shown in Table 2 (Kobielska et al., 2018). They enter the environment through geological and anthropogenic sources. They are indispensable, relatively poisonous at low concentrations, and can be found within the different environmental compartments (Alonso-Magdalena et al., 2019). Commonly, heavy metals are found in rock-forming minerals. Copper, cobalt, manganese, nickel, zinc, and vanadium are examples of heavy metals that easily leach because of mineral weathering (Chen et al., 2019). Soil represents another medium for heavy metals storage. Heavy metals are found in soils in different forms. Contaminated surface waters can lade heavy metals over great distances (Richards et al., 2019). Other factors such as physical, chemical, and biological are considered large contributors to heavy metals contamination. Whereas, anthropogenic sources are implicated in groundwater contamination. Groundwater is the main source of drinking water. It is mainly polluted by both inorganic and organic contaminants from various anthropogenic sources. This includes runoff from agricultural sites, sewage systems, motorways, railways, landfills, urban areas, and so on. Human industrial activities are considered to be major contributors to environmental pollution by toxic heavy metals (Ibrahim and Fakhre, 2019). Considerably, several industrial activities such as coin production and coal combustion generate a huge amount of heavy metals laden wastewater. It includes arsenic, cadmium, cobalt, copper, chromium, lead, zinc, iron, manganese, nickel, mercury, and others. These effluents are dumped into water systems without proper manipulation (Liu, J. et al., 2019a). Heavy metals are classified into non-essentials and essential components necessary to biological and metabolic activities. As, Pb, Cd, and Hg are categorized as non-essential components to metabolic functions. Because of their non-biodegradability and bioaccumulation behavior, they are very toxic even at trace levels (Huang et al., 2019). Therefore, due to their deleterious nature, they have been ranked in the top 20 list of dangerous substances as reported by the United States Environmental Protection Agency (US EPA) and the Agency for Toxic Substances and Disease Registry (ATSDR). Whilst Cu, Co, Fe, Mn, Zn and Cr(III) are essential components for human health. They are associated with multiple metabolic processes of biota including cytochromes and enzymes. However, excessive exposure to these metals can lead to severe hazardous impacts on different biota. Maximum contamination levels of different heavy metals are set by World Health Organization (WHO). It confirms that zero or only the allowable levels should be in water sources and hence ensure water safety for human usage or consumption. Severe detrimental effects on ecosystems as well as health hazards are generated form existence of heavy metals in water systems. Their presence in water courses can pose major threats to living creatures. They are very toxic to aquatic ecosystems, adversely affect on plants, invertebrates, fish and amphibians (Sun et al., 2019). Additionally, they cause serious health problems on human beings. Various diseases such as autoimmunity, cancer, developmental retardation, kidney damage and even death in severe cases are associated with exposure to heavy metals (Arshad et al., 2019).
| Heavy metal or metalloid | Anthropogenic sources | Toxicity | Provisional maximum tolerable daily intake (PMTDI) (g L−1) | References |
|---|---|---|---|---|
| As | Animal feed additive, algaecides, herbicides, insecticides, fungicides, pesticides, rodenticides, sheep dip, tanning and textile, pigments, veterinary medicine, ceramics, special glasses, metallurgy, electronic components, non-ferrous smelters, electrical generation (coal and geothermal), light filters and fireworks. | Phytotoxic (toxic to plants), arsenicosis (i.e. Blackfoot disease), keratosis, possible vascular complications, and carcinogenic. | 0.01 | Cui et al. (2021) |
| Cd | Neutron absorbers (within nuclear reactors), nickel-cadmium batteries, anti-corrosive metal coatings, alloys, plastic stabilizers, coal combustion, and pigments. | Phytotoxic, bio-accumulative, itai-itai disease, and carcinogenic. | 0.003 | Nandi and Chowdhuri (2021) |
| Cr | Data storage, plating, ferroalloys manufacturing, textiles and leather tanning, wood treatment, passivation of corrosion of cooling circuits, and pigments. | Cr3+ not detrimental to mammals, Cr6+ very toxic and carcinogenic. | 0.05 | Kobielska et al. (2018) |
| Cu | Water pipes, chemicals and pharmaceutical equipment, kitchenware, roofing, alloys, and pigments. | Relatively not detrimental and narrow tolerance for plants. | 2 | Costa et al. (2020) |
| Pb | Alloys, ceramics, plastics, glassware, lead-acid batteries, cable sheathings, sheets, solder, pipes and tubing, sheets, ordinance, antiknock agent, tetramethyllead, and pigments. | Pb poisoning (a world-wide issue) through gasoline, plumbing, and paints. | 0.01 | Cho et al. (2020) |
| Hg | Amalgamation (the process of metal extraction), electrical and measuring apparatus, catalysts, dental fillings, Hg vapor lamps, X-ray tubes, pharmaceuticals, fungicides, scientific instruments, electrodes, rectifier, oscillators, and chloralkali cell's mobile cathode. | Biomagnification in aquatic environments and Minamata disease. | 0.006 | Frachini et al. (2020) |
| Ni | An alloy in the steel industry, computer components, catalysts, ceramic and glass molds, electroplating, nickel-cadmium batteries, dental and surgical prostheses, arc-welding, rods, and pigments. | Contact dermatitis, asthma, and chronic respiratory infections carcinogenic. | 0.07 | Özdemir et al. (2021) |
| Zn | Zn alloys, PVC stabilizers, gold precipitation from cyanide solution, in chemicals and medicines, anti-corrosion coating, cans, barriers, rubber industry. | Relatively not detrimental to mammals (may affect cholesterol metabolism in humans). | 0.3–1.0 mg kg−1 of body weight per day | Kobielska et al. (2018) |
Copper(II) as an example of heavy metals is a chemical element naturally abundant in different compounds. It is the third most widespread metal commonly used by mankind in the industrial sector. Its annual discharge rate is estimated to be 939,000 metric tons (Blagojev et al., 2019). It is primarily found in a variety of cells and tissues. It has many oxidation states but the divalent state Cu(II) is the most toxic oxidation state. Copper-laden wastewater is originally discharged from different industrial activities (do Nascimento et al., 2019). Despite its dangerous nature, it is considered to be an essential micronutrient to human health. Its required daily intake value varies between 0.9 and 2.2 mg for adults (Moreira et al., 2019). WHO and US EPA have set the threshold limits of 2 (mg L−1) and 1.3 (mg L−1) for copper, respectively in drinking water (Safari et al., 2019). Excessive intake of copper is harmful to animals and humans because of its bioaccumulation character. At high concentrations, it can cause harm to the skin, brain, myocardium, liver, pancreas, kidney, and reproductive system (Peng et al., 2019). Its high existence is also associated with depression, central nervous system disorders, gastrointestinal irritation, cirrhosis, lung cancer, and even death (Liu et al., 2018).
4. Biosorption
4.1. Biosorption fundamental
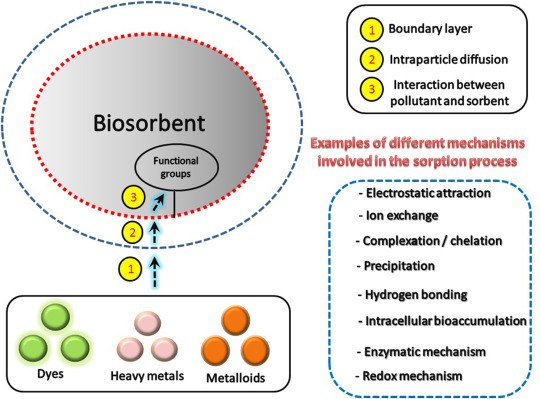
Biosorption is a practical application branch of sustainable development (biotechnological approach) (Işıldar et al., 2019). It is counted upon as an eco-friendly, economic, and efficient technique for water treatment (Gupta et al., 2018). It minimizes the concentration of different water pollutants to the acceptable limits recommended by different federal regulations (Krstić et al., 2018). This green protocol is in coincidence with the principles of green chemistry. The involved fundamentals of biosorption process related to its different ingredients should be comprehended. Briefly, it is defined as a metabolism-independent process (passive uptake), built on using of bio-wastes for the elimination of different water pollutants. Generally, numerous benefits are achieved due to the recycling of these biomasses (bio-wastes). Their utilization in their native and/or modified forms directly participates in wastes minimization. This can rectify many ecological and environmental problems (Gupta et al., 2019). It also characterizes by remarkable merits such as low operating and manufacturing cost and high efficiency.
4.2. Biosorption strategy
In recent years, biosorption conception as a multidimensional effective process has been evolving. It is perceived as an alternate admirable wastewater treatment compared with other traditional technologies (Demey et al., 2019). Sorption is mainly described as Physico-chemical phenomenon by which sorbate molecules concentrate on the surface of another substance (sorbent). This provides purified effluents with high qualities. Despite denoting a biological entity involvement by using the “bio” prefix, biosorption term is also simply defined. Both bioabsorption and biosorption dimensions are involved in terms of sorption mechanism. Absorption is the incorporation of a substance in one state into another substance with a different state. It includes the absorption of gases by water or liquids by a solid). While adsorption is a physical attachment in which sorbate interacts with a sorbent resulting in a sorbent-sorbate interface (Fomina and Gadd, 2014). Briefly, biosorption is a passive, metabolically-independent process covering all interaction aspects between any sorbate and biological matrix (biosorbent). It plays a crucial part in many processes naturally occurring in different scientific disciplines.
4.3. Selection of biosorbents
The suitability of biosorbent is known to be the foremost important step in controlling their selection. Biomass cost and its origin represent a vital criterion to be considered during biosorbent selection. Dead biomass has priority over the viable ones to be used for the preparation of different biosorbents (Elwakeel et al., 2012). Employing of dead biomass has many merits. It is summarized as (1) absence of the need of growth requirements (i.e. media, nutrients) to be contained in the feed solution; (2) absence of toxicity limitations; (3) possible reusability and recovery of saturated biosorbent and sorbed pollutants, respectively; (4) easier mathematical and statistical modeling of pollutants uptake. Additionally, the selected biosorbent should have numerous requisite aspects such as eco-friendly, availability, biocompatibility, and feasibility. This ensures its potential capacity to detoxify different water pollutants. Other diversified admirable features characterized by biosorbent are required. This includes its high sorptive performance towards target pollutants, good stability, and its reusability. Recyclability and adaptability of biosorbent to different designs (i.e. batch, fixed bed systems) should significantly be considered (Elwakeel, 2010). Based on wastes as wealth, utmost attention should be given to the available wastes. Their utilization offers multiple benefits because of their environmentally benign nature. It is economically beneficial because it overcomes disposal problems and generates revenue for different industries. In reality, the plentiful biological materials are substantially different in structure. They consist of a variety of ligands like alcohol, amino, aldehydes, carboxylic, hydroxyl, phosphate, thiol, ketones, phenolic, and ether groups. They are present at varying degrees and hence being capable to interact with target pollutants via different mechanisms.
Biosorption represents a promising and alternative to conventional processes. It depends on using bio-wastes for different water polluter's removal. Its theory is built to make a double benefit from these bio-wastes. This is done by recycling it to directly participate in wastes minimization in addition to extract maximum benefits from it (Gupta et al., 2019). Consequently, it allows reaching the abatement levels endorsed by international or national regulations and WHO (Hespanhol and Prost, 1994) It characterizes by remarkable merits such as low operating and manufacturing cost, flexibility, ease of operation, and high efficiency. Various biosorbents based on: (i) chitosan (Almughamisi et al., 2020); (ii) biochar (Lei et al., 2019); (iii) activated carbon (Elwakeel et al., 2015); (iv) bio-nanocomposites (Jung et al., 2019); (v) bio-hydrogels (Qi et al., 2019); (vi) macroalgae (Elgarahy, Ahmed M. et al., 2019); (vii) agriculture wastes (Toumi et al., 2018); and (vii) bio-calcium carbonate (Arslanoğlu, 2021) were used for water refinement from different pollutants including toxic dyes and heavy metals.
Marine algae are considered as alternative and effective based sorbents for the manipulation of wastewater. It considers renewable resources spread all over the world. It can be categorized based on its color and colloid content. Generally, three basic groups are normally found in oceans: Chlorophyta, Phaeophyta, and Rhodophyta algae (Mokhtar et al., 2017). The use of microalgae as a sustainable alternative has been demonstrated in recent studies. Afshariani and Schneider (Afshariani and Roosta, 2019), for example, studied the sorption of methylene blue by batch and continuous processes in aqueous solutions. At pH 9 and a temperature of 30 °C, the maximum sorption reached 87.69 ± 3.22 mg g−1. The use of defatted microalgae biomass (microalgae biofuel waste) as an alternative leather dye adsorbent was examined (da Fontoura et al., 2017). Biosorption experiments were carried out with Acid Blue 161 aqueous dye solutions (AB-161). A dye was adsorbed with a maximum amount of 75.78 mg g−1 and 83.2 mg−1 at 25 °C and 40 °C, respectively. The findings showed that biomass decreased dye concentrations substantially 76.65% from true tannery wastes effluents. Polysaccharides are the main components of the algal cell wall which consists of alginate, carrageenan, and polycolloid. These constituents have the capabilities for the removal of different water pollutants (Daneshvar et al., 2017). Both macro and microalgae were utilized as excellent candidates for different organic dyes (da Rosa et al., 2018); as well as heavy metals elimination from aquatic systems (Chen et al., 2019). The ability of green macroalga (Enteromorpha flexuosa) to capture both crystal violet (CV) and methylene blue (MB) from aqueous solutions was evaluated (Elgarahy, Ahmed M. et al., 2019). The results showed that percentage removal of 90.3% and 93.4% were obtained under optimum conditions of variables for CV and MB, respectively (Elgarahy, Ahmed M. et al., 2019). Green algae mainly consist of cellulose associated with a high percentage of proteins combined with polysaccharides to form glycoproteins (Jayakumar et al., 2014). These compounds characterize by possessing numerous functional groups (i.e. amino, hydroxyl, and carboxyl). They play a prominent role in the sorption process (Rangabhashiyam et al., 2016).
Brown algae characterize by possessing a broad range of metabolites such as alginates, fucoidans, mannitol, laminarins, fucoxanthin, halogenated compounds, polyphenols, and terpenoids (Saravana et al., 2018). Alginates are a mixture of calcium, phosphate, and sodium salts. Sodium salts are the major constituents naturally present in the brown seaweed cell wall. It is nearly up to 30–40% in its weight. It is linear anionic and water-soluble polysaccharides (Fernando et al., 2019). Different pre-extraction treatment methods were used for obtaining alginate from brown seaweed (Saravana et al., 2018). There is a great use of these natural biopolymers in different environmental applications (Cardoso et al., 2017). Referring to possessing different functional groups, it is widely used as a potential sorbent for treating wastewater from various water pollutants (Elgarahy et al., 2021).
Biochar derived from microalgae pyrolysis as another type of sorbents for heavy metals removal from aqueous solution was investigated by several researchers (Amin and Chetpattananondh, 2019). Many experiments on the efficiency of the Co(II) removal biochar have been performed in a batch system. The equilibrium data were well fitted for Freundlich, Temkin, and D-R isotherms. The Langmuir biosorption capacity reached 1.117 mg g−1 (Bordoloi et al., 2017). Biochar produced from water hyacinths (Eichhornia crassipes) was demonstrated to be an effective sorbent for the removal of certain heavy metals and as a means of control for this highly invasive species. Harvesting water hyacinth biomass as feedstock for biochar has the additional advantage of reducing its impact as an invasives species on sensitive aquatic habitats. biochar-alginate capsules were examined to remove Cd(II) from an aqueous solution with the maximum sorption capacities ranging from 24.2 to 45.8 mg g−1 (Liu et al., 2020).
Recently alginate and sericin particles chemically crosslinked with poly(vinyl alcohol) (SAPVA) for batch biosorption of rare-earth element ytterbium from the aqueous medium was investigated (da Costa et al., 2020), The equilibrium study showed that the maximum biosorption capacity for ytterbium was 0.642 mmol g−1 at 55 °C (da Costa et al., 2020). To increase the sorption properties of alginate gel beads, sodium alginate-based beads with different amount of pore-forming agent were prepared with calcium carbonate as the pore-forming agent (Hu, X. et al., 2020). The experimental results showed that the adsorption capacity of Cu(II) increased by at least two times (from 13.69 mg g−1 to 33.88 mg g−1) (Hu, X. et al., 2020). Another recent way for alginate modification was carried out, alginate-PEI beads were functionalized by phosphorylation and applied for the sorption of Nd(III) and Mo(VI) (Wei et al., 2020). The sorption of Nd(III) is strongly increased by phosphorylation, while for Mo(VI) the enhancement is rather limited by phosphorus groups. The phosphorylation of alginate-PEI beads increased Nd(III) maximum sorption capacity from 0.61 to 1.46 mmol g−1, The enhancement of Mo(VI) uptake is much less marked (from 1.46 to 2.09 mmol g−1) because of the high affinity of molybdate species for amine groups (Wei et al., 2020).
Chitosan is a derivative of chitin. It is made predominantly from shellfish waste items like crab or shrimp shells (Elwakeel, 2010). The comparison of the sorption properties of 6 different crosslinking agents crosslinking chitosan sorbents (3 ionic agents: sodium citrate, sodium tripolyphosphate, sulfosuccinic acid, and 3 covalent agents: glutaraldehyde, epichlorohydrin, trimethylo propane, triglycidyl ether) was examined. (Jóźwiak and Filipkowska, 2020). Ionic crosslinking significantly affected the sorption capacity of the chitosan hydrogel towards Reactive Black 5 dye (Jóźwiak and Filipkowska, 2020). The sorption potential of 46.7% and 37.2% for chitosan crosslinked with sodium citrate and sulfosuccinate was higher compared to non-crosslinked chitosan after 24 h of the sorption process (Jóźwiak and Filipkowska, 2020). Conversely, chitosan crosslinked with glutaraldehyde and trimethylopropane triglycidyl ether showed to have a 35.3% and 26.6% lower sorption potential after 24 h of sorption than that of unmodified chitosan. Once the sorption equilibrium had been reached, the highest sorption capacity (2307 mg g−1) was displayed by the unmodified chitosan, whereas sorption capacities of the ionically crosslinked hydrogels were in the range of 2005–2164 mg g−1 and these of the covalently crosslinked hydrogels were in the range of 2083–2183 mg g−1 (Jóźwiak and Filipkowska, 2020). To remove cationic crystal violet (CV) and anionic methyl orange dye (MO) from wastewater, polypyrrole decorated chitosan-based mag-sorbent was prepared. Removal performance was obtained to be 88.11 and 92.89% for CV and MO at the optimum conditions. The pseudo-second-order represented the adsorption of CV well, while the pseudo-first-order kinetics better followed that of MO. Langmuir adsorption isotherm with a maximum monolayer sorption potential of 62.89 and 89.29 mg g−1 for CV and MO, respectively, was well matched to the adsorption equilibrium data for both dyes. Another way for chitosan modification is to be immobilized on another polymer. For example, chitosan was successfully modified with 4-methyl-2-(naphthalen-2-yl)-N-propylpentanamide-functionalized ethoxy-silica (Jabli, 2020) then tested for methylene blue (MB) and Acid blue 25 (AB25). Using composite beads, the adsorbed yield of MB increased three times more than the capacity of chitosan beads, and in the case of AB25, it improved 1.4 times. Another chitosan hybrid composite was prepared by doping a very small amount of chitosan and Y(III) ions onto the acid-modified fly ash (named as MFA) the saturated adsorption capacity reached up to 627 mg g−1 (Li and Ren, 2020). A lot of effort was done by the researchers to increase the selectivity of chitosan towards specific metal ions. For example, the grafting of 2-mercaptobenzimidazole onto chitosan microparticles allows developing highly selective sorbents. The incorporation of magnetite particles is also a strategic aspect for facilitating the use and recovery of chitosan microparticles (Elwakeel et al., 2021a). The sorbent showed high selectivity for precious metals against base metals. The combination of chitosan and 2-MBI allows designing a very efficient sorbent for the recovery of precious metals from acidic leachates (Elwakeel et al., 2021a). The adsorption capacities of the heavy metal ions (manganese, iron, cobalt, nickel, copper, and zinc) on chitosan were investigated in dependence on their corresponding anions sulfate, chloride, and nitrate by batch experiments (Weiβpflog et al., 2020). The selectivity of the different heavy metal ions was analyzed by column experiments. The heavy metal cations of the sulfate salts and the sulfate ions themselves adsorb to a significantly higher extent compared to the analogous chloride and nitrate salts, respectively (Weiβpflog et al., 2020). Luo et al. (2020) prepared fluorescent chitosan-based hydrogel incorporating titanate and cellulose nanofibers modified with carbon dots to effectively remove Cr(VI) (Luo et al., 2020). The sorbent had a higher adsorption ability of Cr(VI) (maximum adsorption capacity, 228.2 mg g−1), maybe mainly attributed to the porous structures and the additional titanate and cellulose nanofibers modified with carbon dots improving the sorption ability of Cr(VI) (Luo et al., 2020). A series of magnetically modified chitosan sorbents with core-brush topology were prepared through grafting co-polymerization on the surface of chitosan/Fe3O4 composite particles, and then applied for the removal of two pharmaceuticals (diclofenac sodium) and tetracycline hydrochloride) from water (Zhang et al., 2016). This study provided a strategy for the design of adsorbents from both topological and chemical structures (Zhang et al., 2016). All the modified chitosan sorbents exhibited higher removal efficiencies, due to the enhanced surface areas and functionalization (Elwakeel et al., 2014).
A series of novel chitosan/nanodiamond (chitosan/ND) composites containing NDs with variable surface carboxyl groups and various concentrations were prepared using the solution casting method. Powdery chitosan/ND composites were employed as the adsorbent of a model anionic dye (methyl orange, MO). Experimental results showed that the incorporation of NDs with high carboxylic content (ND-H) into chitosan increased substantially the maximum adsorption capacity of neat CTS from 167 mg g−1 to 454 mg g−1. The remarkable adsorption capacity of dye on chitosan/ND composites was associated with the oxygen-containing groups on the outer surface of NDs which would be beneficial to interact with the dye molecules through hydrogen bonding and electrostatic interactions (Raeiszadeh et al., 2018).
Calcium carbonate (CaCO3) is considered one of the most versatile materials known to man. It is widespread and represents above four percent of the total earth's crust. CaCO3 is found in many common forms as chalk, marble, and limestone. Bio-calcium is mainly produced from shells of aquatic biota such as bivalves, corals, and snails (Elwakeel et al., 2020). It carbonate can be found in three main forms: aragonite, calcite, and vaterite (Hoque et al., 2013). Though all these forms are the same chemically, it varies in other physical aspects as whiteness, homogeneity, thickness, and purity. Due to its special white color, CaCO3 is extensively used in the cement industry. It has also wide applications as a filler and/or coating pigment in coatings facilities as well as plastics, paints, and paper industries (Thilagan et al., 2015). Moreover, CaCO3 is used in industrial settings to neutralize acidic conditions in both soil and water due to its antacid properties (Granados-Correa et al., 2013). Granados-Correa et al. mentioned that CaCO3 benefits the environment through water and wastewater treatment (Granados-Correa et al., 2013). It is widely used in the tissue bioengineering field (Dharmaraj et al., 2015).
Two marine wastes, including sepia shells and bivalve shells, are rich in CaCO3. Shells of the mentioned aquatic biota act as exterior and interior skeletons for protecting the soft parts of the molluscan bodies. Sepia shells (SS) are sub-products of fisheries. They offer mechanical support against predators. Despite being rich in calcium, they are usually disposed of without any commercial valorization. This may cause locally some environmental nuisances especially in Port-Said City, Egypt. The exploitation of this by-product for application in dye removal would make a double benefit. A literature survey showed that very few works were performed on dye removal using cuttlefish bones (Hasmath Farzana and Meenakshi, 2014). Bivalve shells of Anadara uropigimelana were firstly tested as a potential biosorbent for MB recovery from an aqueous solution (Elwakeel, Khalid Z. et al., 2017), Percentage removal of 93.6% were obtained under optimum conditions of variables (initial pH of 10.4, sorbent dose of 1 g L−1, initial MB concentration of 20 mg L−1, and temperature of 25 ± 1 °C) (Elwakeel, Khalid Z. et al., 2017). On the other hand, bivalve shells are composed of 95% CaCO3 in addition to 5% of proteins and polysaccharides (Kanyal and Bhatt, 2015). Due to its structural and superficial properties, it is used as an adsorbent material for the removal of different pollutants from the environment (Chavan and Mane, 2013). Calcium chloride (CaCl2) is one of the calcium carbonate derivatives that can be directly prepared by reacting to CaCO3 with a hydrochloric solution. Discarded oyster shells were used to prepare vaterite calcium carbonate microparticles and explored the removal effects and the underlying mechanism toward several heavy metal ions (Elwakeel et al., 2020). The experimental results showed that CaCO3 exhibited different removal efficiencies for Pb(II) (99.9%), Cr(III) (99.5%), Fe(III) (99.3%), and Cu(II) (57.1%) (Lin et al., 2020). Efficient sorbent based on Sepia shells (cuttlefish bones) was prepared and examined for the sorption of cationic dye (crystal violet, CV) and an anionic dye (congo red, CR). The sorbent was modified by the reaction of sepia shell powder with urea in the presence of formaldehyde (SSBC). Maximum sorption capacities reach up to 0.536 mmol g−1 for CV and 0.359 mmol g−1 for CR, at pH 10.6 and 2.4, respectively (Elwakeel et al., 2020). Using SSBC Maximum sorption capacities reach up to 0.794 mmol g−1 (254.05 mg g−1) for MB and 0.271 mmol g−1 (269.18 mg g−1) for RB5, at pH 10.5 and 2.3, respectively (Elgarahy, A. M. et al., 2019). Recently, an eco-friendly sorbent based on marine brown algae and bivalve shells for subsequent uptake of congo red dye and copper(II) ions was investigated, the maximum sorption capacity reached up to 441 mg g−1 (Elgarahy et al., 2020).
Analyzing the reviewed biosorbents, chitosan and alginate-based biosorbents showed the best sorption capacities for metal ions removal. Chitosan-based sorbents were the most effective sorbents for cationic dyes removal, while alginate-based sorbents were the most effective sorbents for anionic dyes. Alginate was demonstrated to be more chemically stable. However, the addition of cross-linking agents to chitosan composites could overcome such a disadvantage.
Generally, numerous benefits will achieve due to the recycling of these biomasses. Its utilization will participate in wastes elimination. Additionally, it will minimize contaminant concentrations in different water resources. This can rectify many environmental problems. Other remarkable merits such as low operating and manufacturing cost in addition to high efficiency may be achieved.