1. Introduction
Graphene is a single sheet of an atom thickness having sp2 hybridized carbon atoms, arranged in a honeycomb like lattice [1]. In this lattice like structure, each carbon atom is attached to another carbon atom in the same plane viacovalent carbon/carbon bond. While the interlayers are arranged through weak Van der Waal forces. These forces are responsible for softness of this material. Presence of aromatic structure, free π-π electrons and reactive sites on the periphery are the reasons for its diverse usage [2], [3].
Graphene exists in many forms such as graphene sheets, graphene oxide (GO) and reduced graphene oxide (rGO) [4], [5], [6]. Properties which makes GO a material of choice in the field of biomedicine especially theranostics include its biocompatibility and biodegradability [7], [8], its large surface area (2630 m2/g approximately) [9] and high aspect ratio for modifications [10], its un-matched thermal conductivity i.e. 5000 W/m/K [11], its tendency to disperse well in aqueous medium, its better colloidal stability compared to other carbon based materials [12], its capability of traversing the plasma membrane and its cost effectiveness and scalability [13].
Existing challenges for successful commercial applications of GO include reproducibility of the functionalized GO layers or composites [14], [15] and limited and/or contradictory data available on in vitro and in vivo toxicity of graphene as biomaterial [16], [17], [18] However, despite all the hurdles and difficulties in capping a suitable system for biomedical usage, GO has 62.6% research weightage towards biomedical applications in contrast to its non-medical usage [19], [20], [21], [22]. Many of these research products ranging from synthesis procedures to their real-time applications are being patented thereby signifying their potential usage in everyday life [23], [24], [25], [26], [27], [28].
There are two kinds of modifications that are generally carried out to functionalize GO nano-sheets namely, covalent and non-covalent modifications [29], [30]. Covalent functionalization occurs due to mutual sharing between adjoining chemical moieties whereas non-covalent modifications include electrostatic, hydrophobic, physisorption, hydrogen bonding and π-π stacking. Presence of various functional groups such as epoxide, hydroxide and carboxyl groups provide endless possibilities to tailor covalent linkages to make desired system [31], [32]. Both these modification techniques have been employed with variations and both have their own downsides. For example non covalent interactions are weak therefore show instability to external environment in vitro and in vivo while covalent modifications allow less quantity of drugs (aromatic) to be uploaded since GO sheets are also occupied by the coated polymers or other functional moieties [33], [34], [35], [36].
1.1. Graphene oxide composites
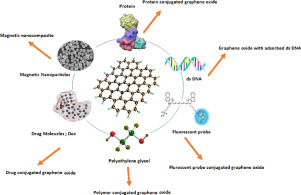
Remarkable properties of GO are mainly associated with chemical modifications & its combine effect with various entities such as polymers and magnetic nanoparticles (Fig. 1). Since GO tends to aggregate under the physiological conditions (due to the presence of salts, ions and proteins) thereby reducing the proposed effectiveness, these modifications not only help retain its effectiveness but also reduce toxicity of the other component.
 Fig. 1. Depiction of modifications that can be made to modify GO.
Fig. 1. Depiction of modifications that can be made to modify GO.Most of the composites include chemical moieties that provide biocompatibility (e.g. poly ethylene glycol (PEG), poly vinyl chloride (PVC)) [37], [38], [39], thermo/stimuli responsiveness (e.g. poly (N-isopropylacrylamide) (PNIPAM) [40], [41], enhance mechanical properties (PMMA, PVC) [42], [43], [44], used for the surface coating of the biomaterials (e.g. dextran, polyamide 11) [45] and enhance colloidal stability (Sulfonic acids, Oleylamine) [46] (Fig. 2).
 Fig. 2. Schematic illustration demonstrating strategies for the use of graphene based materials for theranostics. Adapted with permission from Ref. [47].
Fig. 2. Schematic illustration demonstrating strategies for the use of graphene based materials for theranostics. Adapted with permission from Ref. [47].Therefore, a lot of research highlights the use of GO in drug delivery application [35], [48], [49], magnetic resonance imaging (MRI) [50], [51], [52], fluorescence imaging [53], antibacterial activity [54], biosensors [55], [56], [57], [58], [59], [60] and hyperthermia [61], [62]. Despite of all this available literature, there is a dearth of knowledge towards GO based magnetic nanocomposites and its role in theranostics [63], [64].
This review entails the compilation of studies carried out on the GO composites used in theranostics from 2010 to 2017 (Fig. 3). In contrast to previous studies, this information is sorted and lately discussed to evaluate the potential challenges and advantages of using GO based magnetic nanocomposites for theranostics.
 Fig. 3. Tabulated illustration of theranostic based applications of GO composites.
Fig. 3. Tabulated illustration of theranostic based applications of GO composites.2. Graphene oxide for theranostics (therapeutics and diagnostics)
2.1. Drug delivery applications of graphene oxide (therapeutics)
Maintaining the efficacy of therapeutic drugs is a major driving force behind drug delivery research. In order to maintain the efficacy of drugs, long term sustained release of drug through blood circulation is required. SN 38 is considered as water insoluble drug and its dispersion is an issue which hampers its potency to attack colon cancer cells. Dai et al., used PEG conjugated graphene nanosheets with non-covalent adsorption of SN38 [38]. This non-covalent adsorption was driven by hydrophobic interaction and π-π stacking. Through this system, they were able to attain controlled release along with high potency i.e. IC 50 value 6 nM for Human Colon Cancer Cell line (HCT cells) in comparison to its pro-drug, a hydrophobic analogue Camptothecin (CPT-11). Further this group used PEG-NGO for the targeted delivery of Rituxan (CD 20 antibody) and Doxorubicin (DOX) (Fig. 4). This system exhibited pH dependent drug release [65].
 Fig. 4. A schematic illustration of doxorubicin (DOX) loading onto NGO–PEG–Rituxan via π-stacking. Adapted with permission from Ref. [65].
Fig. 4. A schematic illustration of doxorubicin (DOX) loading onto NGO–PEG–Rituxan via π-stacking. Adapted with permission from Ref. [65].Zhang and colleagues tested the ability of graphene nanosheets to carry multiple anticancer drugs at a time. This approach was one of its kind and significant to reduce drug resistance occurring for many cancer treatments thereby reducing their efficacy over time. They used NGO functionalized with sulfonic acid groups which was decorated later with folate (FA) receptors through covalent binding. FA receptors enable direct uptake of drugs which were loaded to FA- NGO matrix. The loading capacity of Dox and CPT drugs was equal to their single loading. Although therapeutic efficacy was increased but cytotoxicity to Human breast cancer cell line (MCF-7) was reduced. Efficacy was increased due to its specificity to deliver drug only to cancer cells [66].
Following the pursued, Kakran et al., functionalized GO with hydrophilic and biocompatible polymer such as Tween 80, Pluronic F38, maltodextrin and the functionalized GO was further used as a nanocarrier for poorly water soluble anticancer drug, ellagic acid (EA). For the very first time EA was loaded onto functionalized GO using π-π interaction. Release kinetics and cytotoxicity of the loaded drug formulation was evaluated at various pH. This functionalized GO carrying EA was further tested to target MCF-7and human colon Adenocarcinoma cells (HT29) [67]. It was established that GO did not hamper the antioxidant activity of loaded EA.
Another research group led by Yang et al. in 2011 has explored the drug carrying capacity of graphene sheets through dual target functionalization and pH sensitivity. In this research surface of GO sheet was functionalized with targeting ligand FA receptors and super paramagnetic iron oxide nanoparticles(Fe3O4). Multiple functionalized GO was able to demonstrate targeted and pH responsive drug delivery. In this setting, GO was decorated with Fe3O4 while 3-aminopropyl triethoxysilane (APS) was use to coat Fe3O4. This coating served as mediator to attach FA to GO-Fe3O4 composite [68].
Hybrid graphene nano-sheets with chitosan showed improved solubility in acidic medium and such functionalized hybrid showed marked controlled drug release behaviour. The drugs tested in this hybrid system were Ibuprofen and 5-fluorouracil. Microscopic techniques such as SEM and AFM were used to scan the topographic features of the functionalized graphene sheets (Fig. 5). It was first report on the adsorption of aromatic moiety containing drugs tested on graphene sheets and concluded that FGOCs has better cellular penetration and hence has better chances of success in its use in drug delivery [69].
 Fig. 5. Schematic illustration of synthesis of the FGOCs and the dispersion of (a) GO and (b) the FGOCs in an aqueous acetic acid solution (CH3COOH/H2O 0.2/1). Adapted with permission from Ref. [69].
Fig. 5. Schematic illustration of synthesis of the FGOCs and the dispersion of (a) GO and (b) the FGOCs in an aqueous acetic acid solution (CH3COOH/H2O 0.2/1). Adapted with permission from Ref. [69].Controlled release formulation has been made using biocompatible GO and biodegradable chitosan. In this formulation, GO was loaded with Dox and lately encapsulated with FA conjugated chitosan [70]. This formulation had shown its immense potential as targeted and control delivery nanocarrier as this formulation was sensitive to acidic environment.
In another study, GO was grafted via facile amidation reaction with chitosan (CS). This CS-g-GO was tested for its drug & gene delivery potential. It was concluded that the CS-g-GO was able to carry CPT which resulted in higher toxicity towards HepG2 and HeLa cell lines than the pure drug (Fig. 6). Similarly, gene delivery potential was illustrated through the delivery of CS-g-GO with plasmid DNA in stable and complexed form into the HeLa cell lines [71].
 Fig. 6. Schematic of the pH-responsive association behaviour of the amphiphlic GO–CS nanocomposites. Adapted with permission from Ref. [71].
Fig. 6. Schematic of the pH-responsive association behaviour of the amphiphlic GO–CS nanocomposites. Adapted with permission from Ref. [71].Effect of AuNPs on drug delivery was investigated where AuNPs were grown in-situ on the GO sheets. This nanocomposite consisting of AuNP/GO showed efficient drug delivery in the Hela cell lines. Such nanocomposite can also be exploited towards intracellular Raman Imaging [72], [73], [74].
In 2012, Kurapati used GO along with LBL microcapsules (poly(allylamine hydrochloride) (PAH) where the composite was stimuli responsive towards near infrared light. The GO-PAH microcapsule released encapsulated drug Dox in a point wise fashion upon Near Infrared Radiation(NIR)-laser ablation. This laser ablation generates local heating effect which in turn leads to the release of drug from microcapsule. In addition to excellent optical and permeable properties of GO, it has also enhanced the mechanical strength of the microcapsule thereby preventing its breakage during intracellular delivery. Following images (Fig. 7) give a clear illustration how this microcapsule release drug in point wise fashion [74].
 Fig. 7. Illustration of the remote opening of GO–polymer composite capsules using NIR-laser light. Adapted with permission from Ref. [74].
Fig. 7. Illustration of the remote opening of GO–polymer composite capsules using NIR-laser light. Adapted with permission from Ref. [74].GO has not only been used as stimuli responsive drug delivery system but also for gel based drug delivery system. Due to recent emphasis on the use of gel matrices for the delivery of drugs such as doxorubicin hydrochloride, CPT, 5-fluorouracil, paclitaxel, cisplatin and adriamycin, exploiting the strength of novel materials is of significant importance. In order to attain these gel formulations various polymers are generally used through physical and chemical cross linking in addition to the use of chemical cross linkers such as photo initiators are used. These species though contribute good mechanical strength but are deemed inappropriate towards biomedical applications. In a recent research, GO has been used to encapsulate doxorubicin hydrochloride through gel matrix. Edge of this research was that none of the polymers or chemical matrix was used except GO which was used for in situ gelation effects (Fig. 8). Doxorubicin was released in a sustained released manner. GO-Dox gel exhibited good mechanical strength and good inject ability [75]. Another aspect of the use of this GO based hydrogel is their capacity to self-heal, carry various biomolecules (DNA) or dyes [76], [77].
 Fig. 8. Photographs for the formation of the gel matrix based on 6 mg/mL GO nanosheets and 2 mg/mL DOX. Adapted with permission from Ref. [76].
Fig. 8. Photographs for the formation of the gel matrix based on 6 mg/mL GO nanosheets and 2 mg/mL DOX. Adapted with permission from Ref. [76].In another remarkable work exploiting drug carrying capacity of GO, Yang et al., used the principle of multiple supramolecular assembly to create a GO scaffold for drug deliver [78] (Fig. 9). In this approach three components were used 1) folic acid modified β cyclodextrin, a target unit 2) adamantanyl porphyrin as linker and 3) GO as carrier unit. It was observed that due to the presence of folic acid modified β cyclodextrin GO nanocarrier could recognize FA receptors on cancer cells thereby increasing the effectiveness of Dox in comparison to free Dox.
 Fig. 9. Schematic illustration of Synthesis of 1/2/DOX/GO from graphene oxide, DOX, adamantane-modified porphyrin, and folic acid modified cyclodextrin. Adapted with permission from Ref. [78].
Fig. 9. Schematic illustration of Synthesis of 1/2/DOX/GO from graphene oxide, DOX, adamantane-modified porphyrin, and folic acid modified cyclodextrin. Adapted with permission from Ref. [78].In an attempt carried out by Wu et al., it was demonstrated that the effects of drug resistance in breast cancer cells can be avoided using GO loaded with Adriamycin. Adriamycin was physically loaded onto the GO sheets. This GO-ADR caused effective reversal of ADR resistance in MCF/ADR cells with reversal index of 8.35 [79], [80]. Similarly, in another study, dual usage of GO platform was exploited where [81] authors used the ability of GO to carry single stranded DNA/RNA with ease and its loading capacity towards the anti-cancer drugs through π-π interaction was investigated.
Bcl-2 which is considered to be an important anti-apoptotic defence protein which leads to multiple drug resistance (MDR) [82]. This drug resistance can be avoided by knocking down the protein's expression where the role of siRNA is extremely important since knockdown of Bcl-2 expression will not only inhibit MDR but also make cancer cells sensitive towards the anti-cancer drugs. Chemically grafted GO with polyethylenimine (PEI) was used as a nanocarrier to Dox and the Bcl-2 targeting siRNA. This study showed that the PEI-GO can effectively be used as a nano-carrier and for the sequential delivery of Bcl-2 targeted siRNA. This dual effect led to the significantly enhanced chemotherapeutic efficacy. In another study, GO coupled with PEG-FA was used to carry hTERT siRNA for the intracellular delivery of siRNA [83]. In this research, GO was conjugated with PEG-FA in order to make it bio-compatible and selective. In addition to this siRNA was loaded onto the graphene sheets with the help of 1-pyrenemethylamine hydrochloride through π-π stacking. This GO-PEG-FA-PyNH2 carried HTERT siRNA led to significant silencing of mTRET expression in HeLa cell lines. This was confirmed through RT-PCR and the western blotting (Fig. 10).
 Fig. 10. The preparation of functionalized graphene oxide for targeted intracellular delivery of siRNA. Adapted with permission from Ref. [83].
Fig. 10. The preparation of functionalized graphene oxide for targeted intracellular delivery of siRNA. Adapted with permission from Ref. [83].In another study, hybrid of PEI modified GO with oleic acid was created which was further modified by up conversion nanoparticles (UCNP) and superparamagnetic nanoparticles. This hybrid (PEI-GO) was used as nano carrier of hydrophobic nanoparticles and resulted in the transferring of hydrophobic nanoparticles from organic phase to a water-soluble phase. PEI-GO-UCNP hybrid exhibited 100% weight loading of Dox drug. This drug carrying hybrid showed higher killing potential towards cancer cells in in vitroenvironment (Fig. 11) complimented by the luminescence properties due to the UCNP [84].
 Fig. 11. (a and b) Photographs of the phase transfer of OA-coated UCNPs green from chloroform to water. The top layers in (0 and (b) is pure water, and PEI-GO aqueous solution (0.2 mg/ml), respectively; (c) Upconversionluminescenceintensity (k = 541 nm) of PEI-GO-UCNP green in aqueous phase and OA-coated UCNPs green in chloroform during the process of phase transfer. Adapted with permission from Ref. [84].
Fig. 11. (a and b) Photographs of the phase transfer of OA-coated UCNPs green from chloroform to water. The top layers in (0 and (b) is pure water, and PEI-GO aqueous solution (0.2 mg/ml), respectively; (c) Upconversionluminescenceintensity (k = 541 nm) of PEI-GO-UCNP green in aqueous phase and OA-coated UCNPs green in chloroform during the process of phase transfer. Adapted with permission from Ref. [84].In a recent study, Szunerits et al., demonstrated that the composite of magnetic nanoparticles coated with 2-nitrodopamine and GO could be used for the effective delivery of insulin without damaging the native state of insulin in the acidic environment. Loading capacity of GO and GO composite was extremely high for insulin where 100 ± 3% was loaded on to GO sheet while 88 ± 3% was loaded on to GO-MPdoxmatrix. Insulin loaded onto GO-MPdox nanomatrix was protected from gastric secretions and acidic environment while drug was released once exposed to basic environment (pH = 9.2) [85]. The drug delivery potential and effectiveness of GO based composites can be increased using various preparation technique. One of the studies which evaluated the difference in the efficiency of GO composites synthesized using in situ and ferro-fluid techniques. In this study it was exhibited that the GO carrying iron oxide nanoparticles in the form of ferrofluid demonstrated higher toxicity towards the MCF-7 cell lines due to higher iron content, higher loading efficiency of the drug (Anastrazole) and smaller particle size [86].
Recently GO has also been conjugated with targeted peptide of chlorotoxin(CTX) through 1-ethyl-3-(3-dimethyaminopropyl) carbodiimide (EDC) and NHS. This CTX-GO was further loaded with Dox through non-covalent interactions. This CTX-GO-DOX showed significant improvement towards the treatment of gliomas (Fig. 12). Conjugated GO was not only able to deliver drug specifically to the glioma cells but has also proved to maintain sustained release [87].
 Fig. 12. Schematic illustration of the preparation of CTX-GO/DOX. Adapted with permission from Ref. [87].
Fig. 12. Schematic illustration of the preparation of CTX-GO/DOX. Adapted with permission from Ref. [87].In another recent study, GO was deposited inside a conducting polymer, poly pyrrole (PPy) properties (Fig. 13). These electrical properties were further used for the release of dexamethasone which was loaded on a conducting nanocomposite [88].
 Fig. 13. Drug loading into and release from the GO/PPy nanocomposite. Schematic representation of the (a) GO/PPy-DEX nanocomposite and (b) DEX release from the GO/PPy nanocomposite in response to electrical stimulation. Adapted with permission from Ref. [88].
Fig. 13. Drug loading into and release from the GO/PPy nanocomposite. Schematic representation of the (a) GO/PPy-DEX nanocomposite and (b) DEX release from the GO/PPy nanocomposite in response to electrical stimulation. Adapted with permission from Ref. [88].2.2. Bio-sensing applications of graphene oxide (diagnostics)
GO has been exploited as an essential material for bio-sensing applications since electron donor & acceptor molecules are exposed at planar surface making GO an efficient candidate for long range quenching [89], [90], [91].
In a study, it was established that COOH modified GO has an intrinsic peroxidase activity which can extend its use for glucose detection. In this report, catalytic property of GO was investigated through the use of peroxidase substrate (3,3,5,5-tetramethylbenzidine (TMB). GO-COOH has high catalytic ability compared to the naturally occurring Horse reddish peroxidase (HRP) enzyme. This ability of GO-COOH has been exploited for highly selective glucose detection. The level of glucose detected was as low as 1 × 106 mol L− 1 while its linear range of the detection was 1 × 106 mol L− 1 to 2 × 105 mol L− 1 [92], [93]. GO-COOH was found to be not only highly selective but also has advantages such as ease of preparation, low cost and stability. Based on the similar intrinsic peroxidase activity of the GO, it has been further exploited for the immunosensing towards Prostate Specific Antigen (PSA). In this approach, magnetic beads were modified with anti-PSA antibody (Ab1) while GO was modified with Ab2 making the entities an immunocomplex, sandwiching antigen protein PSA. Once the reaction was complete, magnetic beads were removed while the concentration of Ab2-GO was calculated due to colour change (Colorimetric), exhibiting as if it reacts with hydroquinone and H2O2. This study established evidence that GO can be used as selective and point of care tool for clinical diagnosis.
Similarly, in another study, it was reported that the intrinsic peroxidase like property of GO can be enhanced once it was decorated with iron ferrites (Fe3O4) [94]. Kinetic parameters of the study supported that the catalytic activity was enhanced under the provided settings. Following table exhibits the improvement made in the peroxidase activity of the GO based ferrites composite (Fig. 14).
 Fig. 14. Comparison of the apparent Michaelis–Menten constant (Km) and maximum reaction rate (Vm). Adapted with permission from Ref. [94].
Fig. 14. Comparison of the apparent Michaelis–Menten constant (Km) and maximum reaction rate (Vm). Adapted with permission from Ref. [94].In a continuation, another study demonstrated the use of nanocomposite prepared from rGO and cobalt ferrites which exhibited higher reaction and subsequently higher catalytic (peroxidase) activity towards the substrate (TMB). This composite was prepared by the use of PVP as reducing agent and stabilizerand showed not only higher catalytic activity but also higher stability in the presence of different solvents and at various temperatures. However, at optimum conditions, the detection limit of this nanocomposite was 0.3 μM which was higher than many other ferrites based colorimetric sensors [95]. It has also been demonstrated in number of recent reports that both Iron, platinum [96] and cobalt [97], [98] based composites with GO having the ability to mimic enzyme like activity are likely to play an important role in biotechnology (Fig. 15) and environmental detection [99]. Recently Mn ferrites have also been explored for the colorimetric detections/peroxidase like activity [100]. However, composites of GO with Mn ferities for peroxidase activity are still to be explored.
 Fig. 15. Schematic Representation of Colorimetric Detection of Cancer Cells by Using Folic Acid Functionalized PtNPs/GO. Adapted with permission from Ref. [96].
Fig. 15. Schematic Representation of Colorimetric Detection of Cancer Cells by Using Folic Acid Functionalized PtNPs/GO. Adapted with permission from Ref. [96].Some of the fascinating applications of graphene nanocomposites reported so far include its use in DNA sensing [89], [101], protein sensing, and protein assays. To explore the ability of GO as molecular probe in situ and in vivo, Wang and coworkers investigated graphene nanosheet and aptamer with carboxyfluroscein (FAM/GO-nS) where graphene nanosheet was used as sensing plate and aptamer as molecular probe [89], [102]. Since aptamers have specificity and sensitivity for the target, the target in this case was a fungi toxin (Ochratoxin A) secreted by Aspergillus ochraceus and Penicillium verrucosumm. In this case Adenosine triphosphate (ATP) aptamer was used which was non-covalently bonded to GO nanosheets. It was demonstrated that GO sheets have the ability to protect aptamer/DNA molecule from enzymatic cleavage activity and also leads to fluorescence quenching. Both these properties make it a strong contender for DNA/RNA/protein cargo for gene delivery and for bio-labelling for cellular imaging respectively.
Luo et al., in 2012 reported a convenient and enhanced chemiluminescence (CL) biosensor for sequence specific DNA detection. It was previously known that GO has fluorescence quenching property and was presumed that it will have CL property too. In this study, human immunodeficiency virus (HIV) oligonucleotide sequence associated with the HMDNAzyme (PHIV) was used as a model probe. This probe (ssDNA) once mixed with GO was assumed to be adsorbed to GO surface through π-π interaction between ssDNA nucleoside/nucleotides and GO. Since HMDNAzyme stimulates CL in the presence of luminol and H2O2 [102] and the probe ssDNA assumed to hybridize with the complementary target DNA, the presence of dsDNA assumed to be released from the GO causing significant increase in CL emission. This system was further suggested to have potential for sequence specific detection not only for DNA but for other biomolecules as well (Fig. 16).