1. Introduction
Materials for tissue replacement have progressively evolved overtime. The discovery of bioactive glass (BAG) has played a key role in this evolution. The 1stgeneration of materials for tissue replacement had the requirement to be bio-inert without triggering any host inflammatory response [1]. In 1969 there was a revolution in tissue engineering when Professor Larry Hench introduced bioactive glass (a sodium calcium phosphor-silicate glass) which was the first material that exhibited bone bonding ability with an excellent bonding capacity [2], [3]. Ever since, there was a shift in the paradigm of materials for tissue replacement from being bio-inert to bioactive. A material that could interact beneficially with the host tissue to drive its repair and regeneration became the prime need. From this point there was a growth of bioactive materials (glass, ceramics) including modified bioactive glass, calcium phosphate [4], [5], hydroxyapatite [6], [7], [8] and calcium silicates [9], [10], [11]. In the years following its invention, bioactive glass has been extensively researched and the mechanism behind its high bioactivity has been reported. The two main reasons for its bonding ability are as follows; 1. The ions leached from the glass form carbonated calcium deficient hydroxyl apatite (HCA) that binds with the collagen of the tissue 2. The ions also up regulate genes that encode growth factors and stimulate osteogenic cells to secrete bone matrix [2]. Bioactive glass has therefore been used in many applications such as a putty for bone repair [12], [13], coating on implants (bone and dental) [14], [15], scaffolds for bone regeneration [16], [17], [18] and also for cartilage repair [19].
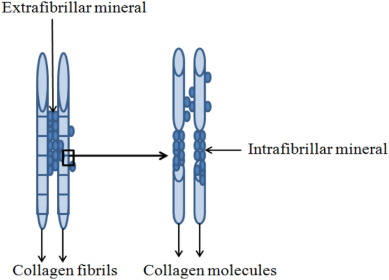
Next to bone regeneration, bioactive glass has found its niche in dentistry. The loss of mineral structures of the tooth enamel and dentin due to caries or erosion is a well-known clinical problem in dentistry [20], [21]. The leaching of ions from bioactive glass and subsequent HCA formation eventually makes it a valuable candidate to be used for remineralization of these dental tissues. Hence, bioactive glasses are now used in tooth pastes [22], [6], [23], air polishing procedures [24] and their ability to treat tooth sensitivity and remineralize enamel has been proven [25], [26], [27]. Dentin remineralization is more demanding than enamel remineralization due to the difference in their composition. As 96% (weight %) of enamel is composed of mineral apatite, the mechanical properties of this tissue is mainly determined by its mineral content. However, the mineral in dentin accounts to only 70% and the remaining 30% is contributed by organic collagen, non-collagenous proteins and water. Furthermore, the design of dentin is such that it is supported by fibril scaffold-form of the collagen in which the mineral apatite is embedded in an extrafibrillar and intrafibrillar manner (Fig. 1). Therefore, the mechanical properties of this tissue not only depend on the overall mineral content but mainly on the intrafibrillar orientation of minerals in the collagen scaffold [28].

Fig. 1. Representation of extrafibrillar and intrafibrillar mineralization in the collagen structure of dentin.
Adapted from [28].Hence, increased mineral content at the tissue after bioactive glass treatment is not enough to remineralize dentin as it is for enamel remineralization. The review of Bertassoni et al. [28] underlines that the improvement in mechanical properties of dentin through intrafibrillar mineralization is crucial to retain the functionality of this tissue. This emphasizes the need to understand the phenomena of remineralization in the context of the tissue it is interfaced with and sufficient characterizations (physico-chemical and mechanical) are of utmost importance to prove the effective remineralization of dentin. Furthermore, remineralization of dentin by bioactive glass can also be complemented by stimulating the odontoblasts or odontoblast-like cells present in the pulp that can secrete reactionary or reparative dentin. However, the new dentin that could be formed is limited only to the pulp dentin interface and the research in this field is at a very evolutionary stage. Therefore, at present, remineralization of dentin in a non-cellular manner through apatite formation by bioactive glass has gained the spotlight. Thus, there is active ongoing research to use bioactive glass as fillers in tooth restorative materials such as tooth pastes, Glass ionomer cements (GICs) [29] and dental composites[30], [31], [32] with the aim to occlude exposed dentinal tubules, improve the bonding at the dentin interface and most importantly to repair the underlying mineral depleted dentin through remineralization and such studies are reporting the efficiency of bioactive glasses for dentin remineralization.
Furthermore, it is well known that the excellent bioactivity of the first developed bioactive glass 45S5 was mainly due to is its finely tuned composition. However, in recent years the glass production methods have become more varied with methods such as sol-gel [33] [34], [35], flame spray [36] and spray pyrolysis [37] on offer in addition to the traditional melt-quench route. These methods give additional textural features such as porosity and fine particle size which increases the surface area of these particles. Recent studies suggest that, besides composition, surface textural properties also play a vital role in the bioactivity of these glasses [38], [39], [40].
Therefore, the aim of this systematic review was to assess if there is a real evidence for bioactive glass to effectively remineralize dentin. We equated effective remineralization of dentin using BAG treatment to improved mechanical properties of this tissue through intrafibrillar mineralization. In addition, we delved into analysing the characteristics of bioactive glass (a consequence of various preparation methods and modifications of material composition) that could highly influence the remineralization of dentin as a part of this review.
2. Methods
2.1. Research question
Prior to the initiation of this comprehensive systematic review, a research question was formulated: “Is there strong evidence for bioactive glass to remineralize dentin?”
2.2. Search strategy
Three individual electronic databases were searched accurately and independently by two reviewers (Delihta Fernando and Nina Attik) following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements for assessing the methodological quality of systematic reviews [41]. The electronic databases searched for identifying the relevant studies included Web of Science, PubMed and Science Direct. The published scientific articles from January 2000 to July 2016 were systematically assessed for this review. The last search was conducted on 20th July 2016 in the Faculté d'Odontologie, Université Claude Bernard Lyon 1, Lyon, France.
2.3. Keywords selection
Three combinations of keywords were independently applied by the 2 investigators on Web of science, PubMed and Science direct. The keyword combination that returned the maximum papers was taken forward for the detailed systematic search.
2.4. Inclusion and exclusion criteria
In order to identify relevant studies, the following inclusion and exclusion criteria were set.
We only included the studies that:
-
(1)
Evaluated BAG effect on demineralized dentin tissue.
-
(2)
Interfaced BAGs on demineralized dentin through indirect (bonding resins, Glass ionomer cements, etc.) or through direct contact.
-
(3)
Have confirmed apatite formation by proper characterizations. Specifically, at least one of these characterizations such as X-ray diffraction (XRD), Raman spectroscopy and Fourier transform infrared spectroscopy (FTIR) and attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) should have been used along with qualitative analysis techniques such as scanning electron microscopy (SEM) either employed alone or coupled with energy dispersive X-ray spectroscopy (SEM-EDX), and confocal laser scanning microscopy (CLSM) to confirm the presence of apatite.
-
(4)
Studies that have reported data on the mechanical properties of the BAG treated dentin in addition to apatite formation were considered beneficial.
We therefore excluded the studies that:
-
(1)
Evaluated BAG effect on demineralized enamel or root cementum.
-
(2)
Used toothpastes containing BAGs for dentin remineralization.
-
(3)
Reported apatite formation based only on SEM imaging or confocal microscopy.
-
(4)
Reported only the mechanical properties without apatite confirmation.
-
(5)
Failed to provide any information about BAG characteristics such as composition, particle size and surface area.
2.5. Paper selection and data extraction
In each database, after performing the initial search using the selected keyword combination, any duplicates were identified and excluded from the total list of articles. In the second phase of evaluation, the titles and abstracts of the remaining papers were carefully read to identify the relevant articles based on the inclusion criteria. The studies that evaluated BAG effects on enamel, root cementum, dental cells for toxicity, bacterial strains for antibacterial effects and review articles were excluded right away. BAGs in tooth pastes and studies that used BAGs in substrates such as resins or cements to evaluate apatite formation without any contact with dentin tissue were also excluded at this stage. The remaining final list of relevant papers were downloaded and the full texts have been thoroughly scrutinized for information provided about the BAGs used, the quality and quantity of characterizations performed to confirm remineralization of dentin. The references of these papers were also checked to include any interesting papers that were missed during the search. If relevant, these new papers were also downloaded and added to the list of papers with full text for similar scrutinization. At this stage, the papers that reported only qualitative evaluation of apatite by SEM or CLSM without any other supporting characterizations such as XRD, Raman spectroscopy, FTIR, ATR-FTIR and TGAwere excluded. Furthermore, the papers that reported only an interaction layer instead of apatite formation were also excluded and the reasons for exclusion were specified. The potentially relevant papers that had passed all these selections stages were included in the final synthesis. Pre-specified data elements were identified from individual studies which included the BAG characteristics such as composition, particle size, surface area, filler wt%, demineralization solution, remineralization solution, time and entered into the tables. The studies were separated based on the bioactive glass mode of application (indirect contact or direct contact) on demineralized dentin.
3. Results
The keyword combination “((bioactive glass OR bio-active glass OR bioglass OR bioceramic) AND (remineralization OR remineralisation) AND (Dental OR tooth OR teeth))” returned the maximum number of papers (Table 1) and hence was chosen for the review.
Table 1. Keyword selection.
|
Keywords ((bioactive glass OR bioglass OR bioceramic) AND (remineralisation OR remineralization) AND (Dental OR tooth OR teeth) AND (enamel OR dentin OR dentine OR root cementum)) |
Database and number of papers
|
|---|---|
| (((bioactive glass OR bio-active glass OR bioglass OR bioceramic) AND (remineralisation OR remineralization) AND (Dental OR tooth OR teeth OR tooth paste OR resin OR composite OR filler OR tooth restoration) AND (enamel OR dentin OR dentine OR root cementum))) |
|
| ((bioactive glass OR bioglass OR bioceramic) AND (remineralization OR remineralisation) AND (Dental OR tooth OR teeth)) |
|
The initial electronic search using this keyword returned 303 articles. At the first phase of evaluation duplicate articles were excluded. The title and abstracts of the remaining 259 papers were screened and 242 papers were excluded as they were not relevant to the inclusion criteria. Finally, 17 relevant papers were scrutinized by downloading the papers and reading the full text. 2 papers were found from the references of the 17 selected papers and these were also assessed in a similar manner. A consensus between the two authors was reached to determine which studies fully met the selection criteria. 16 papers were excluded after reading the full text (Fig. 2). The reasons for exclusion are stated in Table 2. Finally, 3 key papers have been included in this review (Fig. 3).
 Fig. 2. Search strategy.
Fig. 2. Search strategy.Table 2. Studies excluded with reasons.
| Studies | Reasons for exclusion |
|---|---|
| Forsback et al., 2004 [42] | Characterization of apatite only by SEM |
| Schmidlin et al., 2007 [43] | Only mechanical properties characterized, apatite not confirmed |
| Xie et al., 2008 [44] | Characterization of apatite only by SEM |
| Curtis et al., 2010 [45] | Characterization of apatite only by SEM-EDX evaluation |
| Bakry et al., 2011 [46] | The mineral deposit in dentin was brushite, monetite and not apatite |
| Gandolfi et al., 2011 [47] | Analysis of tricalcium silicate, dicalcium silicate, tricalcium aluminate, calcium sulfate, barium sulfate and not a bioactive glass |
| Osorio et al., 2012 [48] | Characterization of apatite only by SEM-EDX evaluation |
| Sauro et al., 2012 [49] | Apatite forming ability of bioglass in resin disks was confirmed by XRD but only CLSM was used to show apatite in dentin |
| Sauro et al., 2012 [32] | Characterization of apatite only by CLSM |
| Lynch et al., 2012 [50] | Apatite forming ability of bioglass was confirmed by XRD but this was not confirmed in dentin |
| Bakry et al., 2013 [51] | The mineral deposit in dentin was brushite and not apatite |
| Chen et al., 2013 [40] | The mineral deposit in dentin was monocalcium phosphate monohydrate and not apatite |
| Khoroushi et al., 2013 [52] | Characterization of apatite only by SEM |
| Profeta et al., 2014 [53] | Characterization of apatite only by SEM and CLSM |
| Osorio et al., 2015 [29] | Only μTBS and SEM image of debonding was characterized |
| Sauro et al., 2015 [54] | Analysis of beta-tricalcium phosphate and not a bioactive glass |
 Fig. 3. Characterization methods and outcomes of included studies.
Fig. 3. Characterization methods and outcomes of included studies.3.1. Study characteristics
3.1.1. BAG characteristics
All 3 papers have used 45S5 (45SiO2:24.5CaO: 24.5Na2O:6P2O5) as the chosen BAG composition. In the study of Wang et al. [55] BAG was modified with polyacrylic acid (PBAG) and in another study with soda lime glass (MBAG) [56]. Only one paper [36] has compared the effect of nano-sized BAG (nano BAG) with micro sized BAG (micro BAG) and the remaining 2 papers have used micro BAG (Table 3, Table 4).
Table 3. Study characteristics of BAG indirect contact.
| Paper | Test material | Particle size (μm) | Weight percent | Resin type |
|---|---|---|---|---|
| [55] | (1) 10% PAA-45S5 BAG | < 30 | 33 | UDMA:Bis-GMA:TEGDMA:HEMA |
| (2) 10% PAA-CS (Calcium silicate) | < 30 | 33 | UDMA:Bis-GMA:TEGDMA:HEMA | |
| (3) 10% PAA-PDP (CS doped brushite) | < 30 | 33 | UDMA:Bis-GMA:TEGDMA:HEMA | |
| Paper | Tooth | Demineralization method | Remineralization method | Time |
|---|---|---|---|---|
| [55] | Human third molar | Solution: 10% orthophosphoric acid |
Application: polymerized resin disk in close contact with demineralized dentin. Solution: artificial saliva Refresh: every 72 h Time: 10 min |
14 days |
Table 4. Study characteristics of BAG direct contact.
| Paper | Test material | Particle size (μm) | Fabrication method |
|---|---|---|---|
| [36] | (1) NBAG (45S5) | 0.03–0.05 | Flame spray |
| (2) BAG (45S5) | 90–710 | Melt-quench | |
| [56] | (1) BAG (45S5) | 30–90 | Not mentioned |
| (2) MBAG (45S5 + 40% Sodalime glass) | 4–80 | Not mentioned | |
| Tooth | Demineralization method | Remineralization method | Time | |
|---|---|---|---|---|
| [36] | Human third molar |
Solution: 17% EDTA pH: 8 Time: 2 h |
Application: BAG in deionised water (172 mg/ml). Dentin stored in the above solution. Remin solution: BAG in water Refresh: no |
1, 10 and 30 days |
| [56] | Human third molar |
Partial: Solution: 0.5 M EDTA pH: 7.4 Time: 5 min Complete: Solution: 0.5 M EDTA pH: 7.4 Time: 15 days |
Application: 20 mg BAG rubbed on dentin with cotton pellet. Time: 1 min Remin solution: artificial saliva Refresh: refreshed every day |
7 days |
3.1.2. Protocol
Briefly, in all studies a caries-free human third molar has been used. The tooth has been cut to expose the dentin and was subjected to demineralization by acid or ethylene-diamine-tetra-acetic acid (EDTA) treatment. Wang et al. have used orthophosphoric acid [55], Vollenweider et al., and Wang et al. have used EDTA to cleave minerals from the tooth [36], [56]. After demineralization, the dentin was brought into contact with BAG followed by immersion in remineralization solution. 2 studies have used artificial saliva [55], [56], 1 study immersed dentin in deionised water for remineralization [36]. At selected time points, the experimental samples were characterized for newly formed apatite (Table 3, Table 4, Fig. 3).
3.1.3. Classification of included studies
Data were divided into 2 types based on the mode of BAG application for remineralization. Wang et al. [55] have used BAG in resins in order to assess the remineralization efficiency of BAG when used in resin based dental restorative materials (BAG indirect contact). The resin was a combination of urethanedimethacrylate (UDMA), bisphenol A diglycidyl ether dimethacrylate (Bis-GMA), triethylene glycol dimethacrylate (TEGDMA) and 2-hydroxyethyl methacrylate (HEMA) (Table 3).
BAGs without any substrate (resin or cement) were used in 2 studies (BAG direct contact). Vollenweider et al. [36] immersed demineralized dentin in a BAG water suspension and Wang et al. [56] rubbed BAG on the demineralized dentin prior to immersion in artificial saliva (Table 4).
3.2. BAG indirect contact
Wang et al. [55] compared the remineralization efficiency of resin containing (33% filler) polycarboxylated BAG (PBAG), polycarboxylated calcium silicate (PCS), and polycarboxylated calcium silicate doped brushite (PDP) microfillers. Unfilled resins contacted with dentin served as controls. The polymerized bioactive resin disks were held in close contact with acid etched dentin while immersed in artificial saliva for 14 days. The authors evaluated the mineral changes through ATR-FTIR, Raman spectroscopy and CLSM. Furthermore, they also performed a micro tensile bond strength (μTBS) test to measure the bonding ability at resin-dentin interface. Overall, they found that both PBAG and PCS exhibited higher levels of apatite formation in dentin compared to PDP and the control. Furthermore, PBAG treated group exhibited the highest mineral matrix ratio after day 14, followed by the PCS group. They also reported that even though there was a significant drop in μTBS after 3 month's storage in artificial saliva the CLSM showed a strong reflection corresponding to the mineral deposit in the hybrid layer. This lead to a reduction in nano-leakage in the BAG treated group supporting the remineralization effects of this bioactive filler.
3.3. BAG direct contact
Vollenweider et al. [36] compared the remineralization effects of nano BAG (30–50 nm) and micro BAG on demineralized dentin. They immersed the ETDA treated dentin in BAG containing de-ionized water for 1, 10 up to 30 days. They used Raman spectroscopy combined with SEM-EDX to identify the newly formed apatite. Furthermore, they also implemented thermogravimetry to quantitatively assess the level of mineral formation. The nano BAG demonstrated substantially faster apatite development compared to the micro BAG due to their increased surface area. The ion release profile (from the BAG particles alone) explained the faster apatite formation induced by nano BAG. Those particles showed a rapid release of calcium and silica, which was 20 times higher than micro BAG at early time points, and also had 10 times higher basicity compared to the micro BAGs treated solution. At 24 h, Raman spectroscopy confirmed a phosphate peak corresponding to apatite in the dentin sample immersed in the suspension of nano BAG whereas no peak was observed in the dentin treated with micro BAG. However, both treatment groups demonstrated apatite development at day 10 which became even higher at day 30. The quantification of mineral content by thermogravimetric analysis showed that nano BAG treatment had a markedly increased mineral content than micro BAG at day 10 and day 30. Further at day 30, the nano BAG treated dentin's mineral content was equivalent to natural dentin. Interestingly, they also found that nano BAG treatment lowered the mechanical properties of the mineralized dentin in comparison to micro BAG treatment group and both of them were much lower than natural dentin.
In 2011 Wang et al. [56], evaluated the dentin remineralization induced by bioactive glass (BAG) and bioactive glass modified with spherical soda lime particles (MBAG). They performed a partial and complete demineralization of dentin with EDTA treatment followed by application of BAG, MBAG for each treatment groups and immersion in artificial saliva for 1, 3 and 7 days. The controls were demineralized dentin without any treatment. They quantified the mineral variation on partially demineralized dentin using ATR-FTIR and on the completely demineralized dentin they performed ATR-FTIR, XRD and SEM-EDX to confirm the apatite formation. A gradual increase in phosphate peak was observed at different time points up to day 7 on both BAG treated groups compared to the control. The mineral matrix ratio also showed that both BAG treated groups resulted in increasing mineral concentration from day 1 to day 7 relative to the control. However, there was no significant difference in mineral content between the BAG and MBAG treatment groups. In addition, XRD confirmed apatite formation in the dentin treated with BAG and MBAG which was similar to the apatite peaks observed in the natural dentin whereas, on the control group, no apatite was detected. The SEM-EDX analysis revealed that apatite spherulites completely covered the dentine tubules in the BAG treated groups. In a similar fashion, MBAG treatment resulted in a homogeneous layer of needle like apatite covering the dentin tubules at day 7 of remineralization treatment. In conclusion, although MBAG has a lower concentration of bioactive glass and a uniform spherical shape compared to BAG both glass systems demonstrated similar potential in dentin remineralization through apatite formation.
4. Discussion
Dentin is one of the most highly mineralized tissues in the tooth after enamel. In the oral cavity, demineralization (loss of Ca2 +, PO43 −, F− ions and subsequent apatite dissolution) and remineralization (gain of Ca2 +, PO43 − and F− ions and subsequent crystallization of apatite) are normal processes that are meant to be balanced. However, inappropriate dietary habits and hygiene thwarts the balance and triggers demineralization to dominate remineralization. This results in initial enamel lesions that progress with time into the dentin. Remineralization process of such lesions in enamel or dentin using bioactive glass involves exchange of ions (Na+, Ca2 +, PO43 −, F−) from the silicate network of BAG with the surrounding oral fluid leading to supersaturating of ions in the fluid which later re-precipitate on the silicate network of BAG in the tissue. Following this, the precipitated amorphous calcium phosphate layer grows and crystallizes into apatite and forms a stable bond to the tissue.
The dentin is not only made of minerals (as in enamel) but also with organic collagen and water. Therefore it is important to note that the functional remineralization of dentin depends not only on increased apatite content in the extrafibrillar collagen matrix but also on improving the mechanical properties of this tissue through intrafibrillar mineralization (ie. apatite positioned in between the collagen fibrils, Fig. 1).
With a growing interest in using bioactive glass for dentin remineralization, this systematic review was performed to assess from the results in this field if there is evidence for bioactive glass stimulating dentin remineralization. We set high standards for inclusion criteria in order to avoid bias on the results. For instance, we excluded bioactive glasses in tooth pastes because the other components in dentifrices in addition to bioactive glass such as sodium fluoride, calcium fluoride are also involved in the process of remineralization. This way the sole effect of bioactive glass mediated remineralization cannot be distinguished. Likewise, we also did not include studies that didn't confirm the apatite in dentin by suitable characterizations such as XRD or FTIR and Raman Spectroscopy. Curtis et al. [45] had interesting findings that showed nano BAGs are efficient in tubule occlusion as they form apatite rods plugged in the dentin tubules; this contrasts with micro BAG performance where an apatite layer covers the tubules. The study was not included in this survey as the apatite phase was not confirmed. Such exclusions were made because the authors wanted to be sure of apatite crystallization on dentin and not just precipitation of a calcium phosphate layer that could appear as mineral by qualitative analysis (SEM or CLSM). In the same way, studies that characterized and reported an interaction layer (brushite, calcium mono phosphate etc.) which are less stable phases that apatite were also excluded [46], [51]. We found that there were some interesting studies [29], [32] that have even characterized the mechanical properties of material-dentin interface after remineralization procedure; however due to the omission of robust characterization to confirm apatite these studies were once again omitted from this review. Sauro et al. studied the therapeutic effects of adhesives containing BAG and BAG doped with Zinc (BAG-Zn) at the resin-dentin interface. They interfaced the demineralised dentin with resin containing BAG and BAG-Zn before immersing them in SBF for up to 3 months. The control was dentin interfaced with unfilled resin [49]. They showed the mineral content by CLSM on different regions of the dentin (e.g. the adhesive layer, hybrid layer and resin penetrated inside the tubules). Most importantly, they also performed mechanical characterizations (elastic modulus and hardness) after the treatment at different positions such as at the adhesive layer interface, on the hybrid, below the hybrid layer and in the intertubular dentin. They found that both the resin with BAG and with BAG-Zn had enhanced mineral formation after 3 months which was reflected as reduced nano-leakage in the hybrid layer. Furthermore the mechanical properties were different at different regions of the dentin and, in general there was a reduction in the elastic modulus at the adhesive layer for all groups after the treatment. However, there was improved elastic modulus in and below the hybrid layer for both BAG-treated resins. The reason why this study was not included in the final 3 papers selected (despite its interesting and well elaborated results) is that the data on apatite confirmation by Raman spectroscopy is from the “resin + bioactive filler” disks immersed in SBF without any contact with the dentin (i.e. from the apatite forming ability of the biomaterial alone). The missing confirmation of the newly formed apatite on the demineralized dentin interfaced with these bioactive materials poses a risk of bias as to whether the changes in mechanical properties and mineral content observed by CLSM is a result of apatite or just an interaction layer in the dentin. Therefore, whilst studies such as these are encouraged, key characterizations must be afforded maximum importance to ensure we fully understand the process and so assess the true potential of bioactive glasses for the remineralization of dentin.
By following this rigorous methodology we arrived at 3 key papers that fulfilled our criteria to be included in this review.
All the three papers in this review have confirmed that bioactive glass results in enhanced apatite formation in dentin (Fig. 3) which is one important aspect for remineralization. Wang et al. have realized apatite formation using bioactive glass in completely demineralized dentin [56]. Apatite formation in completely demineralized dentin is strong evidence that this process was not influenced by seed crystallites in the dentin as in the other studies that employed a partial demineralization procedure. Furthermore, in 2014 Wang et al. demonstrated apatite formation in dentin by using bioactive glass as a filler in dental resin [55]. This emphasizes that the apatite formation has also been possible via indirect contact to the dentin. Regarding the glass characteristics, Vollenweider et al. have shown that nano BAGs lead to faster apatite formation due to their improved surface area [36]. As previously stated, remineralization of dentin also demands mechanical recovery of the mineralized tissue to be similar to the natural dentin. The same study has characterized this aspect and they have found that treatments with both micro and nano BAGs could not match natural dentin in terms of the Young's modulus and flexural bend strength even though there was enhanced mineral content in the nano BAG treated group. This finding stresses the importance of defining remineralization of dentin not just from the overall apatite content but also from the mechanical properties of the remineralized tissue. Furthermore, from the 3 key papers in this review the data on the mechanical characterization of dentin after BAG treatment for remineralization was available from only one paper (Fig. 3). The lack of such important evaluations limits us from drawing firm conclusions. Kinney et al. tested if the hardness and modulus of dentin was proportional to its mineral concentration by comparing the values of normal dentin with dentin that lacked intrafibrillar mineralization [57]. They found that both hardness and Young's modulus decreased evidently in dentin without intrafibrillar mineralization. Based on these outcomes they pointed that mineral concentration alone may not be a sufficient end point for assessing the remineralization of dentin.