Introduction
Organ-on-a-Chip has recently emerged as a new paradigm in enhanced cell culture [1]. The field builds on almost 25 years of developments in microfluidic and associated microfabrication techniques on the one hand and an urge towards ever more physiologically relevant cell culture on the other hand 2, 3. Application of microengineering techniques in cell culture enables the use of flow and associated sheer stress, mechanical strain and allows integration of sensors and systems such as, sample preparation aspects, automated dosing and dilution series preparation. It also facilitates co-culture, 3D culture and application of controlled gradients.
Earliest work in microfluidic cell culture appeared around the turn of the century and includes perfused Transwell systems, multi-organ systems and 3D liver tissue 4, 5, 6, 7. Although many applications have been developed over the last 15 years, it was not until the paradigm shifting Lung-on-a-Chip publication of the Ingber group in 2010 that one could identify Organs-on-Chips as a field in its own right [8]. Since then, the field has expanded tremendously, both in terms of academic publications as well as commercial offerings.
In our 2015 review article, we concluded that the field is currently shifting from a technology focus, aiming to develop prototypes and concepts, towards a biology focus, whereby validation of culture systems and integration of state-of-the-art stem cell and cell culture techniques are key [9]. With this transition towards an application focus, the question poses itself: what efforts are ongoing to promote end-user adoption?
In this critical review, we attempt to take an end-user perspective on Organ-on-a-Chip developments and make an inventory of instrument compatibility, ease of handling, and adoption readiness aspects. In addition, we consider the type of assays that are typically carried out in, or on samples from, these systems, providing insight in the spectrum of techniques that can be deployed for assessing biological properties and responses, and to answer biological, clinical or pharmacological questions.
Overview
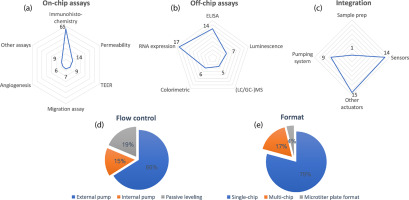
In this review, we catalogued 77 research articles containing the keywords (Organ-on-a-chip) OR (“Organ on a chip”) OR (“microfluidic” AND “cell culture”), which appeared since 2014 on PubMed. Papers that were not found with the search string, but were known to the authors as highly relevant were added to the database. The articles were categorized according to on-chip and off-chip assays, integration aspects, flow control and format in Figure 1 and Supplementary Info.
 Figure 1. Overview of assays and usability aspects of Organs-on-Chips since 2014. (a–c) Relative scores for the frequency of assays and integrations in Organs-on-Chips. On-chip assays: Immunohistochemistry scored the highest followed by permeability. Off-chip assays: RNA expression had the highest score, followed by ELISA. Integration: Other actuators and sensors scored the highest. (d) The distribution of different mechanisms of flow control in Organ-on-a-Chip. More than half of the developed microfluidic models had external pumps. (e) The distribution of different formats: The majority of Organ-on-a-Chip models is comprised of single chip concepts.
Figure 1. Overview of assays and usability aspects of Organs-on-Chips since 2014. (a–c) Relative scores for the frequency of assays and integrations in Organs-on-Chips. On-chip assays: Immunohistochemistry scored the highest followed by permeability. Off-chip assays: RNA expression had the highest score, followed by ELISA. Integration: Other actuators and sensors scored the highest. (d) The distribution of different mechanisms of flow control in Organ-on-a-Chip. More than half of the developed microfluidic models had external pumps. (e) The distribution of different formats: The majority of Organ-on-a-Chip models is comprised of single chip concepts.Although articles referenced in this paper describe many aspects of Organ-on-a-Chip systems, we have chosen to focus solely on usability and compatibility aspects of the solutions proposed. Physiological relevance of the various systems has been extensively reviewed elsewhere 1, ••9, 10, 11.
Figure 1a and b show spider graphs of assays performed in Organs-on-Chips, categorized into on-chip and off-chip assays. On-chip assays include immunohistochemistry, permeability, trans epithelial electric resistance (TEER), migration assays, angiogenesis and other assays (e.g. calcium imaging, colorimetric and luminescence). Off-chip assays consist of enzyme-linked immunosorbent assays (ELISA), luminescence, liquid/gas chromatography–mass spectrometry ((LC/GC-) MS), RNA expression and colorimetric assays. Immunohistochemical staining is the dominant on-chip analysis technique. Almost all publications used immunohistochemical staining to characterize the physiology of their tissue or organ models. We assume that also phase-contrast microscopy is generically used for on-chip assessment of cell morphology and confluence during culturing, however we omitted this from our analysis as it is usually not used as an endpoint or quantified analyses.
RNA expression analysis and ELISA are often used for assessing cellular responses to flow, co-culture or drug compounds. Although very well possible to perform such techniques on chips, in our analysis we find PCRs and ELISA to be exclusively performed off-chip. Although being a highly generic analysis technique, (LC/GC-) MS is used as a readout for Organs-on-Chips only by few 12, 13, 14, 15, 16.
Off-chip assays have the benefit that they are readily available and standardized. However, a disadvantage arises in conjunction with microfluidic chips. Cell culture volumes are typically quite small and dead-volumes in comparison are large. This renders the signal-to-noise ratio low in comparison to classical cell culture techniques. This problem is largely solved by performing assays on the chip. It is for this reason that immunohistochemical staining and other optical readouts are highly popular. Not only is their implementation relatively straightforward, the microfluidic environment also assures excellent imaging quality. Other on-chip assays are reported less often, as they have the disadvantage that they need to be tailored to the microfluidic environment. This puts higher constraints on the engineering skills of the research team, potentially distracting from biological developments.
Microengineering techniques offer ample opportunities to integrate actuators, sensors and complex fluid handling modules on the same chip (see Figure 1c). In recent Organ-on-a-Chip publications, this is predominantly done for sensors and actuators. The trend to induce or measure mechanical strain has led to a relatively large number of publications that use actuators other than for integrated pumping 17, ••18, ••19. Chip-integrated sensors are predominantly electrochemical sensors 20, 21, 22. Relatively few number of papers demonstrate integration of sample preparation aspects to improve the quality of the readout of assays of cell cultures [14].
Since the human physiology consists of a dynamic environment, flow control is a crucial requirement of Organs-on-Chips. While microengineering techniques offer ample precedents for integration of pumps on the chip, the majority of publications make use of external pumps to drive the flow (see Figure 1d). Although convenient in a proof-of-concept phase, none of the publications researched, showed an easy to use approach for connecting an external pump system by non-expert end-users. Passive levelling on the other hand is a very simple technique that is becoming rapidly more popular to drive flow in microfluidic systems 13, 23, 24, •25, ••26. Although very simple to use for the non-expert end-user, the bidirectionality of the flow is seen as a disadvantage by some. Integration of pumps is an alternative solution to this 12, 27, 28, 29, 30, 31.
Strikingly, most publications show Organ-on-a-Chip concepts on single chips, although it is crucial for end-user acceptance to include dilution series, replica's and positive and negative controls (see Figure 1e). A small fraction incorporates multiple microfluidic networks on a single chip and yet in exceptional situations the microtiter plate standard is adopted to enable compatibility ••26, •32.
Usability aspects
The usability aspects of microfluidic devices relate to aspects of compatibility to existing equipment, automation, ease of handling, possibility to generate multiple data points amongst others. We describe here some selected papers that we feel made particular progress on one or more of these aspects. For example, Birchler et al. created an open format hanging-droplet system for microfluidic handling, culturing of single cells and microtissue spheroids in multiple culture compartments (Figure 2a) [33•]. Noteworthy, their system was compatible with fluorescence-activated cell sorting (FACS) to directly sort cells, without the need of intermediate steps, into the desired microfluidic culture compartments. Cells were sorted corresponding to their light-scattering characteristics, which enabled the separation of single cells from cell clusters and assess the biological structure of cells during loading. The open microfluidic chip of Birchler et al. gives an agreeable illustration of a system that is both high-throughput, compatible and can be coupled with standard cell biologytools.
 Figure 2. | Selection of Organ-on-a-Chip systems with particularly interesting usability aspects. (a) Hanging-drop chip network for loading, culturing and harvesting stem cells and microtissue spheroids. This system is compatible with optical imaging and fluidic pumps and was integrated with a FACS machine to accurately and directly sort cells into compartments of the microfluidic network [33•]. (b) The OrganoPlate®, a 384-well microtiter plate format that comprised 96 microfluidic networks for high-throughput assays. A capillary pressurebarrier enables to pattern the extracellular matrix while leaving half the chamber for culture medium in a “membrane-free” manner. Microfluidic flow was induced by passive levelling [26••]. (c) Microfluidic device by Theberge et al. for parallel angiogenesis assays in co-culture. Flow was facilitated by passive pumping [32•]. (d) Microphysiological device with eight independent wells. The device was fully 3D printed and integrated with a strain sensor for continuous electronic readout of cardiac tissue contractions [18••].
Figure 2. | Selection of Organ-on-a-Chip systems with particularly interesting usability aspects. (a) Hanging-drop chip network for loading, culturing and harvesting stem cells and microtissue spheroids. This system is compatible with optical imaging and fluidic pumps and was integrated with a FACS machine to accurately and directly sort cells into compartments of the microfluidic network [33•]. (b) The OrganoPlate®, a 384-well microtiter plate format that comprised 96 microfluidic networks for high-throughput assays. A capillary pressurebarrier enables to pattern the extracellular matrix while leaving half the chamber for culture medium in a “membrane-free” manner. Microfluidic flow was induced by passive levelling [26••]. (c) Microfluidic device by Theberge et al. for parallel angiogenesis assays in co-culture. Flow was facilitated by passive pumping [32•]. (d) Microphysiological device with eight independent wells. The device was fully 3D printed and integrated with a strain sensor for continuous electronic readout of cardiac tissue contractions [18••].Similarly, Wevers et al. reported 3D culturing of neuronal and glial cells in a high throughput 3D cell culture platform using a modified 384-well plate to create 96 independent microfluidic networks for 3D cell culture (Figure 2b) [26••]. The platform employs passive levelling for fluid exchange and 175 μm thin glass for enhanced microscopic imaging. The microtiter plate format renders the platform fully compatible with standard microscopes, automated readers and robot handling, an aspect that was utilized by Wevers et al. for generating dose response curves to toxic compounds. Moreno et al. also used the same platform to differentiate human iPSC-derived neuroepithelial stem cells into functional dopaminergic neurons [24]. Jang et al. used a similar platform for studying drug induced liver injury [34]. The fact that three different universities report use of the platform is a strong indication of transferability of the concept.
Another study reported a chip system for studying angiogenesis (Figure 2c) [32•]. The system consists of an array of 14 microfluidic networks perfused by passive pumping. Each microfluidic network has two main microfluidic channels that are interconnected through microchannels that are substantially lower than the main channels (30 μm vs. 330 μm) enabling exchange of soluble signalling molecules between cells grown in the two separated channels. The authors studied the effects of antiangiogenic factor MMP12 and pro-angiogenic factors secreted by macrophages on endothelial tubule formation. Pipette operation makes the microfluidic device simple to use and although not demonstrated in the publication, the system seems easily made compatible with liquid handling systems.
Lind et al. used 3D printing to develop an assay concept for measuring contractility of cardiac tissue (see Figure 2d) [18••]. The system has integrated soft strain gauge sensors to measure contractile stresses of multiple cardiac micro-tissues. Each device is composed of eight independent wells with multilayer cantilevers, a base layer, an embedded strain sensor, a tissue-guiding layer and electrical interconnects for readout. The tissue-guiding layer promotes the self-assembly of physio-mimetic laminar cardiac tissues. The system developed by Lind et al. enables non-invasive real-time electronic readout of contractile properties inside a cell incubator and can be used to study dose-response of drugs that influence contractile strength or beat rate.
An example of a more complex system is reported by Ramadan et al. [35]. This device contained three parallel cell culture chambers and Ag/AgCl electrode wires to measure TEER of the human skin in vitro under perfusion of culture media. The barrier integrity of human keratinocytes was measured in co-culture with monocytes. A chip comprised three such devices, although due to external pump and open electrode wire connections, the operation of the device is primarily reserved for expert end-users.
Functional assays in Organ-on-a-Chip systems
In vitro microcirculation, induced by flow, is important to mimic organ physiology. Kim et al. demonstrated this with angiogenesis assays in their three-dimensional culture model [36] (see Figure 3a). Blood endothelial cellswere cultured in a microfluidic platform and vasculogenesis was stimulated by fibroblast-secreted pro-angiogenic factors and flow-mediated mechanical stresses. They found that interstitial flow plays a significant role in the growth of angiogenic sprouts. It can either promote or suppress angiogenic sproutingdepending on the direction of flow (Figure 3a). The control over gradients of angiogenic factors and application of interstitional flow for 3D angiogenic sprouting is a strong example of an assay that can be exclusively done by means of microfluidic techniques.
 Figure 3. | Noteworthy assays in Organs-on-Chips. (a) Angiogenesis model developed by Kim et al., showing directional prejudice in response to flow direction. S–N: Downward direction. N–S: Upward direction [36]. (b) Vascularized micro-tumours and their response to drugs. Human colorectal cancer cell line HCT116 (green) and endothelial cells (red) were visualized by confocal microscopy. The results were translated to IC50 ratios (tumour growth and total vessel length) [38•]. (c) Model of human Airway-on-a-Chip by Benam et al. High-speed microscopic imaging showed the beating activity of the cilia on the epithelium under healthy condition, asthmatic phenotype, COPDexacerbation and drug treatment [39•]. (d) Calcium imaging recordings to detect electrophysiological activity of neurons. This assay was used to evaluate compounds effect in the high-throughput 3D culture system of Wevers et al. [26••].
Figure 3. | Noteworthy assays in Organs-on-Chips. (a) Angiogenesis model developed by Kim et al., showing directional prejudice in response to flow direction. S–N: Downward direction. N–S: Upward direction [36]. (b) Vascularized micro-tumours and their response to drugs. Human colorectal cancer cell line HCT116 (green) and endothelial cells (red) were visualized by confocal microscopy. The results were translated to IC50 ratios (tumour growth and total vessel length) [38•]. (c) Model of human Airway-on-a-Chip by Benam et al. High-speed microscopic imaging showed the beating activity of the cilia on the epithelium under healthy condition, asthmatic phenotype, COPDexacerbation and drug treatment [39•]. (d) Calcium imaging recordings to detect electrophysiological activity of neurons. This assay was used to evaluate compounds effect in the high-throughput 3D culture system of Wevers et al. [26••].Wang et al. illustrated different stages of vascular development in a microfluidic system [37]. This system contained a well-defined hourglass shaped communication pore that pins fibrin gel at its vertexes and permits the formation of an endothelial cell monolayer on the fibrin gel interface. Subsequently, the authors induced vasculogenesis, endothelial cell lining, sprouting angiogenesis, and anastomosis by using an optimized interstitial flow and VEGF gradient. This finally enabled the formation of an intact and perfusable microvascular network. The system was used for co-culture with tumour cells for clinical applications [38•] (see Figure 3b). The impact of drugs was assessed on either the tumour directly or to the microvascular network. The growth and assaying of perfused 3D vascular networks is another example of tissue modelling that is inherently performed in a microfluidic setting.
Benam et al. elaborated on their initial Lung-on-a-Chip work to model small airway epithelium comprised of a co-culture of bronchial epithelium and microvascular endothelium separated by a membrane [39•]. A particularly elegant assay was implemented to measure the beating frequency of cilia in response to stimuli. Figure 3c shows the increase in cilia beating frequency in response to the drug Tofacitinib. The extra value of this work in comparison to classical Transwell system is to be sought in the perfusion of the basal compartment and the imaging, but this goes at the cost of ease of handling and throughput.
Wevers et al. demonstrated 3D culture of iPSC-derived neuronal–glial cells of healthy people and Huntington's disease patients in Matrigel, which they showed and quantified with immunohistochemistry [26••]. They measured cell viability following exposure to several neurotoxic compounds. Particularly interesting was the compound mediated modulation of electrophysiological activity that was visualized by calcium imaging (see Figure 3d).
A visualisation of electrophysiological activity was reported by Müller et al. who developed a CMOS chip with high density electrodes to measure spatial voltage distribution at a 17.5 μm resolution [40]. Each electrode was used to measure the electrical activity of individual neurons. Noteworthy, an elegant indirect readout for electrophysiological activity was reported by Uzel et al. that used photosensitized embryonic stem cells to differentiate into neurospheres and interact with muscle tissue [19••]. Photostimulation or glutamate addition caused the muscle to contract as a consequence of action potentials that could be measured by deflection of microfabricated pillars.
Herland et al. demonstrated a blood-brain barrier, the barrier function thereof could be interrogated by leakage of 3 kDa FITC labelled dextran and calculating the Papp value thereof [41].
Chen et al. reported a microfluidic cell culture chip, made of four microchannelsand Petri dish-based cell medium supply system that was used to measure cell migration [42]. They were successful in screening highly metastatic sublines in their system. Interestingly, to perform parallel cell migration with different modes, Ma et al. created a microfluidic system that combines membrane-based cell migration and droplet-based techniques [43•]. Droplets were adjacently positioned on either side of a membrane, enabling gradient formation that was exploited in migration assays. Consequently, multi-parametric gradients were constructed for metastatic assays. The concept allowed multiple in-droplet operations in the nanoliter range and up to 81 assays in parallel.
Molecular assays in Organ-on-a-Chip systems
Organ-on-a-Chip based models have been extensively analysed with help of molecular assays such as ELISA, RNA expression analysis, probing metabolism and immunohistochemistry 14, 44, 45, 46. A particular extensive analysis was done by Kamei et al. who performed global gene expression analysis on human embryonic stem cells and induced pluripotent stem cells that were cultured in a 3D thermo responsive hydrogel in a microfluidic channel [47].
Patra et al. analysed 5000 3D tumour spheroids using flow cytometry after dissociation of the tumour [25•]. In that context, they could perform single cell analysis which they correlated to tumour size, 2D or 3D culture as well as therapy response based on Calcein AM (healthy) and 7-Amino-ActinomycinD (necrosis) and APC-Annexin V (apoptosis). The assays demonstrate the power of large numbers using flow-cytometry, which is a standard instrument in modern cell biology.
A compatible assay setup was demonstrated by Bavli et al. who could distinguish between cell death, healthy cells and on-set of mitochondrial dysfunction in real-time by measuring glucose, lactate and oxygen [48•]. Oxygen was measured by tissue embedded Ru-CPOx oxygen sensors, while glucose and lactate measured by amperometric detection of glucose and lactate oxidase mediated oxidation of H2O2. Time-resolved sampling was supported by an off-chip microfluidic switchboard. The switchboard also enabled automated calibration of the amperometric sensing scheme. Similar electrochemical recordings were demonstrated by Misun et al. in conjunction with hanging drop-based cell culture [22]. The authors project their setup for real-time monitoring applications of glucose and lactate in Body-on-a-Chip type setups.
A rare example of coupling LC/MS based metabolomics techniques with microfluidic cell culture is given by Filla et al. However, no in depth biological analysis was demonstrated in their report [14].
Although being impressive examples in terms of complexity of the assays, the added value of the microfluidics is still limited in many of the above examples. Kamei et al. did not use any of the flow aspects, while both Patra, Bavli and Kamei et al. did not consider co-culture or other aspects that render 3D cell culture more physiologically relevant •25, 47, •48.
Conclusions and future directions
In this article, we assessed usability, compatibility and assay ability aspects in recent Organ-on-a-Chip publications. We discussed examples of efforts to improve usability, incorporate unique assays, or informative analyses using standard laboratory techniques. However, only in rare examples an Organ-on-a-Chip concept ticks all the boxes and can be considered ready for transfer to an end-user. On the contrary, the majority of publications reported single chip-based models, external pumping and use immunohistochemical stains as primary readout. In the review article of van Duinen et al. we concluded that with maturation of the microfluidics field, the focus in Organ-on-a-Chip studies will shift towards validation of models and integration of newest stem cell techniques [9••]. Thus, the multidisciplinary field will become more and more the realm of biologists. However, with an increasing role of biologists in the field, attention for usability aspects, throughput and compatibility is critical. Moreover, compatibility with the full width of biochemical analysis techniques is crucial in order to enhance end-user adoption and full validation of the models. Last but not least, availability of these systems, either in commercial form, or at least producible in significant numbers becomes critical, enabling the end-user to perform his/her optimisation research.
We envision that in coming years, the end-user aspects will dominate engineering aspects in the Organ-on-a-Chip field and that commercial providers will be playing an increasingly dominant role. The availability of easy-to-operate, mass produced systems will enable end-users to focus on what they do best: excellent biology, validation of the models and screening for better medicines. And this will require the availability of proper molecular or physiological readouts to answer clinical, biomedical or biological questions.