1. Introduction
Natural products (NPs), including antibiotics and other bioactive compounds, are widely used in traditional medicine, especially for treatment of cancer, infectious diseases, cardiovascular disease, and diabetes (Newman and Cragg, 2016). The total annual medical consumption of antibiotics worldwide is estimated at hundreds of thousands of tons (Wise, 2002). Actinobacteria, particularly the Streptomyces genus, as a high mol% G + C Gram-positive bacteria of terrestrial and marine origin, is used to produce more than 40% of all known microbial bioactive NPs (Bérdy, 2012), and 35% of all marketed antibiotic formulations contain an active ingredient derived from Actinobacteria (Gomez-Escribano and Bibb, 2014). During the final decades of the last century, after the “golden age” of antibiotic drug discovery in Actinobacteria in the 1980s, only a few antibiotic classes were developed (Hopwood, 2007). However, with the advent of the genomics era, Actinobacteria is again regarded as a robust and rich source to search for novel products, as next generation DNA sequencing has revealed a wealth of hidden biosynthetic gene clusters of unknown NPs within the genomes of Actinobacteria species (Bentley et al., 2002, Harvey et al., 2015). In addition, with the exploration of marine resources, the marine Actinobacteriahave also been revealed as huge resources for discovery of new NPs (Gomez-Escribano et al., 2016, Jensen et al., 2015, Subramani and Aalbersberg, 2013, Xiong et al., 2013).
As a microbial cell factory for industrial production of NPs, kinds of irrational and rational approaches have been applied to improve NP titers in Actinobacteria. For example, by applying traditional random mutagenesis and screening, a tremendous increase in targeted NP production (such as antibiotics) has been achieved. However, as a time-consuming and labor-intensive approach, random mutagenesis and screening not always enable appearance of the optimal solution in terms of specific mutations that are required for the desirable phenotype (Rokem et al., 2007). In recent decades, with the development of metabolic engineering, Actinobacteria have been engineered to produce higher titers of NP. At present, methods to efficiently improve the yield and productivity of NPs derived from Actinobacteria are being carefully investigated to reduce energy consumption, as well as waste and emission generation during industrial fermentation in response to a series of published studies showing very high emissions and pollution generated by the manufacture of pharmaceuticals (Larsson, 2014). To this end, more efforts should be taken to maximize the biosynthetic efficiency of the microbial cell factory of Actinobacteria by engineering of both the producer and NP synthetic pathway. However, due to the poor understanding of the complicated metabolic and regulatory profiles of targeted synthetic pathways, and limited information on the producers, rational and systematical engineering of synthetic pathways of NPs in Actinobacteria is often difficult.
In addition, heterologous expression of genes and gene clusters in Streptomyceshas been used extensively as a means to access new NPs in the genomics era (Komatsu et al., 2013, Luo et al., 2016). Nevertheless, it is worthwhile to note that occasionally the heterologous production of NPs has very low efficiency or is even unable to produce targeted NPs. In Streptomyces, heterologous production of NPs may involve the addition of a precursor and cofactors to regulate transcription of a gene cluster and enzymatic activity of each catalytic protein (Rokem et al., 2007). Therefore, heterologous production of targeted NPs is just a starting point in the drug development process, while the following rational engineering and innovation needed for efficient biosynthesis often remains challenging.
With the emergence of new metabolic engineering methods, the concept of refactoring had expanded strategies to decouple metabolite biosynthesis from complex native regulation or to rewire the NP synthetic pathway. For example, in a model organism, such as Escherichia coli, the synthetic pathways of some chemicals, including biofuels and fatty acids, have been systematically refactored to achieve precise and flexible control of production (Xu et al., 2014, Zhang et al., 2012). In Saccharomyces cerevisiae, the synthetic pathway of artemisinic acid has also been systematically refactored to achieve highly efficient heterologous production (Paddon et al., 2013). These studies also identified promising approaches for synthetic pathway refactoring in Actinobacteria. In this review, the significance of Actinobacteria as a microbial cell factory for NP production will be discussed and several challenges that need to be overcome in order to improve biosynthetic efficiency are also considered. To this end, current approaches toward metabolic engineering of NP biosynthetic pathways are briefly summarized. Lastly, a strategy and perspectives to rationally and systematically refactor the NP synthetic pathway in Actinobacteria are highlighted.
2. Actinobacteria as the microbial cell factory for NP biosynthesis
As the most widely used microbial hosts in metabolic engineering, E. coli and Sa. cerevisiae have many merits, such as well-understood metabolic backgrounds, amenable genetic manipulation, and fast growth. With the developments in metabolic engineering, many breakthroughs in microbial biosynthesis of various types of pharmaceutical precursors, such as 6-deoxyerythronolide B (Pfeifer et al., 2001), taxadiene (Ajikumar et al., 2010), artemisinic acid (Paddon et al., 2013), and thiamine or hydrocodone (Galanie et al., 2015), have been achieved in these microbes. However, as the producer of most NP antibiotics used clinically today (Lewis, 2013), Actinobacteria undoubtedly has more practical significance in pharmaceutical industrial application than E.coli or S. cerevisiae, as Actinobacteria, especially Streptomyces, display many unique advantages for NP biosynthesis.
The advantages of Actinobacteria as a microbial cell factory for NP biosynthesis are summarized in Table 1. First of all, Actinobacteria may be better suited or compatible for the heterologous biosynthetic genes with high G + C content and mega synthases, such as polyketide synthases (PKS) and nonribosomal peptidesynthetase (NRPS). It is known that Actinobacteria has the most extreme codon usage profile, as most of the wobble positions of the codon are a G or C, resulting in a high G + C content in this organism (e.g., the G + C content in S. coelicolor is 71%) (Gustafsson et al., 2004) and the biosynthetic gene cluster with high G + C content from marine Actinobacteria could be efficiently heterologously expressed in Streptomyces (Yamanaka et al., 2014). In addition, the PKS and NRPS genes of Actinobacteria are often composed of tens of kilobase pairs, e.g., in the candicidin (or FR-008) gene cluster and the six genes encoding for type I modular PKS are reported to be as long as 100 kilobase pairs (Chen et al., 2003, Olano et al., 2014). Even so, the large gene cluster (>80 kb) encoding PKS compounds could also be heterologously produced in Streptomyces and other Actinobacteria (Huang et al., 2016, Nah et al., 2015, Sosio et al., 2000). Second, Actinobacteria is a powerful platform for NP biosynthesis due to its abundance of intracellular metabolites and intermediates, which could be used as precursors for formation of various types of NPs and the great potential for delicate post-modification of chemical structures. As reported by Komatsu et al., more than 20 NPs, which are classified into five classes on the basis of the pathway for generation of corresponding precursors, have been successfully heterologously produced in Streptomyces avermitilis (Komatsu et al., 2013). These cases offer a rough idea of the powerful NP biosynthesis capabilities of Streptomyces. In addition, the complicated and delicate post-modification capabilities of Actinobacteria could also be addressed post-modification during biosynthesis of the type I PKS compound spinosyn in Saccharopolyspora spinosa(Mertz and Yao, 1990, Waldron et al., 2001). In the spinosyn biosynthetic gene cluster, 14 of 19 genes are responsible for spinosyn post-modification, which include complicated intramolecular cycloaddition (Fage et al., 2015, Kim et al., 2011) and cross-bridging (Kim et al., 2007), as well as multiple steps of glycosylation (Chen et al., 2009, Hong et al., 2006, Hong et al., 2008, Isiorho et al., 2014, Zhao et al., 2005) and methylation (Kim et al., 2010). After multiple steps of post-modification, these NPs often are too complex for chemical modification. Lastly, as a widely used antibiotic producer in industrial fermentation, Actinobacteria have many other meritorious features, such as cognate antibiotic tolerance (Méndez and Salas, 2001), phage resistance (Chinenova et al., 1982), and mature industrial fermentation and separation techniques.
Table 1. The advantages and challenges of Actinobacteria as the microbial cell factory for natural products production.
| Advantages |
|---|
|
|
|
|
| Challenges |
|
|
|
These merits mentioned above render Actinobacteria as a suitable host for NP biosynthesis. Hence, many Streptomyces spp., such as S. coelicolor (McDaniel et al., 1993), S. avermitilis (Komatsu et al., 2010), and S. albus J1074 (Zaburannyi et al., 2014), have been developed as a universal chassis for the production of heterologous NPs. It is worthwhile to note that S. sp. FR-008, which is more amenable to genetic manipulation and fast growth (the doubling time is about 3.6 h), has also been developed as a Streptomyces chassis by knock-out of several native antibiotic pathways to create a clean background (Liu et al., 2016). In addition, some industrial strains, such as S. hygroscopicus TL01 (Zhou et al., 2011), S. albus BK3-25 (Lu et al., 2016), S. cinnamonensis C730 (Li et al., 2009) and Sa. erythraea (Huang et al., 2016) could be optimized through mutation for production of heterologous NPs composed of similar building blocks. Thus, starting with these industrial strains offers a shortcut to increase NP production (Pickens et al., 2011).
On the other hand, there are also some challenges that must be taken into consideration. As shown in Table 1, the complicated transcriptional and metabolic control necessary in NP biosynthesis (Liu et al., 2013, Shao et al., 2013) and poor understanding of biosynthetic mechanism often hinder metabolic engineering to achieve high yield or biosynthetic efficiency of NPs in Actinobacteria. In addition, possibly due to the lack of rational guidance toward redesign (or edit) of known biosynthetic pathways and discovery of new structures produced by unknown pathways, traditional diversification of NPs or new structure discovery in Actinobacteria are often intensive with low efficiency (Scheffler et al., 2013, Wu et al., 2015). Lastly, many Actinobacteria, such as Saccharopolyspora spinosa and Actinosynnema pretiosum, are not only amenable to genetic manipulation but also with a slow cell growth (e.g. the doubling time of S. spinosa is more than 20 h) (Xue et al., 2013, Gao et al., 2014, Lin et al., 2010), which has hindered their application in NP biosynthesis and discovery. In view of these reasons, possible solutions to these challenges are discussed in the following sections.
3. Current approaches for engineering of NP synthetic pathways
Actinobacteria produce a variety of NPs via secondary metabolic biosynthetic pathways. Primary metabolism provides precursors, reductive power, and adenosine triphosphate for biosynthesis of biomass and secondary metabolites. Various pathways for the generation of precursors include sugars, polyketide, amino acids, shikimate, and the mevalonate (or 2-C-methyl-D-erythritol 4-phosphate) pathways. In secondary metabolism, the transcriptional regulation system in Actinobacteria is responsible for activating or inhibiting the transcription of specific NP biosynthetic pathways in accordance with the extracellular environment and intracellular physiological status (Martín, 2004, Rigali et al., 2008). Lastly, the secondary metabolite biosynthetic pathway acts as a manufacturing workshop to produce various types of NPs by utilization of different raw materials (precursors). Consequently, approaches for metabolic engineering of targeted synthetic pathways for NP overproduction or derivatization mainly revolve around the precursor supply, transcriptional regulation, and biosynthetic genes.
Current approaches for metabolic engineering of NP biosynthesis in Actinobacteria have already been well reviewed (Bilyk and Luzhetskyy, 2016, Pickens et al., 2011). According to these studies, metabolic engineering to improve NP yield are summarized as follows: (1) Overexpression of positive regulatory genes or inactivation of repressors to enhance NP biosynthesis. As is known, both global pleiotropic and cluster-situated regulators are involved in the transcriptional regulation of secondary metabolism in Actinobacteria(Martín and Liras, 2010). Various types of global transcriptional regulators, such as AdpA, have been engineered to increase gene cluster transcription and antibiotic production in high-yield producers, such as S. hygroscopicus TL01 (Tan et al., 2015, Tan et al., 2013). Other global regulators, including the two-component PhoP-PhoR system (Mendes et al., 2007), PAS–LuxR transcriptional regulators (Santos-Aberturas et al., 2011), the nitrogen regulatory protein GlnR (Qu et al., 2015), the WhiB-like protein WblA (Lu et al., 2015, Yu et al., 2014) and the GntR-family transcription regulator DasR (Liao et al., 2015), have also been engineered for NP biosynthesis in Streptomyces and other Actinobacteria. On the other hand, cluster-situated regulatory genes, such as hrmA and hrmB, which regulate hormaomycin production in Streptomyces griseoflavus W-38 (Cai et al., 2013), mibR and mibX, which control microbisporicin production in the rare Actinobacteria Microbispora coralline (Fernández‐Martínez et al., 2015), sgcR1and sgrR, which are involved in the regulation of antitumor antibiotic C-1027 production in Streptomyces globisporus (Li et al., 2015b), and SACE_7301, which positively controlling erythromycin biosynthesis in Sa. erythraea A226 (Wu et al., 2014), have been engineered for overexpression of corresponding NPs. (2) Increase the intracellular precursor supply by overexpression of appropriate biosynthetic genes or elimination of competing pathways. For example, overexpression of the uridine diphosphate (UDP)-glucose pyrophosphorylase gene ugp to increase the supply of UDP-glucose for validamycin overexpression in S. hygroscopicus TL01 (Zhou et al., 2011), duplication of the phosphoglucomutase gene pgm in S. argillaceus to increase the supply of glucose-1-phosphate for mithramycin production (Zabala et al., 2013), and increase the supply of malonyl-CoA and methylmalonyl-CoA by deletion of several potential competitive PKS/PKS-NRPS gene clusters for salinomycin overproduction in S. albus BK3-25 (Lu et al., 2016). (3) Manipulate the biosynthetic gene cluster by duplication or heterologous expression. It was reported that ZouA-mediated DNA amplification (Murakami et al., 2011) has been used for validamycin production by duplicating its gene cluster. In Streptomyces pristinaespiralis, the production of pristinamycin II also has been enhanced by gene cluster duplication (Li et al., 2015a).
Undeniably, many of the approaches mentioned above are sufficient to efficiently increase NP yield by Actinobacteria. However, several issues must still be addressed. First, one of the most important issues is how to rapidly and effectively identify the rate-limiting factors in NP biosynthesis. For example, as an efficient approach to increase the production of NPs, engineering of regulatory genes that control the biosynthesis of bioactive secondary metabolites has been widely used in Actinobacteria (Martín and Liras, 2010). However, it should be noted that manipulation of putative regulatory genes that are annotated by CDS sequence BLAST analysis often result in no improvement in effects or can even reverse NP production because a prerequisite for successful engineering of regulatory cascades for overproduction of targeted products, the corresponding regulatory mechanisms involved in NP biosynthesis are usually complicated and incompletely understood, especially for indirect cascade regulation. Hence, deciphering the regulatory mechanism of targeted biosynthetic pathways in Actinobacteria is often difficult. A second issue is whether the transcriptional or metabolic regulatory mechanism for NP biosynthesis can be redesigned for more precise and flexible control of targeted synthetic pathways to maximize biosynthetic potential. A last issue is how to rationally engineer the synthetic pathway for high-efficiency NP formation. For these reasons, the present study attempted to overcome these issues in the application of Actinobacteria as a microbial cell factory for NP biosynthesis. To address these issues, a methodology termed rational synthetic pathway refactoring is proposed in the following section.
4. Rational synthetic pathway refactoring for NP biosynthesis
The complexity of naturally occurring cellular pathways is often a major challenge in metabolic engineering. However, with the emergence of new metabolic engineering methods, various new biological elements, modules, derives, and systems have been created that utilize these elements for refactoring of NP synthetic pathways. Refactoring cannot only facilitate the creation of new structures by substituting variations of enzymes from homologous clusters, but also permits the exchange of regulatory factors, thereby enabling control of enzyme expression levels in these pathways (Smanski et al., 2016). In the refactoring process, codon-randomized essential structural genes and well-characterized genetic parts (including promoters, ribosome binding sites, terminators, and various types of genetic circuit, etc.) can be employed to rewrite the natural genetics multigene system to render it more amenable to engineering efforts (Smanski et al., 2016, Temme et al., 2012). For NP biosynthesis in Actinobacteria, refactoring has been applied for the activation of silent biosynthetic gene clusters by employing the characterized strong promoters to decouple the synthetic pathway from complex native regulation (Luo et al., 2013, Shao et al., 2013). With the recent development of analytical and detection technology, the NP synthetic pathway could also be rationally refactored under the guidance of in vitro analysis or omics analysis to maximize the biosynthetic potential of targeted synthetic pathways. In the following section, some approaches to rational NPs synthetic pathway refactoring in Actinobacteria are proposed.
4.1. In vitro reconstitution guided synthetic pathway editing
As mentioned above, an important challenge to traditional metabolic engineering is the identification of rate-limiting factors of major importance to improve specific cellular functions. In addition, it was also found that the efficiency of editing the synthetic pathway for NP production or derivatizationis low, due to the complexity of living systems, which results in limited freedom to test biosynthetic potential. Therefore, a simplified in vitro system with more focus on targeted enzymatic pathways could be created to overcome this barrier. Hence, in vitro systems could provide unprecedented freedom to modify and control biochemical systems for technological application and to understand the design principles of biological circuits.
To this end, by removing targeted elements from cells and recapturing their catalytic activities in vitro, which is referred to as in vitro reconstitution, researchers will be able to use a variety of analytical tools to study the fine details of associated chemical transformation. Without construction of a series of mutants by in vitro reconstitution, the rate-limiting steps, as well as the biosynthetic potential of targeted biosynthetic pathway, could be revealed by catalytical analysis (Tan et al., 2016). Briefly, this approach involves four steps, as outlined in Fig. 1. First, the proteins involved in the targeted synthetic pathway are overexpressed and purified. The whole pathway is reconstituted and then the role of each component is analyzed systematically by in vitroassays. Second, engineered strains are constructed under the guidance of information gained from these in vitro assays. Third, the metabolic status of the engineered strains is analyzed at both the protein and intermediate levels. Last, the deviation between the observed data and the optimum conditions provide targets for further engineering. By applying this approach, various types of valuable chemicals, including fatty acids and their derivatives (Guo et al., 2014, Li et al., 2014, Liu et al., 2014), and terpenoid (Zhu et al., 2015, Zhu et al., 2014) could be efficiently produced in E. coli and S. cerevisiae.
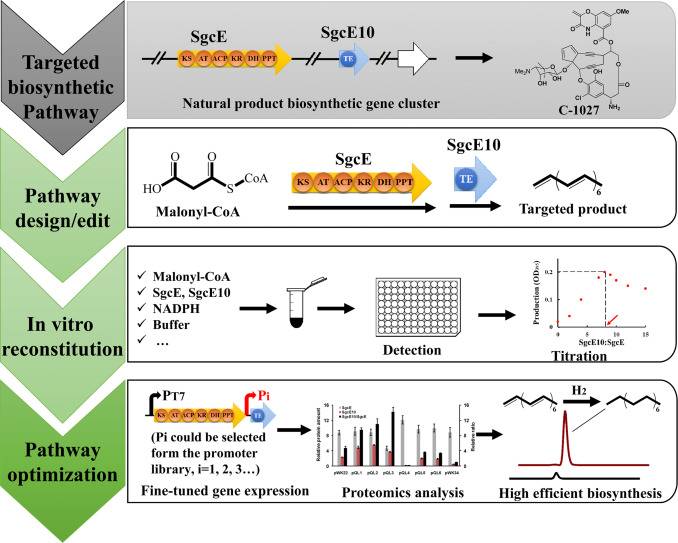
 Fig. 1. In vitro reconstitution guided synthetic pathway refactoring for targeted product biosynthesis. 1) The type I PKS SgcE (203 kDa) and cognate TE SgcE10 (18.5 kDa) derived from the C-1027 biosynthetic pathway in S. globisporus. 2) Synthetic pathway of C-1027 was re-edited to overproduce a single form of pentadecaheptaene (PDH). 3) In vitro reconstitution assays processed with malonyl-CoA and NADPH, and titrated PDH production by measuring absorbance. 4) Fine-tuned adjustments of targeted gene expression by using T7-derived promoter library. Relative amounts of SgcE and SgcE10 could be determined by targeted proteomics analysis. The engineered strain with best production of targeted product could be obtained.
Fig. 1. In vitro reconstitution guided synthetic pathway refactoring for targeted product biosynthesis. 1) The type I PKS SgcE (203 kDa) and cognate TE SgcE10 (18.5 kDa) derived from the C-1027 biosynthetic pathway in S. globisporus. 2) Synthetic pathway of C-1027 was re-edited to overproduce a single form of pentadecaheptaene (PDH). 3) In vitro reconstitution assays processed with malonyl-CoA and NADPH, and titrated PDH production by measuring absorbance. 4) Fine-tuned adjustments of targeted gene expression by using T7-derived promoter library. Relative amounts of SgcE and SgcE10 could be determined by targeted proteomics analysis. The engineered strain with best production of targeted product could be obtained.In regards to Actinobacteria, Liu et al. first reported the use of the StreptomycesPKS-derived protein SgcE for in vitro reconstitution to produce a high-value fuel product (Liu et al., 2015). In their work (as shown in Fig. 1), the biosynthetic pathway of C-1027, which is a potent antitumor antibiotic produced by S. globisporus (Liu et al., 2002), has been re-edited to overproduce a single form of pentadecaheptaene (PDH) followed by its hydrogenation to pentadecane (PD). The purified type I PKS SgcE (203 kDa) and cognate TE SgcE10 (18.5 kDa) derived from the C-1027 biosynthetic pathway was initially reconstituted in vitro to produce PDH. Based on the in vitro reconstitution assays, it was found that PDH production was strongly dependent on the SgcE10: SgcE ratio and a ratio of 8 afforded maximum PDH production. Then, the SgcE10 expression level was subsequently fine-tuned using a T7 promoter-based synthetic promoter library. Finally, the single form of C15 alkane was achieved and the highest titer reached was 140 mg L–1 and the best SgcE10: SgcE ratio calculated in vivo was closer to that obtained from the in vitro assay. This strategy was also successfully applied in Actinobacteria, indicating that the optimized PDH synthetic pathway could also work in S. sp. FR-008 (unpublished results). This study demonstrated the utility of developing in vitro reconstitution systems for the guidance of synthetic pathway refactoring.
In vitro reconstitution could also provide guidance on the refactoring of complicated synthetic pathways. As is known, the biosynthetic pathways for many NPs with complicated chemical structures can often been divided into several biosynthetic modules or blocks. Therefore, the whole complicated biosynthetic pathway could be broken-up into several parts or modules and each targeted part could be refactored separately under the guidance of in vitroreconstitution. For example, a series of methylation reactions often occur during post-modification of polyketide. In order to investigate the function and substrate specificity of the three S-adenosyl methionine (SAM)-dependent methyltransferases involved in the methylation reactions of spinosyn, Kim et al. reconstituted the methylation module in vitro using three methyltransferases (SpnI, SpnH, and SpnK), a substrate, SAM, and Tris-HCl buffer. Systematic analysis demonstrated the preferred reaction sequence of catalyzed reactions and the likely regulation of permethylation of the sugar moiety (Kim et al., 2010). Moreover, through in vitro reconstitution, the ratio of three methyltransferase in the spinosyn biosynthetic module to achieve the greatest bioconversion efficiency was also revealed. These findings also provide guidance for metabolic engineering of spinosyn production.
However, due to the interference occurring in vivo from the existence of competition or a branched pathway, or phosphorylation or acetylationmodification of targeted proteins, an in vitro system cannot always precisely mimic in vivo conditions. It should also be noted that in vitro reconstitution is not suitable for a pathway containing proteins with certain attributes, such as poor solubility, purification difficulty, and susceptibility to activity loss in vitro(Tan et al., 2016). To construct highly efficient cell factories, both the pathways of interest and the whole cell system should be well balanced. Also, approaches to provide further guidance to metabolic engineering of the whole cell system are absolutely essential.
4.2. Omics analysis-guided systematic NP synthetic pathway refactoring
Since the first complete Streptomyces genome was released in 2002 (Bentley et al., 2002), dozens of whole genomes of antibiotic-producing Actinobacteria spp. have been sequenced, indicating that genome-based functional analysis of antibiotic producers could shed new light on antibiotic biosynthesis, development, regulation, and evolution, as well as mining of the rich repertoire of secondary metabolites (Wu et al., 2012). Sequencing of the complete genome will not only help in the discovery of NP biosynthetic pathways and understanding of underlying biosynthetic mechanisms (Lautru et al., 2005, Wang et al., 2015), but also lays a foundation for comparative genomics to link gene variation(s) to the interested phenotype, such as highly efficient antibiotic production (Peano et al., 2014). The use of DNA microarray or RNA-seq next-generation sequencing technologies has facilitated transcriptomic analysis of gene expression profiles (Mutz et al., 2013, Schena et al., 1995). In Actinobacteria, transcriptome analysis has been widely used as an effective approach to discover novel regulatory genes (or mechanisms) and to facilitate systematic analysis of bacteria with the aim to elucidate the molecular basis for metabolic engineering of NPs (Tan et al., 2015). Proteomic analysis refers to analytical methodologies that attempt to determine changes in protein concentrations and structures in given cells. The main theme of proteomics is to elucidate the complete panoramic picture of protein composition and dynamics, and connection of total proteins (proteome) in the cell under specified conditions (Lee et al., 2005). Metabolomics arose from the concept of metabolic profiling with the goal to qualitatively and quantitatively analyze all metabolites that are contained in an organism at a specific time under specific conditions. To date, metabolomics also offer unprecedented tools for NP discovery and production in Actinobacteria (Breitling et al., 2013). About 10 years ago, the concept of “X-omics,” which refers to any omics study currently available, such as genomics, transcriptomics, proteomics, metabolomics, fluxomics, and regulomics, was introduced by Lee et al. (Lee et al., 2005). Obviously, the combined analysis of these X-omics could provide a foundation for systematic and in-depth understanding of targeted biological processes.
In recent several years, various innovations in MassSpec technology, such as the developments in both column and instrument technologies, including ultra-high performance liquid chromatography, core-shell columns, and monolithic columns, have dramatically decreased the analysis time and improved throughput (Raad et al., 2016, Kuehnbaum and Britz-McKibbin, 2013, Nováková, 2013). The developments in high-resolution MS technology could facilitate the emergence of gel-free proteomics and metabolomics. The employment of omics technology could provide great potential to improve Actinobacteria as an intelligent chemical factory to enhance production of useful bioactive compounds. To date, the widespread application of omics techniques and approaches have not only facilitated NP discovery and development (Asamizu et al., 2013, Chen et al., 2013, Doroghazi et al., 2014, Gubbens et al., 2014, Katz and Baltz, 2016, Kersten et al., 2013), but have also helped to elucidate the underlying metabolic mechanisms and to identify new metabolic junctions in Actinobacteria. In addition, by implication of omics analysis, the transcription profile, expression levels of catalytic enzymes, and the accumulation of metabolites involved in targeted synthetic pathways, could be quantitatively analyzed. The information obtained by omics analysis could also provide guidance on synthetic pathway refactoring in Actinobacteria.
The complete process of NP synthetic pathway refactoring under the guidance of omics analysis data is presented in Fig. 2. As is known, heterologous expression of biosynthetic gene clusters in a tractable Streptomyces host is a widely used approach for metabolic engineering of NPs. By employment of currently available methodological approaches, such as Gibson assembly (Gibson et al., 2009), transformation-associated recombination (Yamanaka et al., 2014), Red/ET-mediated recombination (Gust, 2009), cosmid/fosmid library construction (Weber et al., 2015), as well as bacterial artificial chromosomelibrary construction (Sosio et al., 2000), the targeted whole biosynthetic gene cluster could be efficiently cloned and subsequently introduced into a heterologous host for NP production. However, the heterologous production of NPs often has very low efficiency or is unable to produce NPs. In addition to heterologous production of NPs, such qualities are also prevalent in many wild-type producer strains with low yields of NPs. Under these situations, omics data, including transcriptomics, proteomics, and metabolomics, could be applied to systematic analysis to identify the rate-liming steps (parts) or synthetic modules. The information provided by omics analysis could guide subsequent synthetic pathway refactoring in several aspects, including module optimization, pathway optimization, genetic circuit design, and host improvement.

Fig. 2. Omics guided systematically natural product synthetic pathway refactoring.
for yield improvement.
4.2.1. Module optimization
In the NP biosynthetic process, insufficient enzymatic activity of catalytic or synthetic modules could result in the accumulation of corresponding intermediates or low yield of targeted NPs (Xue et al., 2013, Zhou et al., 2011). The abnormal accumulation of intermediates or low transcriptional and translational levels of proteins can be readily detected by omics analysis. Consequently, the catalytic capability of targeted synthetic modules could be enhanced by the use of synthetic promoters (Bai et al., 2015). Moreover, as mentioned above, the synthetic module also could be reconstituted in vitro for further precise engineering using well-characterized synthetic promoters (Tan et al., 2016).
4.2.2. Pathway optimization/edition
By analyzing the transcription, translation, and corresponding intermediates of each gene and protein, the rate-limiting gene(s) or protein(s) involved in the NP biosynthetic pathway could be identified. The following engineering work, such as gene deletion/overexpression, protein evolution, and heterologous gene substitution, could be carried out to identify the rate-liming steps under the guidance of omics information. In addition, the promiscuity of enzymes and the wide substrate tolerance of enzymes often lead to the production of various types of undesired by-products and analogs. By metabolomics analysis, the structures of these components and analogs can be qualified and analyzed. Then, the enzymes and assemblages (e.g., PKS) could be optimized or re-edited according to the metabolomic results.
4.2.3. Genetic circuit design and application
The cells of microorganism are delicate and an abrupt increase (or decrease) in the concentration of regulators by arbitrary overexpression (or inactivation) presumably disrupts the ordered sequence of activation and inhibition of an anomalous sequence of metabolic events (Tan et al., 2015). Most often, intracellular processes should be flexibly and precisely controlled for NP biosynthesis. As is known, various types of precursors are not only used for secondary metabolite biosynthesis, but also biomass production. In this situation, the metabolic switch often plays a critical role in the biosynthetic efficiency of NPs. For example, the naturally existing transcriptional regulator FapR was rewired to dynamically control expression of genes involved in the supply and consumption of malonyl-CoA. Implementation of this metabolic switch resulted in an oscillatory malonyl-CoA pattern and balanced metabolism between cell growth and product formation, yielding 15.7- and 2.1-fold improvement in fatty acid titer (Xu et al., 2014). In addition, Zhang et al. also increased the yield of a biofuel using a FA/acyl-CoA biosensor system that could dynamically sense and adjust pathway expression based on the presence of key intermediate metabolites (Zhang et al., 2012). In Actinobacteria, under the guidance of omics analysis, especially metabolomics, appropriate genetic circuits could be designed for flexibly and precisely controlled NP biosynthesis using various types of sensors to evaluate cell populations and concentrations of specific metabolites.
4.2.4. Host improvement
Information of the precursor supply, cofactor level, redox potential, and energy metabolism in the host could facilitate systematic omics analysis. Therefore, under the guidance of omics analysis, both the synthetic pathway and host could be systematically optimized for NP biosynthesis. It also should be noted that, if necessary, the synthetic pathway of NPs could be optimized repeatedly by using this improved strain to achieve higher yields in industrial applications.
5. Conclusions
Synthetic pathway refactoring provides great potential and possibilities for flexibly and precisely controlled NP overproduction and structural diversification. Compared to model organisms, such as E. coli and S. cerevisiae, in addition to the traditionally metabolic strategies, new emerging approaches for NP synthetic pathway refactoring remain limited in Actinobacteria. However, it should be noted that with the rapid emergence of technologies and tools for recognition and manipulation of complicated living systems, the connotation of synthetic pathway refactoring in Actinobacteria could be gradually extended. At present, with the rapid development of metabolic engineering, various types of Actinobacteria-derived synthetic biological elements, especially sensors, regulators, synthetic Streptomyces promoters, and well-characterized signal cascade systems, could be mined and characterized for genetic circuit design. Under the guidance of in vitro reconstitution and omics analysis, the NP synthetic pathway could be precisely rewired by using these genetic circuits. In addition, other new tools, such as highly efficient biosynthetic platform/modules/enzymes, plug-and-play synthetic scaffolds, as well as an optimized-chassis host, have also been employed for high-efficiency NP biosynthesis. Hence, these approaches and perspectives are expected help to boost an upsurge in pharmaceutical innovation using Actinobacteria.
Acknowledgement
This study was supported by National 973 Program of China (No. 2012CB721001), the Open Project of State Key Laboratory of Microbial Metabolism funded this work (No. MMLKF15-13), and the Young Talents Program of National High-level Personnel of Special Support Program (The “Ten Thousand Talent Program”) to Tiangang Liu. The authors also specially acknowledge the support from the National Natural Science Foundation of China (No. 31500072, 31670090), the Natural Science Foundation of HuBei Province (No. 2015CFB415), and China Postdoctoral Science Foundation Grant (No. 2014M562052).