1. Introduction
Tissue failures such as heart failure, neural disorder and severe burns etc. can cause mortality globally, due to the limited regenerative ability of major organs after initial injury [[1], [2], [3], [4]]. Clinically, organ transplantation is still the first option, while the available organs are severely limited. To solve this unmet challenge, tissue engineering, with the aim of constructing an artificial organ composed of cells, especially stem cells and a bioactive matrix to improve or restore damaged tissue and organ functionality, has emerged as a promising solution [[5], [6], [7], [8]]. The bioactive matrix used in tissue engineering functions as an extracellular matrix (ECM) that provides well-defined biochemical (e.g. growth factors, and surface chemistry) and biophysical cues (e.g., fibrous structure, hydrophilicity and stiffness) to effectively regulate cellular behaviors such as attachment, migration, proliferation and differentiation for restoring the functionality of damaged tissues [9, 10]. Among various biomaterials, polyester biomaterials that have appropriate degradation properties and limited host response have emerged as a versatile tool in preparing bioactive matrix to mimic native ECM for regulation of cell behaviors [4, [11], [12], [13]]. Various polyester materials have been fabricated into different forms of bioactive matrixes for promoting tissue regeneration [[14], [15], [16]]. For instance, a biodegradable polyester with tunable affinity to bone marrow-derived mesenchymal stem cells (MSCs) was synthesized through copolymerization and further modified with controllable amounts of MSCs specific affinity peptide (EPLQLKM, E7) through click-chemistry. The E7 modified polyester scaffolds significantly promote their interactions with MSCs and lead to better cell adhesion and proliferation as compared to the scaffolds without modification [17]. Because of their simple synthesis procedure, hydrolytic degradation properties, and elastomeric characteristics, these polyester biomaterials are highly desirable in tissue engineering, with some already approved by FDA (e.g., polycarprolactone (PCL) and poly-l-lactic acid (PLLA)) [18].
To mimic the fibrous structure of native ECM, polyester materials have been fabricated into multi-scaled fibrous scaffolds by techniques such as electrospinning, phase separation and self-assembly [13, 19]. Among these techniques, electrospinning has been intensively applied due to the merits of simplicity, reproducibility, and diversity in producing fibers with micro- or nano-scaled diameter and with different topographical features [20, 21]. Beyond their fibrous structure, the large surface-area/volume ratio of electrospun nanofibrous scaffolds makes them as ideal bioactive matrix for cell accommodations, efficient bioactive molecules loading, and easy incorporation of functional components (e.g., graphene and carbon nanotubes) to provide enriched biochemical and biophysical cues for enhanced tissue regeneration.
Additionally, inspired by the highly oriented features of native ECMs in tissues such as nerve, heart and tendon etc., aligned nanofibrous scaffolds have been prepared by electrospinning and demonstrated a critical role for topographical cues in the controlled structure and functions of stem cells [22]. For instant, polyester materials such as PCL and PLLA have been electrospun into aligned fibrous scaffolds, which can promote the neuronal differentiation of human MSCs and the elongation of the formed neurites compared to the flat casting film and random fibrous scaffolds [23, 24]. Furthermore, coupled with chemical cues such as the incorporation of various native proteins in terms of collagen, gelatin and bioactive peptides, electrospun aligned nanofibrous scaffolds can further promote stem cells attachment, migration, proliferation and differentiation [25, 26].
Many studies have demonstrated that conventional two-dimensional (2D) biomaterials (e.g., fibrous sheets and biofilms) cannot maintain the phenotypes of cells derived from complex multi-cellular tissues due to the dramatic differences between 2D culture in vitro and the native cellular microenvironment in aspects of physical and chemical properties [27, 28]. To overcome this obstacle, 3D cell culture using biomimetic nanofibrous scaffolds has emerged as an alternative strategy to recapitulate native cell microenvironment in vitro [29, 30]. To create an engineered 3D aligned fibrous scaffolds, Gomes and colleagues' electrospun continuous aligned nanofibers and then explored their assembly into 3D scaffolds using different textile techniques such as twisting, braiding, and weaving, and further characterized their morphological and biomechanical properties. The topographical and biomechanical cues provided by the fabricated 3D aligned nanofibers could direct the tenogenic differentiation of human adipose-derived MSCs and also maintain the phenotype of native tendon cells [31]. Whereas, these aligned nanofibres are usually densely packed and will be denser after textile technique processing, which leads to a lower porosity and limited cell infiltration. To enhance the porosity of the electrospun aligned nanofibers, Baker et al. introduced a water-soluble polyethylene oxide (PEO) nanofiber as a scarified component within the electrospun aligned polyester PCL naonfibers using a dual spinneret system. After immersion in water, the pore sizes of PCL nanofibers was enlarged, allowing sufficient cell penetration and the formation of a highly organized ECM that nearly matched native tissue of the knee meniscus [32]. From the above, we can conclude that 3D aligned electrospun nanofibers may represent an ideal biomimetic ECM with integrated topographic, biomechanical and biochemical cues for precisely controlling cellular morphology, orientation, and other behaviors for the regeneration of tissues such as skin, heart, nerve and tendon [33].
Recent advances in fabrication of 3D nanofibers have been summarized in several excellent reviews usually, which usually focus on summarizing different fabrication methods and their potential applications in energy generation and storage, water treatment and biomedical engineering [[34], [35], [36]]. However, a holistic review about the electrospun 3D aligned polyester nanofibrous scaffolds that can mimic the nano- and micro-topography, and define the structural orientation of the ECM for the regulation of cell behaviors and tissue regeneration is still missing. In this review, we provide an extensive overview on recent efforts for constructing electrospun 3D aligned polyester nanofibrous scaffolds, then the results of cell-specific functions dependent on such physical cues, and discuss their potentials in promoting the regeneration of various tissues in terms of skin, heart, nerve and tendon.
2. Nanotopographical cues in regulating stem cell behaviors
Cells in the physiological niche are constantly challenged by both soluble cues and physical stimuli, and dynamically regulated in the local extracellular matrix (ECM) [37]. The cellular sensing of extra cellular topographical cues through nanoscale architecture and dynamics of cell-ECM adhesions initiates downstream intracellular mechano-transductive events, resulting in topography-sensitive cellular behaviors, including cell adhesion, proliferation, self-renewal, gene expression, morphology, and differentiation [38, 39]. Substrates with topographies such as vertically aligned nanotubes [40], nanoscale ridge/grooves [41], aligned nanofibers [42], and micropatterned surfaces (such as circle, square, rectangle, triangle, and star) [43] have been developed to harness the fate of stem cells for the regeneration of damaged tissues and have shown significant effects on the regulation of stem cell fates. For instance, it has been commonly observed that both rat and human MSCs on nanofiber scaffolds show significantly enhanced osteogenic differentiation compared to conventional tissue culture plates [40, 44, 45]. In addition, the capability of substrates with nanotopographies in regulating stem cell fate is size-depended. For example, TiO2 nanotube arrays directed human MSC differentiation toward the osteoblast lineage even in the absence of soluble inductive factors. In this study, among arrays of 30, 50, 70, and 100 nm diameter TiO2 nanotubes fabricated on Ti substrates, the one composed of 100 nm diameter nanotubes showed the highest potential for promoting human MSC osteogenic differentiation [46].
Among these topographies, electrospun nanofibers with aligned morphology have been widely used to regulate stem cell fate due to their nano-structures of biomaterials that are of vital importance in nerve, bone, tendon and cardiac tissue engineering based on biomimetic ideas [47]. By mimicking the natural extracellular matrix, the nanofibers have been found to provide distinct contact cues to modulate cell activities, including supporting cell proliferation and migration, promoting the stemness and maintaining pluripotency of stem cells [48]. In addition, the alignment of fibers has also been shown to generate topography-induced cues to promote Schwann cell maturation, myotube formation and MSC differentiation [[49], [50], [51]]. Various polyester-based aligned nanofibers have been fabricated to regulate stem cell fates. It has been found that the uniaxially aligned nanofiber substrates enhanced not only neural differentiation efficiency but also neurite out growth along nanofibers, critical for neuronal network development [[52], [53], [54]]. Electrospun aligned polyester nanofibers such as poly(lactic-co-glycolic acid) (PLGA), PLLA and PCL nanofibers have also been found to induce unidirectional alignment of collagen assembly that resembles collagen assembly of a lamellar bone in vivo [[55], [56], [57]]. Instead of studying the aligned topographical cue in regulating stem cell differentiation, Su et al. have studied the effect of aligned naonfibers on the paracrine effect of stem cells and found that as compared with random nanofibers, the electrospun aligned PCL nanofibers can significantly promote the attached stem cells in producing cytokines that are capable of promoting angiogenesis, immunomodulation and tissue healing processes [58].
Although nanotopographical cues have shown great potentials in regulating stem cell fate for tissue regeneration, the underlying molecular mechanisms remain elusive. Currently, the widely accepted mechanism is that topography-induced stem cell differentiation occurs through mechanotransduction [59]. The stem cells that attached on the substrates can probe surrounding ECM viaintegrin-ligand binding to form focal adhesion (FA), which triggers integrin-mediated FA signaling to elicit down-stream biochemical signals in terms of FAK-Src pathway [60], FAK/MEK/ERK [40], PI3K/Akt [61] and BMP and TGFβ/SMAD [62] pathways that are important for the regulation of gene expression to regulate stem cell behaviors such as cell proliferation, self-renewal, and differentiation [63]. Notably, recent studies have shown enhanced ECM proteins adsorption on nanotopographical surfaces [64, 65], which could also contribute to stem cell fate regulation.
Despite the promising outcomes of nanotopographical cues in successfully regulating cell behaviors, the substrates used in the majority studies are 2-dimensional (2D) membrane or mesh, resulting in an experimental design with significant deviation from the innate 3D structure of tissues such as skin, nerve, bone and heart. Therefore, there is an urgent demand in fabrication of aligned nanofibrous scaffold with a 3D structure that can structurally resembling the natural matrix as a suitable biomaterial for tissue regeneration [66]. In this contribution, fabrication of 3D aligned nanofibers and their potential applications in regenerative medicine are discussed below.
3. Fabrication of 3D aligned nanofibers by electrospinning
Various techniques such as colloidal lithography [67], self-assembly [68], solution blow spinning [69], and electrospinning [70] (including melt electrospinning [71] and near-field electrospinning [72]) have been used to fabricate 3D aligned nanofibers. Among these techniques, electrospinning provides a versatile, inexpensive, and straightforward technique to deposit organic and inorganic nano and micrometer-scale fibrous scaffolds with great throughput [34]. With template-assisted collection and post-processing, we can easily obtain the 3D aligned nanofibrous scaffolds via electrospinning. In this section, we introduce the setup, mechanism, key parameters, and different means to obtain 3D aligned nanofibers using electrospinning.
A typical electrospinning setup includes three elements: a high voltage power supply, a syringe pump (including a syringe and a spinneret) and a collector. The fabrication process involves an electrostatic field of the order of 5–30 kV between a collector and the spinneret (usually in the form of a needle). Under electrostatic field, the polymer melt or solution pumped out of the spinneret at a controlled rate is under two major forces, including stretching force provided by the sufficiently high electric field and surface tension of the polymer solution. When the stretching force overcomes surface tension, a thin jet of the liquid flies toward the collector plate [22, 24]. Therefore, parameters that can affect stretching force (such as voltage, solution conductivity, distance between tip and collector) and the surface tension (viscosity, concentration, as well as the humidity and temperature of the surroundings) can influence the morphology and diameter of the electrospun fibers [73].
The aligned nanofibrous scaffolds can be produced by three major methods in terms of conventional electrospinning with the rotating mandrel, electric or magnetic field induced electrospinning, and near-field electrospinning. Conventional electrospinning with the rotating mandrel has been widely employed to deposit aligned nanofibers. When the electrospun nanofibers reach the mandrel, it is the simple pulling of the fibers toward the collector that counteracts the whipping motion of the fibers and generates fiber alignment [74]. This method can produce continuous and aligned nanofibers in a productive manner. However, due to the chaotic whipping of liquid jets during electrospinning, it usually requires high rotating speed (usually 1000 rpm or even higher rotational speeds) of the mandrel to obtain aligned nanofibers, which usually raises safety concerns because of the high rotating speed [75]. Other collector configurations, such as rotating wire drum collector, rotating disc collector, rotating drum with sharp pin inside, have also been engineered and used for fabrication of aligned nanofibrous scaffolds [76]. Field-induced electrospinning has been developed to control deposited fiber alignments through modifying magnetic and electrical fields [77, 78]. However, this method lacks large-scale productivity due to the limited void gap between the magnetic and electrical collectors. In addition, the above-mentioned two methods are not capable of controlling the precise direction of each single fiber. More recently, near-field electrospinning has been introduced as a powerful method to form highly controllable patterns of electrospun fibers on flat surfaces [72]. In near-field electrospinning the distance between needle and collector is very short (500 μm to 3 mm). This relative short distance prevents the bending instability and splitting in electrospun fibers [79]. Therefore, near-field electrospinning provides the ability to write fibers directly in a highly precise manner [80]. Whereas, one of the problems associated with near-field electrospinning systems is that the majority of these systems use complex and expensive multiaxis microscope stages to control the alignment of the fibers in mainly X-Y directions [81]. Additionally, near-field electrospinning suffers from other limitations such as low assembly efficiency and sophisticated setup [82].
The 3D aligned nanofibrous scaffolds can be obtained using the above-mentioned three methods. One simple way is sequential electrospinning or multi-layering electrospinning. In this way, the electrospun aligned nanofibers are continuously collected for a long time (for example, from 20 min to 20 h) till it grows to a sufficient 3D structure. The thickness of the 3D aligned nanofibrous scaffolds can be adjusted by controlling the deposition duration. Post-processing of the electrospun 2D aligned nanofibers is another way to produce the 3D aligned nanofibrous scaffolds. After peeling off the thin filmfrom the collector, post-processing such as bending/folding or stacking the fiber layers into a 3D fibrous structure are usually applied to form pipe or thick mat. In addition, replacing the conventional 2D flat collector by a 3D collecting template can also be applied to obtain 3D aligned nanofibrous scaffolds [34]. For instance, Beachley and colleagues have developed one pair of automated mobile parallel tracks that were turned synchronously at a controlled speed by two separate motors to collect nanofibers from an electrospinning jet. They have obtained 3D loosely assembled aligned nanofibers with good cell penetration properties using this collecting technology [83]. In addition, Wang and colleagues immersed half of a rotating mandrel in an ethanol solution as the collector configuration to fabricate 3D aligned nanofibers. The obtained fluffy 3D aligned nanofibers possess a high porosity, which could be due to the synergistic effect of interaction among the polymer, ethanol, and rotating mandrel [84]. Likely, ethanol post-treatment of the electrospun aligned nanofibers can also obtain the 3D aligned nanofibrous scaffolds with crimp structure as induced by the release of residual stress within the aligned nanofibers upon contact with a plasticizer ethanol [85]. With the advances in engineering collector configurations by integrating the above-mentioned three methods (rotating mandrel, electric or magnetic field induced electrospinning, and near-field electrospinning), the electrospun 3D aligned nanofibrous scaffolds with controllable structure and porosity could be realized.
4. Applications of electrospun 3D aligned polyester nanofibers
Polyester materials such as PCL, PLLA and PLGA have been widely used in tissue engineering [86]. However, their further applications in tissue engineering have been limited by their inherent hydrophobic surface and the lack of functional groups [87]. To address this, considerable efforts have been made to prepare polyesters with functional groups by means of mixing with native proteins, plasma treatment, and conjugation with bioactive molecules. Additionally, inspired by aligned collagen fibers of the native ECM, these functionalized polyester materials have been fabricated into aligned nanofibers viaelectrospinning to provide tunable biochemical and biomechanical and topographical cues to precisely regulate cell fate for tissue regeneration [88].
4.1. Skin tissue engineering
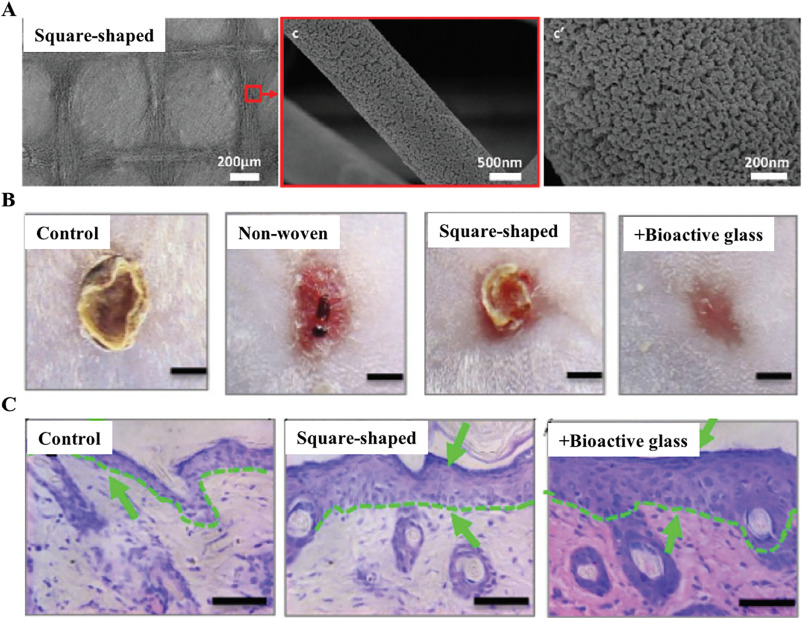
As the protective and regulatory organ in the human body, skin protects us from the invasion of pathogens and excessive water loss, while damages to skin will cause the loss of skin integrity and barrier function. The gold standard treatment for skin wounds such as severe burn injuries in clinic is still using skin autografts, which is strictly restricted by the wound size as the lack of suitable donor sites for autografting. To solve this issue, various polyester materials such as PCL, PLGA, PLLA have been electrospun into nanofibrous scaffolds for wound healing because of their fibrous structure similar to the native skin ECM [5, 89, 90]. Furthermore, incorporation of native proteins such as collagen and gelatin within these electrospun nanofibers can further promote stem cell epidermal differentiation for skin regeneration [91, 92]. In addition to improving the biocompatibility of electrospun polyester nanofibers, tuning the alignment of these electrospun nanofibres has also shown great promise in skin regeneration. For instance, uniaxially aligned nanofibres have been used for skin regeneration because of their capability in guiding cell migration as compared with random nanofibres. Considering that skin wounds are usually in irregular shapes, Xie et al. developed a radially aligned nanofibrous sheet by electrospinning with a point electrode centered in a metal ring as the collector. Cells attached on the radially aligned nanofibers could migrate from the peripheral healthy tissue toward the central injured site along the topographic cues provided by the nanofibers to improve wound healing [93]. However, these radially aligned nanofibers can only be applied for epidermis wound closure, due to their 2D membrane shape, which restricts their applications for the regeneration of the full-thickness skin wound. It is noted that ECMs in dermis have 3D uniaxially aligned morphology when mechanical tensions present in the skin, which is called tension line [94, 95]. Due to such anisotropic nature of dermis morphology, the incision direction would be an important element in order to obtain smaller scar formation and better healing [96, 97]. To further mimic the anisotropic nature of skin, Xu et al. electrospun various aligned nanofibers and then applied pulsed laser deposition (PLD) technology to create a micro-pattern (300–400 μm) on the patterned electrospun nanofibers (Fig. 1A). The combination of patterned electrospun nanofibers with pulsed laser deposition strategies and the hierarchical micro/nano structures in the scaffolds could synergistically improve the efficiency and re-epithelialization of wound healing (Fig. 1B–C) [99].
 Fig. 1. Electrospun the square-shaped nanofibrous yarns for skin tissue engineering. (A) The SEM image of the square-shaped electrospun nanofibrous yarns with bioactive glass at different magnification. (B) Photos of large excision wound healing after 11 days from different treatment groups. Scale bar = 2 mm. (C) Histological sections from the three groups after 7 days post-surgery. Scale bar = 50 μm. Reprinted with permission from reference [98].
Fig. 1. Electrospun the square-shaped nanofibrous yarns for skin tissue engineering. (A) The SEM image of the square-shaped electrospun nanofibrous yarns with bioactive glass at different magnification. (B) Photos of large excision wound healing after 11 days from different treatment groups. Scale bar = 2 mm. (C) Histological sections from the three groups after 7 days post-surgery. Scale bar = 50 μm. Reprinted with permission from reference [98].4.2. Nerve tissue engineering
Nerve injuries caused by physical trauma or neurodegenerative diseases often lead to disability due to the limited regeneration ability of nerve tissue, which greatly affect the life quality of patients worldwide. In this contribution, great efforts have been made to develop tissue engineered nerve grafts using a combination of stem cells and biomaterials to repair or improve the functionality of damaged nerve. Among various bioactive scaffolds developed for nerve regeneration, electrospun nanofibers, especially for the electrospun aligned nanofibers with the capability in guiding and directing neuronal outgrowth, have shown great promises in enhancing neuronal repair and recovery [[99], [100], [101]]. Generally, the electrospun aligned nanofibers can be obtained using three different collectors in terms of rotating mandrel, conductive or magnetic substrates with air gap collection systems. Polyester materials such as PCL and PLA have been electrospun into aligned nanofibers for nerve regeneration [[102], [103], [104]]. However, due to the hydrophobic surface of these polyester materials, the behaviors (e.g., cell attachment and elongation) of cells attached on these polyester nanofibers were restricted due to the poor cell-material interface. Therefore, native proteins such as collagen, gelatin and fibronectin have been incorporated within these aligned polyester nanofibers to improve their biocompatibility, leading to improved cell attachment, proliferation and migration [[105], [106], [107]]. Beyond this, conductive materials such as polypyrole, polyaniline and carbon nanotubes have been incorporated within aligned polyester nanofibers to further promote cells adhesion, neurite alignment and elongation [[108], [109], [110], [111]]. However, these 2D nanofibrous scaffolds are still incapable in mimicking the hierarchical structures of native 3D peripheral nervous system.
To develop 3D scaffold for improved nerve regeneration, Wang and colleagues fabricated the 3D aligned electrospun nanofibers by simply collecting the nanofibers using a rotating mandrel, half of which was immersed in an ethanol solution. The obtained 3D aligned nanofibers can enhance neuron activities and efficiently guide the alignment of attached cells [84]. However, this method is not suitable for preparation of water-soluble scaffold such as 3D aligned collagen nanofibers, due to the potential dissolution of water-soluble materials within ethanol, leading to the destruction of fibrous structure. Instead of immersing the collector mandrel within ethanol, Mo and colleagues developed a dual spinneret electrospinning system using textile method for fiber collection to fabricate a 3D aligned nanofiber yarns, which promoted the proliferation rate and spread morphology of Schwann cells (SCs) as compared to film substrate [103, 112]. Additionally, the synthetic 3D nerve guidance conduits (NGCs) that mimic the structure and composition of an autograft have been fabricated by rolling two layers of electrospun nanofibers into a tube. The inner layer of the tube was aligned nanofibers and the outer layer of the tube was random nanofibers. The performances of the bilayer NGC seeded with Schwann cells in terms of nerve fiber number and maximum isometric tetanic force were comparable to nerve isografts, demonstrating a great potential in axon regeneration and functional motor recovery [113]. The developed 3D NGC from electrospun patterned nanofibers with biodegradable and porous channel walls can allow cell infiltration and efficiently provide biochemical cues and biomechanical cues to the attached cells for advanced nerve regeneration [114, 115]. Mao and colleagues further evaluated the mechanical cue (elasticity) together with the aligned topography of the electrospun 3D hierarchically aligned fibrillar fibrin bundle (Fig. 2A) on cell behaviors such as cell alignment and neural differentiation of human umbilical mesenchymal stem cells (hUMSCs) and the neurite outgrowth (Fig. 2B). The aligned fibrillar fibrin bundle with low elasticity could promote the neurogenic differentiation of hUMSCs as compared with random fibrin bundle and much stiff tissue culture plate (Fig. 2C). Moreover, aligned tissue cables were formed through neural cell migration and axonal invasion along the implanted aligned fibrin bundle in a in vivo spinal cord injury model [47].
 Fig. 2. Electrospun aligned fibrillar yarn for nerve tissue engineering. (A) SEM images of the electrospun aligned fibrillar yarn at different magnifications showing hierarchically aligned organizations. (B) Aligned cell cable formation by hUMSCs attached along aligned fibrillar yarn in vitro. (C) Neural differentiation of hUMSCs on the aligned fibrillar yarn. Reprinted with permission from reference [47].
Fig. 2. Electrospun aligned fibrillar yarn for nerve tissue engineering. (A) SEM images of the electrospun aligned fibrillar yarn at different magnifications showing hierarchically aligned organizations. (B) Aligned cell cable formation by hUMSCs attached along aligned fibrillar yarn in vitro. (C) Neural differentiation of hUMSCs on the aligned fibrillar yarn. Reprinted with permission from reference [47].4.3. Cardiac tissue engineering
Cardiac failure caused by myocardial infarction (MI) is a leading cause of mortality worldwide [116, 117]. Due to the limited regeneration ability of the heart, clinical treatments including coronary bypass, balloon angioplasty, and stent can only temporally maintain cardiac functions instead of bringing back the normal function of the heart. In this sense, engineered cardiac patches made from bioactive scaffolds together with cells, and/or growth factors were developed for improving or restoring cardiac functions after MI [[118], [119], [120]]. Specially, electrospun aligned polyester nanofibers that can mimic the aligned anisotropic nature of the myocardium showed great promise in guiding cell alignment and maturation of cardiomyocytes (CMs) [121, 122]. Beyond that, collagen blending or fibroblast-derived ECM deposition onto the electrospun aligned nanofibers could further enhance cardiomyoblast differentiation and CM maturation [123, 124]. Furthermore, to mimic electrical impulse transition throughout the myocardium in native heart, electrically conductive scaffold was prepared by incorporation of electrically conductive melanin, CNTs or polyaniline within these aligned nanofibers, which could further promote cell elongation, enhance the production of sarcomeric α-actinin and troponin I, and promote the synchronous beating of CMs [[125], [126], [127], [128]].
However, these 2D film-formed scaffolds are not capable of representing the 3D anisotropic cardiac structure (Fig. 3A) due to their simplicity in spatial dimension, thus failing in constructing fully functional and biomimetic cardiac substitutes. To mimic the anisotropic cardiac structure and guiding 3D cellular orientation, Ma et al. fabricated a 3D hybrid scaffold by embedding the electorspun aligned conductive nanofiber yarns within a hydrogel shell (Fig. 3A). CMs cultured on each layer of the developed 3D hybrid scaffold acquired aligned cell morphology with enhanced cell adhesion, viability, and maturation (Fig. 3B). Furthermore, to prolong the survival duration of the 3D cardiac constructs, endothelial cells (ECs) and CMs were co-cultured within the 3D hybrid scaffold and then endothelialized myocardium was formed after 5 days, demonstrating great potential in engineering 3D cardiac anisotropy using this 3D hybrid scaffold (Fig. 3C) [129].
 Fig. 3. Electrospun aligned nanofibrous yarn for cardiac tissue engineering. (A) From left to right, image represents interwoven structure of native cardiac tissue, SEM image of the electrospun aligned nanofibrous yarn and SEM image of the interwoven scaffold. (B) 3D views of fluorescent images of cardiomyocytes on different interwoven scaffolds by staining with F-actin (green) and DAPI (blue) after 5 days of cultivation. Scale bar = 300 μm. (C) The fluorescent images of GFP positive ECs (green), CMs (red) and their merge image, respectively. Reprinted with permission from reference [129]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3. Electrospun aligned nanofibrous yarn for cardiac tissue engineering. (A) From left to right, image represents interwoven structure of native cardiac tissue, SEM image of the electrospun aligned nanofibrous yarn and SEM image of the interwoven scaffold. (B) 3D views of fluorescent images of cardiomyocytes on different interwoven scaffolds by staining with F-actin (green) and DAPI (blue) after 5 days of cultivation. Scale bar = 300 μm. (C) The fluorescent images of GFP positive ECs (green), CMs (red) and their merge image, respectively. Reprinted with permission from reference [129]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)4.4. Tendon tissue engineering
Roughly, one out of four adults will suffer tendon-related condition that greatly affects the quality of life due to the instability and abnormal joint movement after tendon injuries [130]. It is still an ongoing challenge to repair damaged tendon, due to the low cellularity and vascularity of native tendon tissue with limited regeneration capacity [131, 132]. Tendon tissue engineering with the aim to improve or repair the functionality of damaged tendon with the combination of bioactive scaffolds, cells and growth factors has emerged as a versatile tool for tendon regeneration. To mimic the native ECM structure of tendon tissue made up of a hierarchal structure of aligned collagen fibers in both micro and nanoscale [131], various aligned nanofibrous scaffolds have been fabricated to recapitulate the organization of tendon ECM [133, 134]. Among these scaffolds, electrospun aligned polyester nanofibers have shown robust capability in guiding cell alignment, improving matrix deposition and tenogenic differentiation of stem cells, and resembling native tendon tissues [[135], [136], [137], [138]]. Considering the inherent low cellularity and vascularity properties of tendon tissue, treatments for enhancing the biocompatibility of the aligned nanofibers by conjugating connective tissue growth factor or loading a platelet-derived growth factor (PDGF) into the nanofibrous scaffold have been made, with significantly promoted tenogenic differentiation of adipose-derived stem cells (ADSCs) by simultaneously providing aligned topographic cue and PDGF chemical cue to the attached ADSCs [139, 140].
Besides stimulating the healing process of damaged tendon, the bioactive scaffolds used in tendon tissue engineering should also have the ability to resist undue strains [141]. To mimic the nonlinear stiffening behavior of crimped collagen fibrils that provide mechanical support, as well as the highly anisotropic structure of tendon tissue, an aligned electrospun nanofibrous bundle with a crimped structure was developed by treating the aligned nanofibers collected from rotating mandrel with ethanol [85]. The crimped nanofibers exhibited many of the mechanical characteristics of native tendon tissues. Specifically, the crimped nanofibers obtained by ethanol treatment have a yield strain comparable to native tendon, and may therefore be a better choice for tendon and soft connective tissue repair. However, the maximal tensile strength of the crimped nanofibers was only 20 MPa, restricting their application for tendon regeneration due to the load-bearing nature of tendon tissues. Thus, to improve the mechanical resilience of the nanofibrous scaffolds, Domingues et al. incorporated cellulose nanocrystals (CNCs) in the aligned electrospun PCL nanofibrous bundles to reinforce the mechanical properties of PCL nanofibers to tendon relevant range (σ = 39.3 ± 1.9 MPa and E = 540.5 ± 83.7 MPa). The aligned and reinforced nanofiber bundles promoted a remarkable uniaxial cell orientation and induced elongated cell morphology [142].
However, the above mentioned aligned nanofibers still face challenges of limited cell infiltration and lack of dimensions, which hinder the widespread application of electrospun aligned nanofibrous scaffolds in regenerative medicine. To solve these issues, the electrospun aligned nanofiber yarn-based woven textiles in 3D have been fabricated from the electrospun aligned nanofiber yarns using textile techniques such as braiding and weaving (Fig. 4A) [31, 143]. The obtained nanofibrous woven scaffold with significantly larger pore size and obviously enhanced tensile mechanical properties (Fig. 4B) could significantly enhance cell proliferation and infiltration, along with the expression of tendon-specific genes by hADSCs, as compared with those on randomly-oriented or aligned nanofiber meshes. Therefore, the electrospun aligned nanofiber yarn-based 3D nanofibrous woven scaffold holds great potential to synergize the multiple cell interaction and mechanical stimulation for promoting tendon regeneration (Fig. 4C) [31, 143].