1. Introduction
There is a wide variety of biocompatible materials used for different orthopedic applications. Among them, metallic biomaterials have been specifically utilized to produce an assortment of orthopedic implants such as the head of the femoral component of a total hip joint replacement (THJR), the tibial tray in a total knee joint replacement (TKJR), the stem of the total shoulder joint replacement, and the spinal fusion cage [1], [2]. Biodegradable metals are a class of biodegradable materials that degrade slowly in the body with a suitable host reaction elicited by the released degradation products. The biodegradable metal should disappear entirely upon fulfilling the mission of supporting the tissue regeneration leaving no implant residues; hence, no further surgical operation is needed for the implant removal [3].
Appropriate biocompatibility, low in vivo corrosion rate and high strength are the main parameters for the selection of a metal for the mentioned applications [4]. The principal issue of metallic implants is their loosening due to bone resorption caused by stress shielding, weak interfacial bonding between the implant and the bone, and the lack of biological anchorage for the growing tissue [2]. To address these issues, there has been much effort on the development and characterization of metal implants with microstructures and properties close to those of trabecular bone. Accordingly, these materials are called open-cell porous metals, metallic foams, metallic scaffolds, or cellular metals with three-dimensional interconnected pores. The pore sizes are typically between 200 and 500 μm with total porosity of 50–75% [5].
The stability of an implant is mainly dependent on both its mechanical strength and its fixation ability to the host tissue. In the conventional approach, such stability was mostly achieved by means of pins, screws and bone cements [6]. However, in recent experiments, researchers are attempting to improve the fixation by tissue engineering techniques [7]. For this purpose, the metal is made porous to provide a scaffold for the bone tissue to grow into and through the pores thus making a suitable bond to the metallic implant [8]. Present tissue engineering approaches are focused on the development of porous scaffolds made of different biomaterials with the aim of replacing and restoring the pathologically altered tissues by transplantation of the cells [9], [10]. Such scaffolds for engineering of hard tissues need suitable mechanical integrity [11]. A porous 3D material provides the required pathways for cells to grow, proliferate, and differentiate, while the architecture defines the final structure of the newly formed bone [12], [13], [14], [15]. It is worth mentioning that the spontaneous renewal of the bone is restricted to only small defects. The treatment of large bone segments caused by tumors, trauma, implant failure, or osteitis is far more challenging to be addressed. An ideal bone tissue engineering scaffold must be biocompatible, osteoconductive, and biodegradable, with a high mechanical strength to fulfil the necessary load-bearing functions [8]. Also, it must have interconnected porous networks allowing cell migration, vascularization and nutrient delivery [16]. The challenge to achieve the above mentioned properties in existent scaffolds has made bone tissue engineering a very popular research field in the last decade in regards to the material selections and production techniques [13], [17]. Although porosity is necessary for scaffolds, it considerably reduces the scaffold's strength which is vital, in particular, for the repair of large bone defects [18], [19]. The trade-off between the mechanical strength and the porosity is one of the main challenges in designing tissue engineered bone scaffolds [6]. At present, various types of biomaterials have been employed for this aim, which are synthetic or naturally-derived.
2. Advantages and disadvantages of porous magnesium-based scaffold compared to other scaffolds
Early 1970s was the starting date for investigations on porous biomaterials such as ceramic [20], polymeric [21], and metallic materials [22], which identified several promising selections for porous implants that would allow bone in-growth. Although ceramics have shown outstanding corrosion properties, their inherent brittleness makes them difficult to be used for load bearing applications [16]. Porous polymeric scaffolds cannot resist the high mechanical stresses present in bone replacement operations [16]. This has led researchers to study porous metals, based on orthopedic metallic implants, due to their greater compressive strength, fracture toughness, and fatigue resistance, which are needed for load-bearing applications [6], [16]. Due to the excellent physical and mechanical properties of magnesium compared to other permanent (non-degradable) metals, porous magnesium and Mg alloys became good candidates to develop biodegradable scaffolds for bone treatments [23], [24].
Among the metal implants, Mg and a number of its alloys are effective because of 1) their mechanical properties, which are close to those of human bone, 2) their natural ionic content that has important functional roles in physiological systems, and 3) their in vivo biodegradation characteristics in body fluids. Some physical properties of Mg alloys, such as high specific strength and elastic modulus, are closer to those of natural human bone compared to other traditional metal implants [27], [28]. For example, compared to titanium alloyswith the elastic modulus of 110–117 GPa, Mg alloys have lower modulus (41–45 GPa) leading to a decreased stress-shielding effect [29], [30]. Moreover, Mg alloys are 3–16 times stronger than biopolymers and, at the same time, they are more ductile compared to bioceramics, which can reduce the chance of the device fracturing throughout the implantation process. Moreover, compared to polymers, Mg alloys can encourage bone growth, which can help the implant to be properly fixed with the host bone to potentially allow full healing of bone defects after degradation [31], [32]. Mg is an essential ion in metabolism and its deficiency in the body may cause some pathological conditions. Due to such collective properties, Mg based alloys can be employed as biocompatible, bioactive, and biodegradable scaffolds for load-bearing biomedical applications [25], [26]. Such characteristics encourage employing biodegradable Mg and Mg alloys as lightweight metals for load bearing orthopedic implants, which would remain in the body and keep mechanical stability over a time scale of 3–4 months, while the natural bone tissue heals [33], [34].
The main challenge for employing Mg and its alloys as an implant is its high corrosion rate resulting in the rapid release of degradation products in the body [35], [36]. The low corrosion resistance in physiological environments can have an adverse effect on the mechanical stability of the implant prior to bone healing [37]. Mg's low corrosion resistance also leads to the rapid release of hydrogen gas and the formation of subcutaneous gas pockets close to the implant. These side products would collect around the implant and interrupt the tissue healing [38], [39]. In addition, due to the hydrogen release, a local alkalization occurs around the implant, which affects the pH-dependent physiological processes in the surrounding area of the implant [17]. The need for a relatively low corrosion resistance, makes it necessary to take relevant measures to control Mg's high corrosion rate [40]. This review aims to bring together recent studies on Mg and Mg alloys based scaffolds with regards to production techniques, surface modifications, general properties, potential applications and future trends. If Mg based scaffolds with the proper mechanical and corrosion properties can be successfully applied in bone defect treatments, a second operation process for the implant removal will be avoided; which in turn will aid bone healing, minimize the trauma to the patients, and decrease medical costs [41].
3. Design and production methods
3.1. Titanium wire space holder
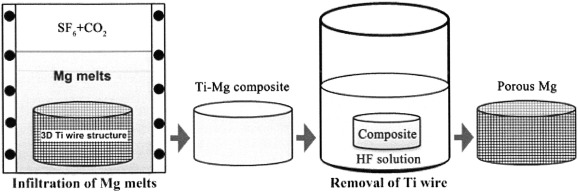
A novel technique for the production of magnesium scaffolds using titanium wires as space holder has been recently developed by Jiang et al. [42]. This technique includes three steps: (a) An entangled three-dimensional shape is made of titanium wire [43], (b) the prepared titanium pattern is utilized as a space holder to generate a titanium/magnesium hybrid system by infiltrating the magnesium cast, and (c) the titanium space holder is dissolved from the hybrid system by hydrofluoric acid (HF) etching at room temperature [42]. With this approach, it is feasible to formulate porous magnesium scaffolds with adjustable pore size and structure [42]. Fig. 1 schematically demonstrates the abovementioned stages [42]. Similar to the shape and dimension of titanium wires, the pores in the magnesium scaffold consist of channels with pipe-like structures with diameter of approximately 270 μm. The results of mechanical tests has shown that the produced magnesium scaffolds with porosities of 54.2%, 51% and 43.2% had elastic modulus of 0.5, 0.6 and 1.0 GPa, and compressive yield strengths of 4.3, 4.6 and 6.2 MPa, respectively. Such results are comparable to the mechanical properties of natural bone, and thus offer potential applications in the treatment of bone defects [42].
 Fig. 1. Schematic design of the titanium wire space holder method for the production of porous magnesium scaffold (reproduced with permission of [42]).
Fig. 1. Schematic design of the titanium wire space holder method for the production of porous magnesium scaffold (reproduced with permission of [42]).3.2. Negative salt pattern molding
Kirkland et al. [44] have developed a new multistep technique with the aim of production of an ordered open cell porous Mg scaffold with controllable porosity and microstructure by a rapid prototyping and casting method. For this purpose, as a supersaturated paste, salt particles were made with high purity (99.5%). Computer-aided design (CAD) models were then engineered as a positive pattern by 3D printing of an acrylic polymer infiltrated in a specific salt slurry. Entire elimination of the polymer by combustion in air using a burnout cycle led to formation of a negative salt pattern which was then infiltrated with melted Mg. Following Mg solidification, the salt was dissolved by washing in NaOH solution. Similar to the macroscopic shapes of the CAD models, a porous Mg scaffold with ordered pores was formed after removal of the salt template by solvent washing. A schematic picture of the entire process is illustrated in Fig. 2 [44]. This research indicates that successful manufacture of topologically ordered porous salt patterns facilitated the negative replication of the ordered three-dimensional configuration utilizing pure Mg [44].
 Fig. 2. Schematic picture of the negative salt pattern molding process: (a) polymeric template produced by rapid prototyping, (b) infiltration of produced porous polymer with salt paste, (c) Burning the polymer template and sintering of salt template, (d) casting of Mg melt into the produced salt template, and (e) final porous Mg scaffold following salt removal (reproduced with permission of [44]).
Fig. 2. Schematic picture of the negative salt pattern molding process: (a) polymeric template produced by rapid prototyping, (b) infiltration of produced porous polymer with salt paste, (c) Burning the polymer template and sintering of salt template, (d) casting of Mg melt into the produced salt template, and (e) final porous Mg scaffold following salt removal (reproduced with permission of [44]).An in vivo study was also carried out on open porous Mg scaffolds which had been produced by a negative salt pattern molding process on biodegradable AZ91D alloy [45]. In this study, a negative template was prepared by pouring the moistened NaCl particles into a core box. The salt template was then infiltrated by Mg melt in a die casting system and the salt particles were then washed out by the NaOH solution. This technique resulted in a scaffold with 72–76% porosity and a pore size distribution of 10–1000 μm [45]. The produced Mg scaffold was implanted into the right knee and an autologous bone graft from the left patellar groove was implanted into the left knee as a control group. Mg scaffolds were significantly corroded after 3 months, and most of the implanted Mg alloy was degraded. These findings confirm that even rapid corroding Mg scaffold has reliable biocompatibility with a desirable inflammatory host response in vivo [45].
3.3. Powder metallurgy
Porous Mg scaffolds can be prepared using a powder metallurgy process by mixing the Mg powder and space holder agents. Normally, a two-step heat treatment is required in order to burn out the space holder agents as well as a sintering process on the pressed powder. Seyedraoufi et al. [46] produced a Mg-Zn alloy scaffold by blending and pressing Mg, 4 wt% Zn and 6 wt% Zn powders with carbamide (CO(NH2)2, 15%, 25% and 35% volume contents) with the particle size of 200–400 μm. The blended powders were pressed at 100 MPa pressure followed by a two-step heat treatment. The first step was adjusted to remove the carbamide particles by heating up to 250 °C for 4 h. The second step involved the sintering process, by heating up to 500, 550, 565 and 580 °C for 2 h. This study found a relationship between the mechanical properties and the porosity content. The compressive strength and Young's modulus of the Mg-Zn scaffolds decreased with increment in porosities at all sintering temperatures. Moreover, the temperature of 550 °C was introduced as the optimum condition for the sintering process since the highest compressive strength and Young's modulus well obtained at this temperature. The results of this study confirmed that the produced Mg–Zn scaffolds with 21–36% porosity could have superior mechanical properties comparable with that of cancellous bone [46].
An example of an open porous magnesium scaffold with interconnected pore microstructure produced by powder metallurgy for orthopedic applications is shown in Fig. 3, reproduced from the work of Yazdimamaghani et al. [47].
 Fig. 3. The cross section of Magnesium scaffold produced by powder metallurgy(reproduced with permission of [24]).
Fig. 3. The cross section of Magnesium scaffold produced by powder metallurgy(reproduced with permission of [24]).The main disadvantage of Mg alloys is their low corrosion resistance. The presence of porosity adversely affects the corrosion resistance by enhancing the surface area exposed to the corrosive media [48]. In order to control the degradability of Mg scaffolds produced by powder metallurgy method, a multilayer polymeric matrix composed of polycaprolactone (PCL) and gelatin (Gel) reinforced with bioactive glass (BaG) particles has been coated on the surface of Mg scaffolds by freeze drying process [49]. In this study, Mg powder was mixed with carbonate hydrogen ammonium particles as the space holder agent with volume content of 35% and particle size of approximately 150–300 μm. The mixed powders were then pressed at a pressure of 400 MPa. Similar to other powder metallurgy techniques for production of Mg scaffolds, the burning out process was carried out at 175 °C followed by a 600 °C heat treatment for sintering. When the samples were immersed in a simulated body fluid for bioactivity tests, the Mg scaffold coated with PCL-BaG presented the best conditions for the deposition of biominerals. Both uncoated and Mg scaffold coated with the PCL-BaG entirely corroded after 3 and 7 days. However, approximately 87% of Mg scaffold coated with multilayer PCL-BaG/Gel-BaG coating remained intact after 14 days of immersion in A simulated body fluid (SBF) [49].
3.4. Hydrogen injection
In hydrogen injection method, Mg melt is poured in a crucible under vacuum, and high pressure hydrogen is introduced into the chamber [50]. As demonstrated in Fig. 4, the melt is superheated in anticipation that the dissolved hydrogen reaches its saturation, and then it is solidified into a water-cooled copper mould. Accordingly, the melt is solidified upwards uni-directionally and straight pores are formed by supersaturated hydrogen during solidification. Changing the cooling part position would change the direction of pore growth [50]. Biological characterization on this type of Mg scaffolds by Gu et al. [51] indicated an acceptable corrosion rate and biocompatibility. However, there are some concerns about the absence of interconnected pores in the scaffolds made by this method of production.
 Fig. 4. Schematic representation of the hydrogen injection technique through the Mg melt (reprinted with permission of [50]).
Fig. 4. Schematic representation of the hydrogen injection technique through the Mg melt (reprinted with permission of [50]).3.5. Laser perforation
Laser perforation technique has been used to produce designed porous Mg scaffolds [11] since Mg has been known as a machinable metal. Porous Mg scaffolds can be developed by laser perforation system by means of a programmable multifunctional laser processing machine on the casted Mg ingots. The advantage of this method is the simplicity to control the structure, size and shape of pores. Normally, interconnected and homogeneous pores with round and honeycomb-like structure can be formed by this technique [11]. Geng et al. [11] produced a Mg scaffold with interconnected pores and honeycomb-like configuration. The optimum parameters in this study were the pulse frequency of 1–10 Hz, width of 0.3 ms, and the effective output of 100 W. Aligned long cylindrical pores with diameter of about 0.5 mm were manufactured within the Mg scaffold. The results confirmed a suitable behavior in mechanical properties due to the fewer flaws and voids resulting from the control of the fabrication technique [11]. Table 1 summarizes the techniques for producing porous Mg-based scaffolds.
Table 1. Summary of the Mg scaffold production methods, their advantages and disadvantages.
| Production methods | Advantages | Disadvantages | References |
|---|---|---|---|
| Titanium wire space holder |
|
|
[42] |
| Negative salt pattern molding |
|
|
[44], [46] |
| Powder metallurgy |
|
|
[47], [48], [49], [50] |
| Hydrogen injection |
|
|
[51], [52] |
| Laser perforation |
|
|
[27] |
4. Biomedical coatings on magnesium scaffolds
As aforementioned above, the main disadvantage of magnesium and magnesium alloy based scaffolds is their high corrosion rate, due to the high electrochemical activity of magnesium metal. Corrosion of magnesium alloy is rapid and inhomogeneous, as it can be localized corrosion. The formation of hydrogen gas cavity due to the faster corrosion rate in comparison to the absorption rate is often observed in Mg scaffolds. In addition, an alkaline pH shift adjacent to the magnesium implant would result in damage to the host tissue. This has led to limiting the potential medical application of magnesium as implant, stents, and bone screws. Corrosion and degradation can drastically reduce the ultimate strength and fatigue life of implants. Increasing the surface area by introducing porosity generally increases the corrosion rates and accelerates the mechanical failure of the implant. To address the long-term safety of porous scaffolds, alloying and surface modification of magnesium scaffolds can be useful approaches. Altering the microstructure and composition by alloying, and surface modification of the scaffolds prior to the implantation, are conducted by a variety of materials and methods [16], [52], [53]. Surface modifying the scaffolds increases the corrosion resistance. The surface modification can be applied by two different processing techniques of conversion coatings or deposited coatings [27]. Conversion coatings are in situchemical or electrochemical interactions of the magnesium scaffolds with the environment which leads to the formation of an inorganic ceramic-like coating on the surface. Deposited coatings are mostly organic coatings applied by different methods such as spraying, painting, spin coating, dip coating, or immersion. Materials used for coating should have specific characteristics such as good corrosion protection, biocompatibility, osseointegration, bioactivity, and controlled biodegradability. Hornberger et al. have comprehensively reviewed the different coating methods [27].
Calcium phosphates (CaPs) with similar composition of the bone mineral component possess several beneficial properties such as excellent bioactivity and osteoconductivity for bone-graft applications [54]. Promoting new bone formation and developing strong interfacial bonding between CaPs biomaterials and the host bone tissue makes this material an appropriate choice for surface modification of magnesium scaffolds.
Hydroxyapatite [Ca10(PO4)6(OH)2, HA], one of different phases of the calcium phosphates (CaPs), has been investigated extensively. Surface modification by hydroxyapatite coating is an effective method for enhancing the corrosion resistance of magnesium scaffolds. Although hydroxyapatite has exceptional biocompatibility, bioactivity properties, and similar chemical and structural nature as bone, it suffers from high brittleness and low strength which limits its application in load bearing conditions [55]. Therefore, utilizing biodegradable magnesium scaffolds with appropriate mechanics, coated with hydroxyapatite to enhance corrosion resistance, and to enhance bone forming ability can be a promising scaffold for bone tissue engineering in load bearing applications [56]. Different techniques such as sputtering process, electrophoretic deposition, sol–gel process, and biomimetic deposits can be used to coat metallic surfaces by hydroxyapatite. However, due to the poor heat resistance of magnesium, none of these techniques can be used efficiently for hydroxyapatite coating of magnesium scaffolds since a high temperature treatment will be required to densify the coating [56]. Therefore, one of the promising options to surface modify magnesium scaffolds is using electrochemical deposition which includes low deposition temperature and is capable of creating uniform coatings with controlled thickness and chemical composition [56], [57], [58].
Hydroxyapatite crystal structure size and morphology can also be manipulated by electrolyte concentration and electrochemical potential. Recently, researchers found that the morphology of hydroxyapatite coating can affect its osteoconductivity and biocompatibility. Healing process of bone defects can be accelerated by loose hydroxyapatite coating which allows easier infiltration and ingrowth of new bone tissue. [57], [58], [59].
One of the main concerns of using magnesium scaffolds is the amount of Mg ions and hydrogen gas released by its corrosion. Incorporating Zn element in contents of less than 3% can improve the corrosion resistance [60] and the mechanical properties of magnesium scaffolds [56], [61]. Seyedraoufi et al. [59]investigated and optimized different parameters of pulse electrodeposition such as peak current density, temperature, and duty cycle to surface modify porous Mg-2 wt% Zn scaffolds with nano hydroxyapatite (Ca10(PO4)6(OH)2) coating upon post-treatment in alkali solutions. Porous Mg-2Zn (wt%) scaffolds were prepared using a powder metallurgy process. Under optimized pulse electrodeposition of 1 h, 40 mA cm− 2 peak current density, 85 °C temperature, and 0.1 duty cycle, followed by post-treatment in alkali solutions, uniform nano hydroxyapatite coatings were generated on the scaffolds. As shown in Fig. 5, perpendicularly needle-like hydroxyapatite crystals with 2–3 μm length with less than 100 nm in diameter were created on the surface of the scaffolds. Loose needle-like hydroxyapatite crystals highly resemble the inorganic apatitestructure of natural bone [59].
 Fig. 5. SEM micrographs in the different magnifications of perpendicularly needle-like hydroxyapatite (reprinted with permission of [59]).
Fig. 5. SEM micrographs in the different magnifications of perpendicularly needle-like hydroxyapatite (reprinted with permission of [59]).Tricalcium phosphate [Ca3 (PO4)2, TCP] as coating material exhibits desirable biocompatibility and bioactivity. However, TCP shows poor mechanical strength and crack-growth propagation resistance. In contrary to HA which has poor rate of biodegradability, TCP is biodegradable. TCP has three polymorphs and among them, β-TCP with hexagonal crystal structure and R3CH space group has a slow-degrading rate [62]. In addition, β-TCP shows desired osteoimmunomodulatory properties which activates the immune response leading to accelerated bone healing process. Favorable osteoimmunomodulatory properties adjust the balance between osteogenesis and osteoclastogenesis which improve bone regeneration. Chen et al. prepared [63] magnesium scaffolds with the laser perforation technique and coated them with β-TCP, in an attempt to exploit the advantages of β-TCP coating such as osteoconductivity, biocompatibility, high chemical stability, slower degradation rate compared to magnesium, and osteoimmunomodulatory [63]. In this study, the importance of β-TCP coating in immune cells and bone cells response was emphasized, and the role of immune cells in adjusting osteogenesis and osteoclastogenesis balance was investigated. Osteoimmunomodulatory property of β-TCP coating demonstrated the activated M2 phenotype of macrophage and release of bone morphogenetic protein 2 (BMP2). Subsequently, osteogenic differentiation of bone marrow stromal cells was observed due to the presence of BMP2 [63], [64]. Activated macrophages secrete BMP2 and transforming growth factor b (TGF-b). It has been shown that the discharging macrophages result in the inhibition of in vivobone formation [65], [66], [67], [68]. Thereby, magnesium scaffolds coated with β-TCP showed desired osteogenesis over osteoclastogenesis, due to the upregulation of osteoinductive molecules secreted by macrophages in contact with β-TCP coating [63].
In a similar study, Geng et al. manufactured [69] interconnected porous magnesium scaffolds utilizing laser perforation technique and coated the samples with β-TCP [69]. In vitro biodegradation mechanism of surface modified scaffolds was evaluated and the osteosarcoma cell (UMR106) attachment and proliferation were studied. Fig. 6a shows a macrograph of a fabricated porous magnesium scaffold with cylindrical aligned pores with 48% porosity before surface modification. Fig. 6b is a SEM image of the β-TCP coated scaffolds obtained by chemical process of immersion in the mixture of Ca(NO3)2and Na2HPO4·12H2O after alkali-heat pretreatment.
 Fig. 6. (a) Porous magnesium scaffold prepared by laser perforation technique before coating. (b) SEM micrograph of the β-TCP coated scaffolds (reproduced with permission of [69]).
Fig. 6. (a) Porous magnesium scaffold prepared by laser perforation technique before coating. (b) SEM micrograph of the β-TCP coated scaffolds (reproduced with permission of [69]).Comparing the pH change of uncoated magnesium scaffold with coated scaffolds incubated in cell culture medium indicates that the pH of the β-TCP coated scaffolds was similar to the pH of the control group, while the uncoated scaffolds have significantly increased pH value. This result confirms the barrier property of β-TCP which increases the corrosion resistance of the scaffold and decreases the magnesium ion release, and control the pH of the medium [69]. In addition, β-TCP coating acts as reservoir of calcium and phosphate ions around the scaffolds and triggers the bone growth. It has been reported that the local supersaturation of the body fluid with calcium and phosphate ions enhances the growth of bone [70], [71].
Degradation of β-TCP coating on the magnesium scaffold is coincident with the regrowth of new calcium and phosphate coating on the surface. This effect occurs by two simultaneous mechanisms namely increased nucleation and increase of pH values. First, degradation of β-TCP increase the magnesium ions release, which in turn leads to an elevated calcium concentration that is desired for nucleation and growth of the Ca-P compound on the magnesium scaffold [69], [72], [73], [74]. Concurrently, enhanced ionic activity of apatite at higher pH, due to the degradation of magnesium, can increase the apatite nucleation and formation of new Ca-P coating [75].
In an attempt to decrease the degradation rate and to increase the possible application potential of magnesium scaffolds in the field of tissue engineering, further biomedical coating studies have investigated various biodegradable materials such as polycaprolactone (PCL) [47], [76], polycaprolactone-bioactive glass (PCL-BaG) [40], and gelatin-bioactive glass/polycaprolactone-bioactive glass (Gel-BaG/PCL-BaG) coatings [49]. The results showed drastic enhanced corrosion resistance and compressive strength during the immersion in physiological saline solution (PSS). The open porous magnesium scaffolds were manufactured by a powder metallurgy technique. While the uncoated scaffolds degraded completely and lost 100% weight after 72 h, coated scaffolds with 3% w/v and 6% w/v PCL showed 36% and 23% weight loss, respectively. In addition, 41% and 83% improvement in compressive strength, and 24% and 100% enhancement in the elastic modulus have been observed, respectively, in 3% w/v and 6% w/v PCL coated magnesium scaffolds [47], [76]. In addition, incorporating bioactive glass (BaG) into the coating layer led to improved bioactivity and mechanical integrity compared to the uncoated scaffold. Formation of an apatite layer on surface modified scaffolds was observed after immersion in SBF, which should stimulate biological bone ingrowth and prevent micro-cracks and pore channels propagation [40]. In the next step, utilizing a freeze drying process, gelatin (Gel)-bioactive glass (BaG) porous layer was added on top of the PCL-BaG layer of the porous magnesium scaffolds. As shown in the SEM images of Fig. 7, porous structure of magnesium scaffolds, PCL-BaG inner layer coating, and Gel-BaG outer layer are distinguishable from each other. While the Mg scaffold/PCL-BaG/Gel-BaG coating almost remained intact after 14 days in SBF, the non-coated Mg scaffold was fully degraded after 3 days and Mg scaffold/PCL-BaG was fully degraded after 7 days. In conclusion, the Mg scaffold with PCL-BaG/Gel-BaG coating showed better bioactivity, higher mechanical integrity and corrosion resistance compared to other scaffolds [49].
 Fig. 7. (a,b) low and high SEM magnification of magnesium scaffold. (c,d) top and cross-sectional view of magnesium scaffold coated by PCL-BaG layer. (e, f) cross-sectional view of magnesium scaffold coated with PCL-BaG inner layer and outer layer of Gel-BaG. (g) Inside and (h) top view of coated scaffold (reprinted with permission of [49]).
Fig. 7. (a,b) low and high SEM magnification of magnesium scaffold. (c,d) top and cross-sectional view of magnesium scaffold coated by PCL-BaG layer. (e, f) cross-sectional view of magnesium scaffold coated with PCL-BaG inner layer and outer layer of Gel-BaG. (g) Inside and (h) top view of coated scaffold (reprinted with permission of [49]).5. General properties and evaluations techniques
5.1. Mechanical properties
An appropriate mechanical strength and stability is required for scaffolds being used in musculoskeletal tissue engineering. Among all possible candidates, magnesium alloys attract considerable attention due to their desirable mechanical and biodegradation properties. During the last decades, researchers have shown increasing interest towards magnesium as a potential biodegradable material for bone and cartilage tissue engineering [29], [40]. In terms of mechanical properties, magnesium alloys show better characteristics than biodegradable polymers such as polyglycolic acid (PGA) or polylactic acid(PLA). Furthermore, degradation and biomineralization of bioactive materialson the surface on the magnesium scaffolds promote osteoblastic activity and ingrowth of the new tissue [35], [45].
Cancellous bone is an interconnected porous structure with porosity varying within the range of 30% to 95%. It is well known that inclusion of porosity into the implants to make the scaffolds, can increase the similarity to the bone structure and enhance the ingrowth of new tissue, however, it can also compromise the mechanical properties. Scaffolds must have adequate strength and stiffness to tolerate the physiological loads. The main concern about the use of metallic implants, such as magnesium alloys, is the mismatch of Young's moduli of the implants and the adjacent bone tissue. This inconsistency between mechanical properties results in the inadequate loading of the bone that leads to bone stress shielding, can subsequently cause bone resorption, implant loosening, formation of cracks within the implant, and implant migration [77], [78], [79], [80]. Thus, implants should have appropriate strength and stiffness matching those of bone tissue. By changing the material of the implant, one can achieve optimal elastic moduli close to the bone to prevent stress shielding. When stress shielding is reduced by changing the material, stiffness mismatch can still exist. In order to overcome the stiffness mismatch, researchers have made porous implants (scaffolds). Porous materials have reduced stiffness mismatches and increased the tissue scaffold interaction by bone ingrowth which helps in the fixation of implants in their place [16]. However, it is noteworthy to mention that the scaffold suffers from drastic decreases in fatigue strength.
It has been observed that the production method of porous magnesium can affect the mechanical behavior and the collapsing mechanism of the sample. A short elastic deformation in the initial stages of the stress-strain curves of compressive loading has been observed in specimens produced by the mechanical perforation method [81] and by melt casting [42]. This behavior indicates the bulk deformation of scaffolds under the compression load, however, porous magnesium specimens produced by metal foaming method [82] and powder sintering method [48] show local collapse of pores with shortened elastic deformation. Following the linear elastic deformation ending at the yield strength, normally magnesium samples exhibit a long plateau of constant stress [82].
Changing the pore size, pore shape, porosity, and pore distribution can affect the mechanical properties [48], [83], [84]. Increasing porosity results in the decrease of compression strength, yield strength, and Young's modulus [48], [83], [84]. The mechanical properties of porous magnesium prepared by the green compacts method using carbamide particles as the space holder and under 100 MP uniaxial pressure were studied to demonstrate the dependence of mechanical properties on pore size and porosity [48]. Scaffolds with pore size of 70–400 μm and porosity of 35–55% were prepared. Compressive strength tests showed a decrease in compressive strength and Young's modulus with increase in porosity and pore size. Scaffolds with 35% porosity and pore size of 250 μm demonstrated 1.8 GPa of Young's modulus and 17 MPa peak stress, and scaffolds with pore size of 73 μm and porosity of 45% showed 1.3 GPa of Young's modulus and 16 MPa peak stress. Considering the mechanical properties of cancellous bone with compressive strength and Young's modulus of 0.2–80 MPa and 0.01–2 GPa [85], mechanical properties of porous magnesium fall in the range of cancellous bone which makes this material a suitable option for hard tissue regenerative scaffolds.
5.2. Bio-corrosion behavior
In contrast to inert materials such as PLA, the increase in bone formation and decrease in healing time observed with magnesium implants indicates the potential osteoconductivity and bioactivity of magnesium [29], [35]. The Osteoconductivity of magnesium is induced by the precipitation of calcium phosphate on the surface of the implants in an in vitro environment due to corrosion [86], [87]. Biomimetic calcium phosphate coatings enhance osteoblast response, impart osteoconductive capacity, and reduce the healing time for bone defects. Moreover, enhanced cell attachment and ingrowth due to calcium phosphate precipitation can lead to reduced corrosion rate [29].
Exposing pure magnesium to air will result in the formation of a gray oxide filmof magnesium hydroxide on the surface. Recent studies [31], [34], [49], [88], [89], [90], [91], [92], [93], [94], [95] proposed the formation of magnesium hydroxide and release of hydrogen bubbles in the electrolytic physiological environment upon degradation of magnesium, through the oxidation and reduction reactions which are summarize in the following reactions:
Magnesium hydroxide is not highly soluble in water. The reaction between magnesium hydroxide and chlorine ions from the tissue fluid or any simulated biological fluids leads to production of highly water soluble magnesium chloride. The release of hydroxide ion from magnesium hydroxide upon reaction with chlorine ions results in the local enhancement of the pH value near the host tissue. The presence of calcium and phosphate ions in the tissue fluid triggers the production of MgxCay(PO4)z(OH)n compounds, such as calcium phosphate and/or calcium magnesium phosphate by the interaction between Mg2 +, Ca2 +, and PO43 −. These complex bioactive mineral products form a deposition layer on the surface of magnesium scaffolds and inhibit further corrosion and increase of pH value. It has been shown that degradation, and consequently deposition of the bioactive passive layers containing calcium, promotes osteoblast growth [96], [97].
5.3. Cell/scaffold interactions
For newly designed materials, cytotoxicity tests including extract based assays, direct contact, and indirect contact for investigating the cell-scaffold interaction, are conducted by applying International Organization for Standardization (ISO) standards 10993-5 and 10993:12. Although direct contact cell cytotoxicity methods may introduce additional information, extract based assays are the most common ones [98]. These cytotoxicity testing standards are developed for non-degradable materials, thus applying them on the degradable magnesium scaffolds may not be suitable. Upon immersion of pure magnesium inside the extraction medium, visible gas evolution is detectable. Strong degradation locally increases the pH value which is evident by tuning normally red medium to colorless medium by the deprotonization of the phenol red. Rapid degradation of magnesium scaffolds in aqueous environment and cell culture media produces highly concentrated extracts with magnesium, strong hydrogen production, very high osmolalities and pH-values.
Osmotic shock by this harsh environment can be lethal to the cells in vitro and makes the in vitro cell culture evaluation nearly impossible for magnesium scaffolds with ISO standards. Active transport processes in in vivo environments regulate most of these fluctuations. Therefore, most research groups bypass the in vitro tests for magnesium scaffolds and directly perform in vivo animal tests, which may be problematic from an ethical point of view. In order to address these issues one can use an in vitro bioreactor with dynamic flow system to simulate the human body situation. However, bioreactors are not always available and the need for in vitro experiments for magnesium scaffolds can be addressed by establishment of reliable test systems. Fischer et al. [99] proposed that in vitro cytotoxicity tests for magnesium materials must be done under physiological conditions corresponds to in cell culture medium containing 10% fetal bovine serum. A physiological condition is a CO2 level of 5%, an O2 level of 20%, temperature of 37 °C, and relative humidity of 95%. As proposed, 10 times more extraction medium should be used comparing to the recommended volume by the ISO standards (EN ISO standards 10993:5 and 10993:12 suggest specimen weight to extraction medium to be 0.2 g/mL) to have reliable results for cytotoxicity [99]. Dilution of the pure extracts decreases the osmolality and high magnesium concentration to avoid cell cycle arrest or apoptosis. It is noteworthy to remember that a high dilution may eliminate most toxic ionic content from the extracts and prevent real cytotoxicity evaluation. On the other hand, cytotoxicity studies on biodegradable magnesium scaffolds by the tetrazolium-salt-based assays such as MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) and XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-((phenylamino)carbonyl)-2H-tetrazolium hydroxide) are prone to experimental errors. Corroded magnesium can interact with tetrazolium salts and convert it to formazan, causing misleading results in static in vitro assays. Therefore, using these assays should be conducted with high level of caution. It is suggested [100] to use luminescence-based (BrdU) cytotoxicity assays which do not have any interference with the corroding magnesium.
In vitro studies of biodegradable open porous magnesium scaffolds confirmed the different response of human osteoblast cells (HOB) and fibroblastic cell line (L929) to osmotic and ionic changes. HOB showed better survival in higher osmotic solutions but exhibited lower proliferation rate comparing to L929 cells in high osmotic extracts [101]. This behavior may be explained by the cell-type-dependent proliferation to cell volume. Cell proliferation can be altered by osmotic swelling or shrinkage. Osmotic swollen cells exhibit more cell proliferation compared to osmotic shrunk cells. Osmotic shrunk cells demonstrate inhibited or delayed cell proliferation in hyperosmotic solutions [102].
Zhang et al. Showed extracellular magnesium ions have regulatory effects on intracellular free ionized calcium ([Ca2 +]i) [103]. Magnesium ions can affect the [Ca2 +]i and K+ channels. It was confirmed that [Mg2 +]o regulates the level of [Ca2 +]i and modulate cell shapes and metabolism of cultured vascular smooth muscle cells to control vascular contractile activities. It is known that [Ca2 +]iconcentration can critically change the cell cycle, and K+ and Cl− channels can influence cell proliferation and cell cycle progression [101], [102], [103], [104]. By having these facts in mind and considering the release of magnesium ions upon corrosion of the scaffold, one can correlate the effect of excess magnesium ion inside the extracts and the cell cycle arrest or apoptosis. Selective interaction of magnesium ions with each type of cell culture media extracts can change the behavior of the magnesium ions, resulting in distinct cytotoxicity responses for different cell types. For instance, corrosion of magnesium scaffolds in RPMI-1640 + 10% FBS and DMEM + 10% FBS showed similar degradation rate under the same cell culture conditions. However, significant differences in magnesium ion concentrations in two extracts were observed [101]. Both original cell culture media, namely RPMI-1640 + 10% FBS and DMEM + 10% FBS, have similar magnesium ion concentration and protein content. The difference in magnesium ion concentration between two media can be explained by the higher concentration of l-glutamine amino acids in DMEM, which can create complexes with magnesium [101].