1. Introduction
Improvements in surgical technique and perioperative care have gradually enhanced outcomes of solid organ transplantation. Immunosuppressive handling is crucial for allograft and patient survival. During the early years of transplantation, steroids and azathioprine were the only available agents to manage the host immune response against the graft; currently, several compounds can guide the donor–recipient interaction [1], [2].
Numerous studies have been conducted to identify the most effective and less toxic immunosuppression regimen to protect both the graft and recipient [3], [4], [5]. Unfortunately, few studies have adhered to the five criteria defined by Jadad: randomization, blinding, adequate description of the randomization and blinding procedures, and intention to treat follow-up with mention of all dropouts or withdrawals from the study. This partly explains the ongoing search for an ideal treatment regimen [6]. A detailed literature review covering the period 2001–2021 identified only seven double-blinded, prospective, and randomized controlled trials (RCTs) with 50 or more participants; four failed to afford any relevant conclusions for clinical practice (Table 1 [7], [8], [9], [10], [11], [12], [13], [14]). Despite the initial observations by Starzl [1] regarding graft acceptance from both large animals and humans, multi-agent immunosuppression resulted in the best means to prevent “repudiation of the allograft.” This policy often generates over-immunosuppression, which is responsible for the development of potentially fatal metabolic (40%), cardiovascular (20%), renal (20%), and oncological and infectious complications (10%–20%) in a high proportion of recipients [15], [16]. These side effects explain why long-term outcomes post-transplantation have not significantly improved during the last 20 years and why recipient death with a functioning graft is the most common cause of late graft loss [3], [4].
Table 1. Double-blind, placebo-controlled RCTs in liver transplantation (LT) during the period 2000–2021.
| Reference | IS | Design | No. of pts | Study completion/exclusion criteria | Endpoints | BPAR | CR | HCV evolution/metabolic impact | GS | PS | Composite endpoint |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wiesner et al., 2001 [7] | MMF vs AZA in triple CyA-based IS | MUC and TC | 278 vs 287 | +/–62% at 6 months and +/–55% at 12 months/yes |
BPAR + TBPAR within 6 months; GS + PS at 12 months; HCV evolution |
38.5% vs 47.7%, MMF better, P < 0.025; 31.0% vs 40.0%, graft loss censored, P < 0.060, at 6 months; 30.6% vs 41.1% in HCV pts, P < 0.040 | 3.8% vs 8.2%, MMF better, P < 0.020 | MMF better in HCV-negative pts at 6 months but NS | Similar at 12 months, NS | Similar at 12 months, NS | NA |
| Neuhaus et al., 2002 [8] | Basiliximab vs placebo in triple CyA-based IS | MUC and TC | 188 vs 196 | +/–83%/yes |
BPAR + GS + PS at 6 and 12 months; HCV evolution; composite: BPAR + GS + PS |
Similar; NS | Similar, NS | Basiliximab better in HCV-negative pts but NS | Similar, NS | Similar, NS | Similar at 6 and 12 months, NS |
| Pageaux et al., 2004 [9] | Steroids vs placebo in triple CyA-based IS | MUC France |
90 vs 84 |
+/–75%/yes |
BPAR + TBPAR; GS + PS; HCV evolution; metabolic impact; all at 6 months |
24.1% vs 38.1%, steroids better, P = 0.030 | Similar, NS | Similar, NS; similar, NS; similar, NS | Similar at 6 months, NS | Similar, NS | NA |
| Filipponi et al., 2004 [11] | Steroids vs placebo in triple CyA-and Basiliximab-based IS | MUC Italy | 74 vs 66 | +/–75%/yes |
BPAR; TBPAR; GS + PS; HCV evolution;composite (pts + graft loss + withdrawal); all at 12 months |
Similar; NS | NA | Similar, NS | Similar, NS | Similar, NS |
8.0% vs 15.6%, P = 0.030 |
| Moench et al., 2007 [10] | Steroids vs placebo at 14 d after LT in TAC-based IS | MOC Mainz | 54 vs 56 | +/–64%/no |
TBPAR; CR; GS + PS; metabolic impact; all at 12 months |
Placebo better, P = 0.016 | Similar, NS | NA; LDL cholesterol placebo better at 6 months, P = 0.033; similar at 12 months, NS | Similar, NS | Similar, NS | Similar, NS |
|
Lerut et al., 2008 [12] and Lerut et al., 2014 [13]a |
Steroids vs placebo in TAC-based double IS | MOC Brussels | 78 vs 78 | 100%/no |
BPAR; TBPAR; GS + PS; TAC monotherapy; metabolic/renal impact; all at 3 and 12 months |
Similar; NS; similar at 3 and 12 months; NS | Similar, NS, TBPAR better in steroids group at 3 months, P = 0.040; similar at 12 months, NS | NA; placebo better at 12 months but NS; similar, NS | Placebo better at 12 months, P = 0.03 | Similar, NS | NA |
| Iesari et al., 2018 [14]b | rATG single shot vs no induction in TAC-based monotherapy IS | MOC Brussels | 97 vs 109 | 100%/no |
BPAR; TBPAR; GS + PS; TAC monotherapy; all at 3 and 12 months |
Similar, NS; similar, NS at 3 and 12 months; similar, NS; similar, NS | Similar, NS | NA | Placebo better at 3 and 12 months but NS | Placebo better at 3 and 12 months but NS | NA |
Underlined items represent the primary endpoint of the respective study.
IS: immunosuppression; MMF: mycophenolate mofetil; AZA: azathioprine; CyA: ciclosporin; TAC: tacrolimus; rATG: rabbit-antilymphocyte globulin; MUC: multicentric study; TC: transcontinental study; MOC: monocentric study; pts: patients; BPAR: biopsy-proven acute rejection; TBPAR: treated BPAR; CR: chronic rejection; GS: graft survival; PS: patient survival; HCV: hepatitis C virus; NA: not applicable; NS: not significant; LDL: low-density lipoprotein.
- a
-
Ref. [13] reports the long-term results of TAC monotherapy concept.
- b
-
Placebo-controlled impossible.
Herein, we critically review the definitions of rejection and optimal immunosuppression, and aim to propose a more rational use of immunosuppression in liver transplantation (LT), as well as provide guidance for future clinical research in the field of (liver) transplantation.
2. Historical note on immunosuppression
In the initial liver and kidney transplantation experiences in Denver, immunosuppression essentially comprised the “secret cocktail BW322,” that is, prednisone and azathioprine. Subsequently, locally produced antilymphocyte globulins were administered as steroid-sparing agents [1]. These pioneering series presented unsatisfactory survival rates (approximately 20% in the long term), thereby igniting the search for more robust immunosuppressants. A retrospective analysis of these reports indicated the need for a more sophisticated interpretation of these results. Eighty percent of grafts were lost owing to technical reasons, poor organ preservation, and cardiorespiratorycomplications, with a loss of 20% attributed to immunologic factors. More importantly, several patients reached a tolerogenic state more than 20 years later due to this “light” immunosuppression regimen [17], [18].
The unspecific “steroid–azathioprine” mix of the 1960s was replaced in the 1980s by the calcineurin inhibitor (CNI)-based immunosuppression, followed by regimens based on mechanistic target of rapamycin (mTOR) inhibition, co-stimulation inhibitors, and monoclonal antibodies in the 2000 era [19], [20], [21]. The CNIs cyclosporine and tacrolimus transformed the field, given their selective mechanism of action, resulting in the minimization and tolerogenic immunosuppression. One- and five-year patient and graft survival rates have rapidly leaped to 75%–90% and 60%–70%, respectively [2]. Information from personal communications in the pharmaceutical industry has revealed that more than 500 CNI- and mTOR-inhibitor (mTORi)-based multicenter immunosuppression studies have been performed worldwide, with the objective of reducing the incidence of allograft rejection or spring the renal function. Despite the promising potential of several minimization regimens, quadruple- and triple-drug regimens are routinely used in clinical practice [22]. Following the death of Starzl in 2017, the drive for an immunosuppression minimization regimen and the interest in broader-scale tolerogenic immunosuppression strategies have faded. A select group of researchers performed research on tolerance, as shown by a systematic search of the electronic database Medline–PubMed, covering the period from 2012 to June 2022, using the medical subject headings: clinical studies/trials, tolerance, cell therapy, immunosuppression, and LT. The search identified only 19 papers. Unsurprisingly, the immune tolerance network has decided to prioritize tolerance trials in 2022 (personal information).
Past clinical observations and experiences need to be considered when reassessing definitions of rejection and standard immunosuppression for prophylaxis and treatment.
3. Liver acceptance reconsidered
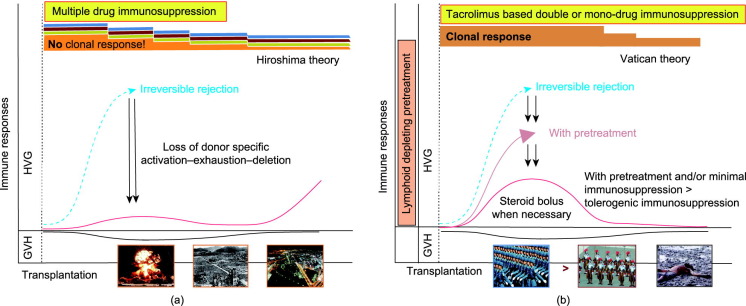
In LT immunology, the first observation was that rejection and tolerance are steps of the same continuum. Consequently, eradicating early acute T-cell-mediated rejection (a-TCMR) against any odds may be counterproductive for long-term graft survival [1], [23], [24]. Second, prolonged organ engraftment during immunosuppression is a sign of partial tolerance. The interaction between donor and recipient immune systems has proven favorable with regard to outcomes [25]. In 1993, the work by Starzl et al. [26] on cell migration and chimerism after solid organ transplantation revealed that graft accommodation and acceptance without long-term immunosuppression were more than a pipe dream. In 1969, Starzl stated: “It is almost certain that the continuous presence of a transplanted organ in a host being treated with immunosuppressive therapy often leads to a selective loss of responsiveness to the antigens of the homograft” and “large doses of immunosuppressants early after transplantation may erode the mechanism of tolerance, and so preclude the goal of minimal dependence on (or independence from) long-term immunosuppression treatment” [1], [26], [27], [28], [29]. Graft acceptance is based on the dynamic interplay between specific clonal activation, deletion, and exhaustion (Fig. 1[29]). Eliminating this interaction halts the route of graft acceptance. Observations from small animals with normal graft function after temporary low-dose tacrolimus administration and clinical experiences with immunosuppression minimization encourage transplant physicians to pursue this strategy toward tolerogenicity [29], [30], [31], [32]. Thirdly, the liver is an immunologically privileged organ. However, this advantage is largely disregarded, as confirmed by the scarcity of investigations on drug minimization and tolerance induction and the lack of integrated documented biological and histopathological long-term follow-up. The quote by Demetris, “the biopsy is the science of transplantation,” must be considered in this context [13], [32], [33], [34], [35], [36], [37], [38]. These three historical observations are essential for the selection process of patients for immunosuppression withdrawal [39].
 Fig. 1. (a) Strong multidrug immunosuppression eliminates the process of donor-specific activation–exhaustion–deletion. Therefore, immunosuppression weaning may lead to delayed (chronic) rejection when withdrawing immunosuppression. This situation is comparable to the aftermath of the atomic bombing. The rare survivors are the strongest opponents of nuclear war (the Hiroshima theory). (b) Low CNI-based immunosuppression favors the donor–recipient interaction, which eventually warrants steroid bolus. Pretreatment may attenuate this interaction by reducing the T and B cell “armies” (the Vatican theory). The ensuing-controlled donor–recipient interaction possibly leads to a subsequent tolerogenic status. The pink and black curved lines indicate the usually observed temporal distribution of graft-versus-host (GVH) and host-versus-graft (HVG) reactions, respectively. Reproduced from Ref. [29], © 2003.
Fig. 1. (a) Strong multidrug immunosuppression eliminates the process of donor-specific activation–exhaustion–deletion. Therefore, immunosuppression weaning may lead to delayed (chronic) rejection when withdrawing immunosuppression. This situation is comparable to the aftermath of the atomic bombing. The rare survivors are the strongest opponents of nuclear war (the Hiroshima theory). (b) Low CNI-based immunosuppression favors the donor–recipient interaction, which eventually warrants steroid bolus. Pretreatment may attenuate this interaction by reducing the T and B cell “armies” (the Vatican theory). The ensuing-controlled donor–recipient interaction possibly leads to a subsequent tolerogenic status. The pink and black curved lines indicate the usually observed temporal distribution of graft-versus-host (GVH) and host-versus-graft (HVG) reactions, respectively. Reproduced from Ref. [29], © 2003.4. Liver rejection reconsidered
A precise definition of rejection is of utmost importance for drawing relevant conclusions from clinical studies examining immunosuppression. The incidence of a-TCMR peaks during the first postoperative week, plunges during the second year (about 5%), and further decreases in the following years (about 2%) [2], [12], [23], [24], [36]. The heterogeneity between reports, the different definitions of rejection, and diverse inclusion and exclusion criteria clarify the broad range of TCMR incidence (median ≈ 40%, ranging from 10% to 80%) [31], [39], [40], [41]. For example, older age, fragility, and hepatitis B virus (HBV)- and alcohol-associated liver disease are associated with reduced immune competence. Better graft quality, particularly from live donation, and administration of depleting antibodies, as induction agents, reduce the risk of immune reactions, while autoimmune liver diseases enhance the risk of adverse immunological events [23], [24], [42], [43], [44], [45], [46], [47].
The clinical course of early a-TCMR is typically benign, as most patients respond to increased doses of CNI and/or high-dose steroids. Late a-TCMR, usually defined as an episode occurring 3–6 months after LT, exposes the recipient to a higher risk of developing chronic rejection (CR) and graft loss [23], [24]. Moreover, the prophylactic and therapeutic use of corticosteroids markedly differs between studies in terms of type, amount, number, route of administration, and duration [48], [49] (Table 2 [14], [21]). Differences in doses or schemes of immunosuppressants can influence the incidence of TCMR and, importantly, corticosteroid-resistant rejection, defined as a rejection that is unresponsive to a given, center-dependant methylprednisolone dose, eventually followed or not by steroid tapering [8], [9], [10], [12], [14], [23], [48], [49]. Collectively, these elements clarify the different interpretations of the efficacy of a given immunosuppression regimen (Table 2).
Table 2. Confounding factors in clinical studies about immunosuppression in (liver) transplantation.
| Confounding factor | Weak study design | Strong study design |
|---|---|---|
| Study design | Multicentre | Uni-/pluri-centre |
| Transcontinental | National or regional | |
| Industry-driven study | Investigator-driven study | |
|
Non-randomiseda Inappropriate method of randomisationa |
Randomised Appropriate method of randomisation |
|
|
Absence of double-blindinga Inappropriate method of blindinga |
Double-blinding Appropriate method of blinding |
|
| Absence of placebo controls | Presence of placebo controls | |
| No information about withdrawals and dropoutsa | Description of withdrawals and dropouts | |
| Prophylactic immunosuppressionb | Induction therapy (delaying rejection)c | No induction therapy |
| Corticosteroid administration | No or short-term (2-to-3-month) corticosteroid administration | |
| Triple- or quadruple-drug therapy | Mono- or bi-drug therapy | |
| No harmonisation of immunosuppressive regimen between study arms | Harmonisation of concomitant immunosuppressive drugs | |
| Donor and recipient selection | (Highly) selected patients | Unselected, consecutive patients |
| Exclusion of auto-immune, HCV-infected, and acute-liver-failure patients | Inclusion of auto-immune, HCV-infected, and acute-liver-failure patients | |
| Exclusion of high-MELD-score patients | Inclusion of all patients, regardless of MELD score | |
| Exclusion of ICU patients | Inclusion of all UNOS categories | |
| Exclusion of patients dependent on organ support (renal replacement therapy, ventilation, etc.) | Inclusion of all patients, regardless of organ support needs | |
| Exclusion of patients with renal failure (exclusion of patients with creatinine > 1.5 mg·dL–1 or creatinine clearance < 40 mL·min−1 per 1.73 m2) | Inclusion of all renal conditions | |
| Exclusion of fragile patients | Inclusion of all patients, regardless of nutritional status | |
| Exclusion of younger and older adult donors and recipients and of extended criteria donors | Inclusion of all categories of age of both recipient and donors | |
| Exclusion of long ischaemia times | Inclusion independent from ischaemia time | |
| Exclusion of EBV negative CMV, HBV, and HCV positive recipients and donors | Inclusion of all patients regardless of viral status | |
| Exclusion of grafts from DCD | Inclusion of all donor types | |
| Definition of rejection | Clinically suspected rejection | BPAR |
| Only per-cause biopsies | Per-protocol and per-cause biopsies | |
| Absence of Banff score or RAI | Banff score or RAI | |
| Absence of immunostaining (C4d, CK19, etc.) | Specialised transplant pathology reading | |
| Counting of all rejection episodes | Rejection episodes within delay of 14 d considered as one single rejection episode | |
| Local biopsy reading | Centralized biopsy reading | |
| Definition of steroid-resistant rejection | No response to steroid pulses | No response to 250–1000 mg methylprednisolone pulses |
| No response to 5.00 g methylprednisolone | — | |
| No response to 3.00 g methylprednisolone | — | |
| No response to 1.00 g methylprednisolone | — | |
| No response to 0.50 g methylprednisolone | — | |
| No response to 0.25 g methylprednisolone | — | |
| Intravenous vs oral pulse | — | |
| Single or two courses | Single course | |
|
Steroid pulse followed by tapering (200–160–120–80–40–20 mg) |
No tapering | |
MELD: the Model for End-Stage Liver Disease; ICU: intensive care unit; UNOS: United Network for Organ Sharing; EBV: Epstein–Barr virus; CMV: cytomegalovirus; DCD: donation after cardiac death; CK19: cytokeratin 19.
- a
-
Five items that correspond to the Jadad scale.
- b
-
Prophylaxis, as opposed to therapy, implies the use of immunosuppressants to prevent, rather than to treat, an immunological event.
- c
-
Induction therapy is considered as a potential confounder in case the primary endpoint of a trial is only BPAR (scored following Banff or rejection activity index (RAI)). Induction is known to reduce tissue inflammatory changes compared to no-induction regimen. When considering both histopathology and clinical evolution (e.g., the necessity to treat), the advantage of induction therapy may disappear [14], [21].
One to five percent of LT recipients can develop CR. The definition of CR is not linked to the time elapsed since LT [12], [13], [24], [39], [50]. The hallmark lesion is the vanishing- or vanished-bile-duct syndrome (VBDS), ushered by the disappearance of bile ducts in 50% of portal tracts in a representative tissue sample containing at least ten portal tracts. This should always be considered in the clinical context owing to its implications in treatment. Biliary complications are observed in approximately 30% of LT recipients, and medications that induce some degree of hepatotoxicity, such as the frequently used amoxicillin-clavulanate, ciprofloxacin, trimethoprim-sulfamethoxazole, and carbamazepine, can mimic the histopathology of CR [51], [52] (Table 3). VBDS can also result in immunosuppression reduction or withdrawal, either undertaken for medical reasons or decided by the recipient. Non-compliance or non-adherence was found to be the highest (up to 14%) among LT recipients [53]. Fortunately, reintroducing the previous immunosuppression level can resolve such “provoked” under-immunosuppression in most cases [29], [32], [54], [55].
Table 3. Drug-induced small bile duct injury after LT.
| Drug | Induced bile duct lesions | |
|---|---|---|
| Acute | Chronicb | |
| Allopurinol | + | (–) |
| Amitriptyline | + | + |
| Amoxicillin-clavulanatea | + | (–) |
| Ampicillin | + | (–) |
| Azathioprine | + | (–) |
| Barbiturates | + | + |
| Carbamazepine | + | + |
| Chlorothiazide | + | + |
| Chlorpromazine | + | + |
| Cimetidine | (–) | + |
| Ciprofloxacin | + | (–) |
| Erythromycin | + | + |
| Fenofibrate | + | (–) |
| Flucloxacilline | (–) | + |
| Glibenclamide | + | (–) |
| Glycyrrhizin | + | + |
| Chlorpromazine | + | + |
| Haloperidol | (–) | + |
| Ibuprofen | (–) | + |
| Imipramine | (–) | + |
| Itraconazole | + | (–) |
| Propafenone | + | (–) |
| Saint John’s Wort | + | + |
| Terbinafine | + | + |
| Ticlopidine | + | (–) |
| Trimethoprim-sulfamethoxazole | (–) | + |
- a
-
Amoxicillin-clavulanate (Augmentin®; GlaxoSmithKline, Belgium) is one of the most prescribed drugs worldwide; ciprofloxacin, erythromycin, itraconazole, carbamazepine, and trimethoprim-sulfamethoxazole are used very frequently after LT.
- b
-
Chronic bile duct injury can mimic chronic allograft rejection.
Severe graft dysfunction, parenchymal necrosis, bile duct destruction in histology, C4d positivity on immunofluorescence, and donor-specific antibodies in plasma measurements help identify antibody-mediated rejection (AMR). Early AMR, also named hyperacute or fulminant rejection, hemorrhagic necrosis or seventh-day syndrome, has been observed under both mild and strong immunosuppression [1], [24], [56], [57], [58]. Compared with other causes of severe allograft dysfunction, including acute rejection, histological examination reveals a markedly high number of apoptotic hepatocytes. After excluding other injuries that cause a similar injury pattern (e.g., hepatic artery thrombosis), this diagnosis can be confirmed by the evidence of donor specific antibodies (DSAs) and tissue complement activation, that is, positive C4d immunohistochemistryon the microvasculature. In ABO-compatible LT, AMR is extremely rare (< 1%), whereas the AMR incidence ranges between 7% and 10% in cases of ABO incompatibility (ABOi). At several Korean and Japanese transplant centers, ABOi LT currently represents up to 30% of all LT. These experiences have largely increased knowledge regarding immune handling in this context [59]. The splenectomy-free approach, which combines preoperative rituximab, a monoclonal anti-CD20 antibody, and multiple plasmapheresis sessions, to reduce natural circulating ABO antibodies, has transformed ABOi living donor LT (LDLT) into a valid opportunity, offering 90% and 80% one- and three-year survival rates, respectively [25].
The wide range in the incidence of TCMR warrants establishing a more precise definition [23], [24], [40]. Thus, four principles must be implemented. First, biopsy is the gold standard to differentiate rejection from other causes of allograft dysfunction and validate rejection treatment [25], [35], [37], [39], [50]. The invasiveness and risk of serious complications have deterred the widespread use of per-protocol biopsies, despite their key role in immunosuppression management decision-making. Studies based on per-protocol biopsies, which have revealed that only one-third of recipients with normal liver tests exhibit normal histological features, represent a guide for adjusting immunosuppression [35], [36], [37]. Given the poor agreement between transplant physicians on clinically suspected rejection, several treatments are still blindly pursued, posing risks for severe complications, frequently caused by subsequent, unnecessary reinforcement of the immunosuppressive burden [60]. These observations largely outweigh the fear of blank biopsy. Several studies have shown that surveillance biopsies can be safely performed. Complications, reported in 0.35%–5.50% of procedures, can often resolve within one week. Bleeding complications can typically be controlled using interventional radiology, and biopsy-related cholangitis often results from underlying, unknown biliary problems [40], [61], [62], [63]. Second, the Banff classification is not only useful for grading rejection but also for comparing the results of different experiences in a manner resembling the role of the tumor-node-metastasis (TNM) classification in oncology [35], [60]. Third, centralized biopsy reading should be promoted, especially in large transcontinental multicenter studies that frequently assemble dozens of centers with distinct transplant expertise [7], [8], [64]. In most immunosuppression trials, biopsy readings by several pathologists lead to a major bias regarding the endpoint. Fourth, biology, intended as the evolution of surrogate plasma analytes, and histopathology should be jointly analyzed before reaching any therapeutic decision. Generally, robust immunosuppression can reduce inflammatory infiltrates and, consequently, the Banff score. In this case, the drug or regimen under assessment could be erroneously interpreted as more effective, despite the absence of an impact on clinical practice. In LT, TCMR in per-protocol biopsies does not necessarily require additional immunosuppression if biological parameters do not confirm clinical impairment [24], [41]. Not every case of severe biopsy-proven acute rejection (BPAR) warrants treatment [12], [14], [23], [24], [65], [66]. Remarkably, episodes of treated acute rejection do not necessarily lead to decreased patient or graft survival. This evidence highlights the tolerogenic potential of a controlled alloreaction that renders the graft less susceptible to further immune attacks [23], [24], [67].
CNIs have turned the traditional stigmata of rejection, including fever, abdominal pain, tenderness, and graft swelling, which are rare and unreliable. Therefore, noninvasive, cost-effective, rapid, reproducible, sensitive, and specific biomarkers of rejection need to be developed. Liver tests, cytokine profiles, inflammatory markers, adenosine triphosphate (ATP) activity, peripheral T-cell clustering, and complex “-omics” signatures have been previously explored [34], [68], [69]. More recently, cell-free biomarkers such as circulating microRNAs (miRNAs) and donor-derived cell-free DNA have also been incorporated into the diagnostic arsenal. These biomarkers might hopefully gain momentum as noninvasive replacements for biopsy for monitoring and predicting allograft rejection [70], [71]. In a trial examining tolerance, Shaked et al. [72] reported that rejection was detected by miRNA measurement up to 40 d prior to clinical manifestations.
Nonetheless, the ideal candidate biomarker remains difficult to identify owing to a marked overlap between rejection and several confounding factors, such as graft steatosis, ischemia-reperfusion injury (IRI), focal or systemic infections, biliary and vascular complications, de novo or recurrent viral infections, and drug-induced liver abnormalities. Cytolytic enzymes (aspartate and alanineaminotransferase) and cholestatic enzymes (γ-glutamyl transferase and alkaline phosphatase) exhibited low accuracy and poor correlation with the severity of rejection. Their dynamics are a surrogate for IRI repair [24]. Progressively increasing serum bilirubin and peripheral eosinophil counts are early a-TCMR markers. Eosinophilia is strongly linked with moderate-to-severe a-TCMR [73], [74], [75], [76]. Platelets have been poorly investigated in a-TCMR, although they play a critical role in liver regeneration and the IRI response [77], [78], [79]. The platelet count usually begins to grow five days after LT, regardless of the selected immunosuppressive regimen. When thrombocytopenia-inducing drugs, such as azathioprine, mTORi, and antivirals, are avoided, hepato–splenic sequestration and immunologically mediated endothelial graft damage explain the known initial postoperative platelet count decrease. Exocytosis, via the release of von Willebrand factor from platelet surfaces, triggers circulating platelet consumption [80]. An increasing platelet count indicates endothelial repair; conversely, endothelial injury further decreases the platelet count. This type of platelet dynamics has been well-documented in AMR and xenotransplantation [25], [59], [81]. Several cytokines are expressed during acute rejection, including interleukin 6 (IL6), which recruits eosinophils. The dynamics of these cytokines fail to clearly distinguish rejection from infection, rendering their clinical use unviable. The same is true for identifying cytochrome polymorphisms and T-cell clusters of differentiation proteins [68].
Since 1995, the team from Université catholique de Louvain, Brussels, has prospectively investigated the correlation between the Banff score on per-protocol or per-cause biopsies and the aforementioned biological markers to objectively define early a-TCMR in LT. The selected biological parameters include increasing serum bilirubin, progressive eosinophilia, decreased platelet count, and an absolute eosinophilia count > 600 cells·μL−1 from days 5 to 7 post-LT [11], [12], [13], [14], [73]. In this model, more than two biological markers plus a Banff score ≥ 6 (indicating moderate-to-severe rejection) pivot the histological picture of TCMR into a clinically relevant depiction. Clinical rejection implies a reinforced immunosuppression load by acting on CNI trough levels or dispensing high-dose steroid boluses. In case of non-responsiveness, anti-lymphocytic sera are the most commonly prescri-bed [12], [23], [24], [65], [82], [83]. This approach has drastically reduced the application of anti-lymphocytic antibodies for corticosteroid-resistant rejection. This “seven-up score” was named after the timing, i.e., postoperative day 7, when a-TCMR occurs most frequently and protocol biopsies are performed. The usefulness of this score was investigated in two prospective, all-inclusive, and investigator-driven RCTs undertaken at the University Hospitals Saint-Luc in Brussels. These studies compared tacrolimus monotherapy plus placebo to tacrolimus plus a short-term, two-month steroid therapy; and tacrolimus monotherapy to tacrolimus plus one single, intraoperative, high-dose polyclonal rabbit-antilymphocyte globulin (rATG) [12], [14]. The second study was not placebo-controlled because rATG requires a mandatory cutaneous test; however, the transplant team was unaware of the intraoperative rATG administration by anesthsiologists (Table 1 [7], [8], [9], [10], [11], [13], [13], [14]). The very strict adherence to both study protocols led to several important conclusions: ① a light tacrolimus-based monotherapy regimen generates comparable early and long-term survival rates to heavier regimens; ② only 10% of moderate-to-severe histological rejections require treatment; ③ corticosteroid-resistant rejection rarely occurs in patients receiving tacrolimus-based immunosuppression; ④ induction of immunosuppression significantly reduces the day 7 Banff score but does not affect the incidence of clinical rejection and, thus, the need for treatment; and finally ⑤ minimal immunosuppression affords renal protection without endangering graft survival [12], [13], [55], [65], [82].
To date, tacrolimus monotherapy has been a successful immunosuppressive regimen in more than 800 recipients. Such findings have shed distinct light on several assumptions drawn from many 21st-century RCTs assessing immunosuppression.
5. Liver standard immunosuppression reconsidered
Standard immunosuppression is considered to achieve a balance between pharmacological side effects and organ and patient survival rates. The annual Scientific Registry of Transplant Recipients reveals that the triple immunosuppression regimen, containing a CNI (mostly tacrolimus), an antimetabolite (mostly mycophenolate or mTORi), and corticosteroids, is the most frequent strategy in LT (approximately two-thirds of recipients). This type of regimen is also markedly common in renal transplantation [84], [85]. In the past decade, the use of induction therapy has been persistently employed; approximately one-third of recipients have been selected for this approach [86]. Induction includes monoclonal anti-IL2-receptor antibodies, polyclonal anti-T lymphocytes, or anti-thymocyte antibodies. Tacrolimus monotherapy remains an immunosuppressive regimen in a minority (10%) of recipients [84]. The optimal trough blood level of tacrolimus in multidrug immunosuppressive regimens is conventionally between 6 and 10 ng·mL−1, whereas many recommendations, regulatory authorities, and pharmaceutical industriessuggest even higher levels during the first weeks [82], [87]. The Consensus on Managing Modifiable Risk in Transplantation (COMMIT) report suggests avoiding underimmunosuppression, that is, tacrolimus levels < 6 ng·mL−1, in the absence of induction or concomitant immunosuppressants. The same report deters immunosuppression minimization [87].
However, triple immunosuppression, as the standard immunosuppressive regimen in LT, has to be disputed for several reasons. First, is corticosteroid truly necessary? If so, for how long? Is mycophenolate superior to azathioprine? Do mTORIs protect renal function and reduce the recurrence rates in cancer recipients? Finally, does induction therapy offer any advantages? Some accumulated evidence may shed light on these questions. First, multidrug immunosuppression regimens do not radically reduce clinically relevant TCMR episodes. Such regimens may be counterproductive even when administered to low-risk transplant candidates [65], [66], [83], [87], [88].
Several drug “cocktails” have been designed to counteract the adverse effects of a given drug used during early and late post-transplant periods. This strategy mainly focuses on avoiding CNI-mediated renal and neurological toxicities. Several combinations have proven beneficial in relation to the endpoint under examination. Unfortunately, chronic immunosuppression still compromises the long-term outcomes of transplant recipients [15], [16], [89]. These disconcerting side effects should be the main drivers for reducing or eliminating the burden of long-term immunosuppression. The first step in this strategy is early withdrawal or complete avoidance of the most detrimental immunosuppressant, i.e., corticosteroids [65], [90]. Padbury et al. (the Birmingham group) [90] were the first to demonstrate that this approach is safe, and this experience has been repeatedly and independently validated [9], [10], [12], [15], [48], [55]. Three systematic reviews examining induction with anti-IL2-receptor and anti-T-lymphocyte antibodies established that induction therapy is not substantially beneficial considering reducing TCMR episodes [21], [91], [92]. Similar evidence is available in relation to mycophenolate against “good old” azathioprine, suggesting that markedly inferior costs favor azathioprine therapy [93]. To date, an RCT performed by Wiesner et al. [7]remains the only available report that compared mycophenolate with azathioprine. The authors showed that BPAR, censored for graft loss, was reduced in the mycophenolate group during the first six postoperative months (p < 0.06). One-year patient and graft survival rates were however similar [7]. Based on these findings, mycophenolate has nevertheless almost universally replaced azathioprine in clinical practice. Nonetheless, these findings should be critically reassessed in light of statistical flaws, such as censoring grafts for causes other than rejection, high (36% and 46% at 6 and 12 months, respectively) withdrawal from the study for different reasons, and lack of competing-risk analysis. Germani et al. [93] revealed a significantly elevated incidence of thrombocytopenia in mycophenolate cohorts. Azathioprine-induced hepatitis and nodular regenerative hyperplasia were not documented [94], [95]. The antagonism between mycophenolate and azathioprine has recently been explored in kidney transplantation, where no difference was detected between the two drugs in terms of rejection [96].
The use of mTORi for renal-sparing approaches and transplant oncology has also been reappraised [97], [98]. Most studies that seek to reduce CNI nephrotoxicity compare standard treatment with either delayed CNI and/or anti-IL2-receptor antagonist-based induction or mTORi [83], [91], [97]. A large multicenter RCT, including 719 recipients with similar baseline estimated glomerular filtration rate (eGFR) at randomization (postoperative day 30), showed that the experimental arm, which received everolimus, low-dose tacrolimus, and corticosteroids, exhibited a significantly better eGFR than the standard-level tacrolimus group (eGFR, 80.9 vs 70.3 mL·min−1 per 1.73 m2 at 12 months, and 78.7 vs 63.5 mL·min−1 per 1.73 m2 at 36 months, respectively, p < 0.001) [97]. However tacrolimus trough levels in the experimental arm were maintained at approximately 6 ng·mL−1, while blood levels were considerably higher in the control group than those currently sought after in clinical practice: 8–12 ng·mL−1 during the first four months, followed by 6–10 ng·mL−1 [82], [97], [99], [100], [101]. Notably, De Simone et al. [97] showed that renal function was significantly better in the third study arm, that is, the group receiving everolimus with tacrolimus elimination. Unfortunately, this arm had to be terminated prematurely owing to the high rate of BPAR. These findings confirmed that early CNI-free immunosuppression should be avoided [97]. A similar observation was reported for mycophenolate. This drug might offer some renal protection, along with several side effects, among which gastrointestinal disturbances and bone marrow suppression remain of particular concern. During monotherapy, this medication is suboptimal because of the high incidence of rejection [102]. Likewise, the Silver study [98], a large multicenter sirolimus-based RCT including 525 recipients, focused on the recurrence of hepatocellular cancer after LT. The sirolimus-based regimen failed to present long-term advantages when compared with sirolimus-free immunosuppression [98]. However, surprisingly, the authors concluded that mTORi-based immunosuppression is beneficial for renal function and tumor recurrence after LT, a strategy that the worldwide transplant community has internalized. In contrast, while low-dose CNI-based regimens and avoidance of unnecessary TCMR treatment diminish tumor recurrence rates, both approaches have rarely been discussed in the literature [103], [104], [105], [106]. The added value of mTORi has emerged in the case of persistant vital tumor tissue in the hepatectomy specimen and in case of tumor recurrence.
6. Liver optimal immunosuppression reconsidered
The most important cause of graft loss is the death of a recipient with a functioning graft. Consequently, assuming that immunosuppression-induced comorbidities are a major cause of fatal events in recipients, an optimal immunosuppression approach should naturally imply minimization. Currently, the standard trough levels for tacrolimus and cyclosporine range from 6 to 10 and 150 to 250 ng·mL−1, respectively [87], [100], [107].
Minimization implies bringing the patient to the lowest possible well-tolerated immunosuppression level [5], [12], [82], [87]. This process mostly starts with optimal CNI levels based on two agents and gradually evolves after 3, 6, or 12 months. These adaptations depend on the experience of the transplant team and the underlying disease of the recipient, directed toward a single-drug regimen. Of note, upfront monotherapy has ben shown to be also safe and effective [12], [29], [48], [54], [55], [99].
Minimization of immunosuppression must include CNIs to avoid severe early a-TCMR episodes [54], [87], [97]. Accumulated literature has revealed that there is no difference in efficacy between standard twice-daily formulations and prolonged-release tacrolimus when used in a monotherapy regimen. In stable recipients, the enhanced bioavailability of prolonged-release formulations offers more consistent exposure and trough levels. However, the use of prolonged-release formulations can be associated with variable absorption and bioavailability during the first “unstable” post-transplant days. This variability increases in cases of initial graft dysfunction, an element of concern when donor selection criteria are progressively extended to cope with graft shortages. Accordingly, during the early post-transplant period, twice-daily tacrolimus could allow easier and more rapid adaptation of plasma trough levels, especially in cases of renal failure [108].
Delayed monotherapy can include a CNI, an antimetabolite, or a mTORi, the final choice depending on the occurrence of nephrotoxicity or neurotoxicity, dysmetabolism, and de novo or recurrent tumor or allograft disease [10], [12], [13], [29], [55], [99], [109].
Infra-therapeutic monotherapy is the next-level option if liver tests remain stable for a prolonged period [28], [29]. This approach has been proven safe and beneficial, as it contributes to a better metabolic profile, renal function, and quality of life when initiated early enough post-transplantation [10], [12], [82], [87], [109]. Several studies that examined the impact of immunosuppression withdrawal on pre-existing complications of long-term immunosuppressive drugs failed to detect a regressive effect. This lack of effect is likely a consequence of overly late initiation of withdrawal. Conversely, early drug weaning leads, most invariably, to drug rejection. Selecting an optimal time for withdrawal needs to consider markedly early and markedly delayed withdrawal [110], [111].
In summary, further progress can be achieved by overcoming several fixed dogmas regarding immunosuppression handling, which remains crucial for the evolution of clinical operational tolerance (COT) (Table 4). Regrettably, this path has been deranged by low-quality literature and the changeable long-term immunosuppression handling of recipients. The continuity of care has never been distinctly analyzed with regard to the decision-making process in immunosuppressive treatments. Growing numbers of long-term surviving recipients, new generations of differently trained transplant physicians and surgeons, the governing of patient care by many subspecialties, and progressive patients’ diaspora clarify this lack of continuity of care. This is well-confirmed by the scarcity of reports examining 10-, 15-, and 20-year follow-ups post-LT. Successful immunosuppression handling implies a continuous, life-long adherence to a uniform scheme (and philosophy) based on reliable and up-to-date literature and, most importantly, on familiarity with recipients: “know your patients” (Table 5). This is the core reason recipients should ideally be followed up by the same transplant team using a centralized patient chart.
Table 4. Ten immunosuppressive dogmas (or beliefs) to reappraise in LT.
| No. | Immunosuppressive dogma |
|---|---|
| 1 | Every episode of moderate-to-severe TCMR (Banff score > 6) requires treatment |
| 2 | Per-protocol liver biopsies are not worthwhile in the early or in the long-term post-LT follow-up |
| 3 | Immunosuppression including steroids is more effective compared to steroid-free regimens |
| 4 | Mycophenolate is more effective than azathioprine |
| 5 | Induction therapy offers relevantly increased protection compared to induction-free immunosuppression |
| 6 | Multidrug anti-rejection prophylaxis is better than one-drug tacrolimus-based immunosuppression |
| 7 | mTORi-based immunosuppression better protects renal function compared to tacrolimus-based minimisation immunosuppression |
| 8 | mTORi-based immunosuppression decreases the risk of recurrence of hepatobiliary cancer after LT |
| 9 | Tacrolimus-based minimisation immunosuppression is dangerous |
| 10 | COT is unrealistic |