1. Introduction
Pyrite is the most abundant sulphide mineral in base and precious metals deposits. Due to its abundance and close relationship with other minerals, pyrite processing has significant economic and environmental implications. For the most part, pyrite is considered a gangue mineral; thus, it is the main sulphide in mining waste and tailings storage facilities unless it contains significant amounts of valuable metals. Once pyrite is exposed to oxygen and water, it can be rapidly oxidized, releasing sulphuric acid and potentially toxic elements, creating a wide range of environmental problems (Evangelou and Zhang, 1995, Grieco et al., 2021, Ogola et al., 2002, Salomons, 1995). For example, pyrite is the primary source of acid rock drainage (ARD) in waste rock dumps, deep underground mining, and tailings, potentially contaminating soil and water bodies (Balci and Demirel-Floyd, 2017, Kefeni et al., 2017, USEPA, 1994). Therefore, it is the primary obstacle hindering land rehabilitation after mine closure (Commonwealth of Australia, 2016, Kuter, 2013, Lottermoser, 2010).
In new prospects and current mining operations, pyrite processing represents a significant problem in achieving production goals (Grano et al., 1990, Mu et al., 2015). If not depressed, pyrite floats along with valuable minerals producing a low-quality concentrate that causes substantial problems for smelting operations (Finch et al., 2007, Mu et al., 2015, Wang and Forssberg, 1996, Young et al., 2006). During smelting, pyrite is decomposed at high temperatures releasing sulphur vapour and sulphur dioxide, which can later react with atmospheric water vapour to form sulfuric acid, in a phenomenon known as acid rain (Dold, 2008).
As conventional high-grade metal orebodies become depleted, there is an increasing need to process pyrite-rich complex orebodies to meet metal demand (Tayebi-Khorami, 2018). Unfortunately, these complex orebodies tend to be non-responsive to traditional methods for pyrite depression (Nabais Figueira, 2018, Voigt et al., 2017). The necessity for higher pyrite rejection causes projects and operations to resort to complicated processing flowsheets(Akop, 2014, Bulatovic, 2007). These process developments can be economically, operationally, and environmentally challenging as it often involves complex reagent schemes (Bulatovic and Wyslouzil, 1985). Thus, understanding why pyrite's flotation performance varies within orebodies and the features affecting its floatability is critical for an efficient depression and possible reclamation of pyrite.
This paper aims to summarise the vast literature on pyrite, generated from various science disciplines, and provide tools for understanding the mechanisms by which different pyrite types affect flotation behaviour. Considering this, this paper provides tools for flotation performance predictions.
2. Pyrite variations
Natural pyrite can be present in many crystal structure forms and textures within an orebody and are never purely FeS2. Pyrite can incorporate impurities during formation, commonly As, Co and Ni, which are likely to either occur as small inclusions of other minerals or as ion substitution in the crystal lattice(Craig et al., 1998, Osborne, 2013).
2.1. Textural variations of pyrite
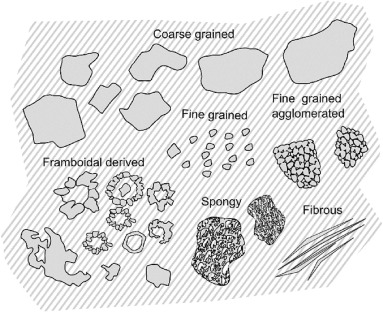
Depending on the formation conditions, pyrite can crystalize in variable structures (Craig et al., 1998). Pyrite textures are commonly classified based on grain size, spatial relationship with other minerals, the level of crystallization, or combinations of those features. Where pyrite is classified based on grain size, they are referred to as fine (4–40 µm), medium (40–300 µm), or coarse-grained (above 300 µm) (Basori et al., 2018, Croxford and Jephcott, 1972, Grondijs and Schouten, 1937, Li et al., 2018, Maguire-Olstad, 2016, Raymond, 1996). Pyrite textures are considered disseminated, compacted, or agglomerated to express their spatial relationships (Basori et al., 2018, Croxford and Jephcott, 1972, Grondijs and Schouten, 1937, Ma et al., 2022). Disseminated textures generally describe dispersed small grains, while agglomerated pyrite textures refer to juxtaposed fine grains and compacted refer to grains squished tightly. Pyrite can be referred to as euhedral, subhedral or anhedral textures to express their degree of crystallization or how well the crystals are formed with easily recognized faces. (Basori et al., 2018, Chen et al., 2020b, Li et al., 2018, Maslennikov et al., 2020). Fully formed crystals with recognizable faces are referred as euhedral, while anhedral refers to no recognizable crystals or faces, and subhedral to somewhere in between. Spongy, often referred to as Melnikovite, and framboidal pyrite varieties have also been reported in a separate class, ranging between 5 and 40 µm in size (Basori et al., 2018, Connell, 2016, Craig et al., 1998, Grondijs and Schouten, 1937). A schematic representation of some of the mentioned pyrite textures is shown in Fig. 1.
 Fig. 1. Pyrite textures schematic examples.
Fig. 1. Pyrite textures schematic examples.One key texture that impacts flotation performance is framboidal pyrite. Framboidal pyrite texture refers to spherical microcrystal pyrite aggregates resembling raspberries (England and Ostwald, 1993). Typically, they range between 5 and 40 µm in size. Whilst often associated with organic matter, framboidal pyrite formation does not necessarily depend on biological activity (Wacey et al., 2015, Wilkin and Barnes, 1997, Zhao et al., 2018). Examples of natural framboids are shown in Fig. 2.
 Fig. 2. Examples of natural pyrite framboids (Ohfuji and Rickard, 2005).
Fig. 2. Examples of natural pyrite framboids (Ohfuji and Rickard, 2005).Framboidal pyrite has been found to contain organic matter either in between grains or as membranes covering the framboids. It has been speculated that organic matter is the remanent of the organic material on which pyrite crystallized (Love, 1965).
However, the term “pyrite framboid” has been used to describe other framboid-derived structures, such as atoll structures, throughout the mineral processingliterature (Can et al., 2021, Croxford and Jephcott, 1972, O’Donnell and Muller, 2018, Taylor et al., 2010). For example, at Mount Isa, the term framboidal pyrite or carbonaceous pyrite has been extensively used to describe pyrite atoll structures (O’Donnell and Muller, 2018), as shown in Fig. 3. Therefore, when referred to Mount Isa ores, the pyrite atoll structures will continue to be referred to as framboidal pyrite throughout this paper.
 Fig. 3. Example of carbonaceous or framboidal pyrite at Mount Isa (O’Donnell and Muller, 2018).
Fig. 3. Example of carbonaceous or framboidal pyrite at Mount Isa (O’Donnell and Muller, 2018).2.2. Pyrite textural variations at Mount Isa
The Mount Isa sediment-hosted deposits in northwest Queensland comprise the Mount Isa, Hilton (P49) and George Fisher (L72) Pb-Zn orebodies and the silica-dolomite breccia-hosted Mount Isa Cu deposit (Lilly et al., 2017). It was initially exploited exclusively as a Pb-Zn mine in 1927. In the 1930s, copper was discovered in the mine premises. The exploitation and production of Cu concentrate began in 1947 through Concentrator 2, until 1973 when the copper concentrator (Concentrator 4) was commissioned (Lilly et al., 2017, Perkins, 1991, Rankin et al., 2013).
Early mineralogical studies on the Mount Isa deposit identified two prominent pyrite textures in the literature: Pyrite I and Pyrite II. Pyrite I refers to finely disseminated close-packed pyrite with grain sizes ranging from 5 to 10 µm. Pyrite II refers to coarse euhedral and subhedral pyrite with grain sizes ranging between 0.5 and 5 mm (Davey and Slaughter, 1970, Grondijs and Schouten, 1937, Gulson et al., 1985).
Pyrite I has also been reported to present framboidal or atoll structures and be closely associated with carbonaceous matter. Grondijs and Schouten (1937)studied 250 polished ore surfaces from the Mount Isa deposit, identifying pyrite atoll structures with diverse sulphide core replacements of pyrite and pyrite grains surrounded by graphite shells (Fig. 4). O’Donnell and Muller (2018)determined by point counting that about 50% of pyrite in the Cu concentrator feed was framboidal. It is important to note that the samples used in the study represent the concentrator ore feed on a specific day and do not represent the deposit. This finding suggests that the presence of framboidal pyrite is significant in the copper orebodies at Mount Isa; however, more work is needed to determine the prevalence of framboidal pyrite in the deposit.
 Fig. 4. Examples of pyrite grains present at Mount Isa deposit. (A) Replacement structures of various kinds of pyrite. Left, irregular atolls; right, pyrite aggregate originally with skeleton structure; white with relief, pyrite; very light grey, galena; grey, sphalerite; black, gangue. (B) Dark grey carbonate, grey sphalerite, white (high relief) pyrite, white galena, light grey tetrahedrite; grey in various shades around pyrite is graphite with strong double refraction. Galena on grain boundaries between pyrite and graphite and filling fissures in graphite. (Grondijs and Schouten, 1937).
Fig. 4. Examples of pyrite grains present at Mount Isa deposit. (A) Replacement structures of various kinds of pyrite. Left, irregular atolls; right, pyrite aggregate originally with skeleton structure; white with relief, pyrite; very light grey, galena; grey, sphalerite; black, gangue. (B) Dark grey carbonate, grey sphalerite, white (high relief) pyrite, white galena, light grey tetrahedrite; grey in various shades around pyrite is graphite with strong double refraction. Galena on grain boundaries between pyrite and graphite and filling fissures in graphite. (Grondijs and Schouten, 1937).Grano et al. (1990) reported the presence of graphitic carbon on pyrite surfaces, particularly in samples where framboidal pyrite was present. X-ray photoelectron spectroscopy (XPS) analysis was carried out on the naturally floatable material, confirming the presence of a graphitic carbon layer, as shown in Fig. 5. However, the instrument used had a limited spatial resolution, with the area analyzed by the X-ray being in the order of 10 mm2. Therefore, the photoelectron signal generated would be an average signal of all the particle surfaces in the area. Given the technology's limitations, it cannot be asserted that the graphitic carbon detected belongs exclusively to the framboidal pyrite grains and not other surrounding grains.
 Fig. 5. XPS spectra for concentrate and tails samples of pyrite-rich Mount Isa copper ores (Grano, 1990) showing the presence of carbon in the pyrite (Binding Energy (eV), g-graphitic, c-carbonate). Test carried out under nitrogen purge conditioning at pH 8, in the absence of collector.
Fig. 5. XPS spectra for concentrate and tails samples of pyrite-rich Mount Isa copper ores (Grano, 1990) showing the presence of carbon in the pyrite (Binding Energy (eV), g-graphitic, c-carbonate). Test carried out under nitrogen purge conditioning at pH 8, in the absence of collector.The structures found at the Mount Isa deposit do not resemble classic framboidal structures. Instead, they resemble atoll structures, often comprised of a pyrite rim with core replacement of another sulphide, either galena, chalcopyrite or sphalerite (Croxford et al., 1961, Grondijs and Schouten, 1937). They most likely result from infilling framboidal clusters (England and Ostwald, 1993).
A detailed conceptual model of framboidal formation was developed by England and Ostwald (1993) based on textural evidence from base-metal sulphide deposits within the Tasman Fold Belt, New South Wales, Australia (Fig. 6). The authors suggest that pyrite framboids can become infilled by other mineral species. The diffusion of additional pyrite into the structure can form solid cores, atoll structures, and eventually euhedral pyrite, depending on the composition of mineralized fluids during ore formation events. Therefore, the atoll structures at Mount Isa likely represent intermediate stages between pyrite framboids and euhedral crystals. Although not centred on atoll structures, Gu et al. (2003) and Zhao et al. (2018) propose a similar morphological transition from framboid to euhedral grains.
 Fig. 6. Conceptual model of framboidal pyrite formation based on microstructural observations from Drake copper–gold field, Halls Peak, Sunny Corner and Cangai base metal sulphide deposits, New South Wales, Australia (England and Ostwald, 1993).
Fig. 6. Conceptual model of framboidal pyrite formation based on microstructural observations from Drake copper–gold field, Halls Peak, Sunny Corner and Cangai base metal sulphide deposits, New South Wales, Australia (England and Ostwald, 1993).The mechanism of framboidal pyrite and atoll structure formation has been extensively studied in the literature and will not be examined further in this paper. See Ohfuji, 2012, Ohfuji et al., 2002, Ohfuji et al., 2005, Ohfuji et al., 2003, Ohfuji and Rickard, 2005, Wilkin and Barnes, 1997, Wilkin et al., 1996, Zhao et al., 2018 for more details on pyrite framboid genesis and structure, and England and Ostwald, 1993, Wacey et al., 2015, Zhao et al., 2018 for framboidal pyrite derived structure formation and evolution.
2.3. Variations in the chemical composition of pyrite
Natural pyrite contains small amounts of other minor and trace elements in its crystal structure. Common minor elements in the pyrite structure are Co, Ni and As, though the concentrations of those elements are generally low (below 0.1 wt%) (Craig et al., 1998). These elements form part of the pyrite structure, either by stoichiometric or non-stoichiometric substitution of either Fe or S in the crystal structure. Additionally, other elements like Cu, Zn, Pb, Ba, Bi, Au, Ag and Sb, when present, are considered as inclusions of different phases (Arrouvel and Eon, 2018, Huston et al., 1995, Large et al., 2011, Large et al., 2007, Mukherjee et al., 2020).
Pyrite is the most abundant sulphide mineral in many types of precious and base metal ore deposits and is relatively stable during metamorphism. Thus, extensive work has been carried out on understanding the chemical composition of pyrite from a deposit interpretation and prospectivity perspective (Craig et al., 1998, Huston et al., 1995). The minor and trace element variations in pyrite grains and textures within an orebody are used to interpret the deposit formation events and metal mobilization and deposition processes (Maslennikov and Large, 2021). For example, Co and Ni are more abundant in high temperature ores (e.g. Volcanic hosted massive sulphide deposits), whilst As is more abundant in low temperature ores (e.g. Orogenic gold deposits) (Abraitis et al., 2004). A summary of the volume of pyrite samples (n = 3040) evaluated for mineral exploration by deposit type, considering studies where pyrite texture was referenced as an evaluating factor, is shown in Fig. 7.
 Fig. 7. A summary of the volume of pyrite samples evaluated for mineral exploration by deposit type. Based on the work of Arehart et al., 1993, Basori et al., 2018, Belousov et al., 2016, Chen et al., 2020b, Conde et al., 2021, Deditius et al., 2011, Deditius et al., 2008, Dmitrijeva et al., 2020, Fleet and Mumin, 1997, Huston et al., 1995, Keith et al., 2016, Large et al., 2011, Large et al., 2007, Ma et al., 2022, Reich et al., 2013, Steadman et al., 2018, Wei et al., 2020, Wells and Mullens, 1973.
Fig. 7. A summary of the volume of pyrite samples evaluated for mineral exploration by deposit type. Based on the work of Arehart et al., 1993, Basori et al., 2018, Belousov et al., 2016, Chen et al., 2020b, Conde et al., 2021, Deditius et al., 2011, Deditius et al., 2008, Dmitrijeva et al., 2020, Fleet and Mumin, 1997, Huston et al., 1995, Keith et al., 2016, Large et al., 2011, Large et al., 2007, Ma et al., 2022, Reich et al., 2013, Steadman et al., 2018, Wei et al., 2020, Wells and Mullens, 1973.For exploration geology studies, pyrite textures tend to be classified differently based on the suggested ore formation events unique to each deposit. A summary of minor and trace element composition of pyrite by recorded mineral texture is shown in Fig. 8. Although different texture classifications are used, studies seem to agree that coarse euhedral pyrite textures have a lower concentration of minor and trace elements compared to other fine-grained, subhedral or anhedral textures (Basori et al., 2018, Chen et al., 2020b, Craig et al., 1998, Li et al., 2018, Ma et al., 2022, Maslennikov et al., 2020). In low temperature Au bearing ores, where pyrite is present with more than one texture and mineral associations, it was found that As and Au were typically found in higher concentrations in framboidal pyrite, followed by fine-grained pyrite, and to a lesser extent in coarse grain pyrite (Abraitis et al., 2004, Gregory et al., 2015, Maslennikov and Large, 2021, Simmons, 1997, Tesh, 2020). However, the implications of those compositional variations on pyrite surface chemistry and flotation tend to be outside their scope. In general, complex pyrite textures seem to present a higher content of impurities such as As, Au, Ag, Zn, Pb, and Cu, as shown in Fig. 8. Depending on the elements present, the occurrence of impurities in pyrite can be considered either beneficial or undesirable in mineral processing. For example, pyrites rich in precious and critical metals, like Au, Co or Ni, in mine waste and tailings are now considered prospective for valuable metals production (European Commission et al., 2019; Geoscience Geoscience Australia, 2022, Van der Ent et al., 2021). On the other hand, the presence of toxic elements, especially those which form oxyanions in solutions, like As, Mo or Cr, is undesirable due to its potential to contaminate soils and ground and surface water, pressing an environmental hazard (Dold, 2008, Savage et al., 2000). Independently, the presence of impurities in pyrite crystal structure influences its electrochemical properties and flotation behaviour in various ways.
 Fig. 8. Minor and trace element composition of pyrite by texture class. Pyrite textures referenced as framboidal, spongy, microcrystalline, cataclastic and sooty have been condensed into the complex texture category. Based on the work of Arehart et al., 1993, Basori et al., 2018, Belousov et al., 2016, Chen et al., 2020b, Conde et al., 2021, Deditius et al., 2011, Deditius et al., 2008, Dmitrijeva et al., 2020, Fleet and Mumin, 1997, Franchini et al., 2015, Gregory et al., 2015, Huston et al., 1995, Keith et al., 2016, Large et al., 2011, Large et al., 2007, Ma et al., 2022, Maslennikov et al., 2020, Mukherjee et al., 2020, Ogilvie, 2014, Reich et al., 2013, Steadman et al., 2018, Thomas et al., 2011, Wei et al., 2020, Wells and Mullens, 1973.
Fig. 8. Minor and trace element composition of pyrite by texture class. Pyrite textures referenced as framboidal, spongy, microcrystalline, cataclastic and sooty have been condensed into the complex texture category. Based on the work of Arehart et al., 1993, Basori et al., 2018, Belousov et al., 2016, Chen et al., 2020b, Conde et al., 2021, Deditius et al., 2011, Deditius et al., 2008, Dmitrijeva et al., 2020, Fleet and Mumin, 1997, Franchini et al., 2015, Gregory et al., 2015, Huston et al., 1995, Keith et al., 2016, Large et al., 2011, Large et al., 2007, Ma et al., 2022, Maslennikov et al., 2020, Mukherjee et al., 2020, Ogilvie, 2014, Reich et al., 2013, Steadman et al., 2018, Thomas et al., 2011, Wei et al., 2020, Wells and Mullens, 1973.3. Sulphide mineral properties affecting flotation
This section will cover various aspects that arise from textural and compositional variations of sulphides and the way that they can impact flotation behaviour. Particular focus will be given to how the floatability of sulphide minerals can be affected by changes in their electrochemical properties or by the presence of mineral associations or micro-inclusions of naturally floatable material.
3.1. Electrochemistry and flotation
Sulphide minerals are considered semiconductors, and their electrical conductivity is low compared to metals (Leja, 1982, Rimstidt and Vaughan, 2003). Charge carriers on their surfaces promote electron transfer and electrochemical reactions in the sulphide mineral flotation system. These reactions can include surface oxidation, interactions with other components, collector adsorption, or precipitation of metals on the surface, determining their basic flotation behaviours (Moslemi et al., 2012).
The floatability of non-hydrophobic sulphides under natural conditions is greatly affected by the electrochemical reactions occurring on the mineral surfaces (Leja, 1982). Collectorless flotation behaviour would occur due to the slight oxidation of the mineral surface, forming hydrophobic species such as elemental sulphur and SxOy species (He et al., 2005). On the other hand, hydrophilic species such as hydroxides or sulphates will be formed with increased oxidation, and self-induced flotation will not be observed (Moslemi and Gharabaghi, 2017, Trahar et al., 1994, Woods, 2003). The pH and Eh significantly affect the oxidation products' nature and flotation behaviour, as shown in Fig. 9 (Heyes and Trahar, 1979, Tao et al., 2018). Furthermore, cations produced by the oxidation of sulphides and the cations introduced by the gangue minerals will interact in different ways in different mineral systems, making the reduction and oxidation species hard to determine (Guy and Trahar, 1984). For example, the presence of cations like Cu and Pb has been found to activate the surface of pyrite and sphalerite, either by adsorption or precipitation of metal species onto the mineral surface, enhancing natural floatability or their affinity towards collectors during flotation (He et al., 2005, Pecina et al., 2006, Peng et al., 2012, Yamamoto, 1980).
 Fig. 9. Pyrite flotation recovery as a function of solution potential at different pH's (Tao et al., 2018).
Fig. 9. Pyrite flotation recovery as a function of solution potential at different pH's (Tao et al., 2018).The main factor affecting the electrochemical processes is the electrochemical potential at the mineral and solution interface (Hu et al., 2009). This potential is determined by the presence of oxidizing and reducing species in the solution. The rest potential of minerals indicates how reactive or prone to oxidation they are, and each sulphide oxidizes at different rest potentials. The higher the rest potential, the more noble the mineral; in other words, it is harder to oxidize. Comparatively, pyrite has the highest rest potential and is thus less reactive than other sulphides. Therefore, the difference in rest potential between sulphides in a flotation system can enhance the oxidation reaction on the mineral surfaces during grinding and flotation (Chen et al., 2020a, Gardner and Woods, 1979, Moslemi and Gharabaghi, 2017, Moslemi et al., 2012, Tao et al., 2018, Vasil'yeva et al., 1990, Woods, 2003), and affect flotation recoveries (Ekmekçi and Demirel, 1997, Forbes et al., 2018, Owusu et al., 2013, Owusu et al., 2014, Peng et al., 2012, Pozzo and Iwasaki, 1989, Trahar et al., 1994, Yang et al., 2022).
3.2. Mineral texture and chemical composition on electrochemical properties
Sulphide minerals can have variable contents of minor and trace elements (Craig and Vaughan, 1990). For example, pyrite typically presents a wide variety of minor and trace elements within the mineral lattice, including As, Au, Co and Ni. Naturally occurring sphalerite always contains other transition metal ionssubstituting Zn, mostly Fe. In the same way, Ag, Bi and Sb can also be found in galena or Pb-sulfosalts. (Craig and Vaughan, 1990, Fallon et al., 2019, Pridmore and Shuey, 1976). The presence of other metal impurities in the sulphide minerals affects their crystal structure, electric and magnetic properties, and increases reactivity, thus influencing how it interacts with the flotation reagents. (Babedi et al., 2021, Wang et al., 2019). For example, Xian et al. (2012)found that Co-substituted and intercrystalline Au pyrites adsorbed more xanthate and were more stable than pure pyrite and As-substituted pyrite due to having lower band-gap values.
Sulphides' electrochemical properties and surface reactivity are also affected by their mineral texture. For example, grain size greatly influences oxidation reactions; smaller grain sizes have a higher surface area, which leads to increased oxidation (Fox et al., 1997, Pugh et al., 1984, Weber et al., 2004). Furthermore, the way sulphides are associated or inlaid in a mixed mineral system can affect their reactivity towards oxidation due to galvanic interactions (Abraitis et al., 2004, Hu et al., 2009).
3.3. Texture and flotation
The effect of grain size and liberation of sulphide minerals on flotation has been widely studied (Bradshaw et al., 2019, Evans et al., 2015, Hunt et al., 2011b, Trahar, 1981). It is generally agreed that the coarser-grained the mineral, the higher its degree of liberation (Bradshaw et al., 2019, Gay, 2004, King, 1979, King and Schneider, 1998, Tungpalan et al., 2018, Vink, 1997). For fully liberated sulphide minerals, recoveries and concentrate grades have been observed to increase as mineral grain size increases in a wide range of sulphides (Evans, 2010, Mishra et al., 2013, Seaman et al., 2012, Sutherland, 1989, Tungpalan et al., 2019). Recoveries as a function of the metal content increase until a peak is reached and recoveries decline, most likely due to hydrodynamics conditions in the flotation cell (Jameson, 2012, Welsby et al., 2010).
Mineral associations significantly impact flotation performance; sulphides associated with non-floatable material are less likely to be recovered than those associated with naturally floatable material (Bradshaw, 2014). However, complex mineral associations and micro-inclusions of sulphide minerals and other naturally floatable minerals can reduce the selectivity of flotation and lead to low metal recoveries due to poor mineral liberation (Cropp et al., 2013). For example, in complex polymetallic deposits, finely disseminated intergrowths of galena and sphalerite are likely to float together into a bulk concentrate, making it challenging to separate Zn and Pb into individual concentrates (Grossou-Valta et al., 1980). Similarly, the presence of naturally hydrophobic gangue minerals such as graphite and talc as micro-inclusions in sphalerite and pyrite can exert natural floatability on the particle and report to the concentrate reducing their grade (Healy, 2005, O’Donnell and Muller, 2018).
4. Pyrite flotation
Previous sections have examined the different types of pyrite textures that occur in base metal sulphide-bearing ores, as well as looking at the effect that the properties of base metal sulphides in general have on mineral flotation. This section focuses specifically on pyrite and how its mineral texture, chemical composition and electrochemical properties affect its flotation behaviour.
4.1. Effect of elemental composition on electrochemical properties
It has been suggested that the impact of pyrite's minor and trace element composition on its electrochemical properties depends on the mechanism by which the element substitutes Fe or S in the pyrite's crystal structure (Deditius et al., 2008, Vaughan et al., 1997, Xian et al., 2012). Non-stoichiometric substitutions are believed to create a charge imbalance on the crystal surfaces, thus impacting its electrochemical properties. The incorporation of As by substitution for S is the primary non-stoichiometric substitution in pyrites (Blanchard et al., 2007, Huston et al., 1995, Lehner et al., 2007).
Pyrite electrochemical properties have been widely studied under acidic conditions due to their role in acid mine drainage and ore enrichment processes (Lehner et al., 2007, Lottermoser, 2010, Rimstidt and Vaughan, 2003). However, the literature is not consistent on how the electrochemical properties of pyrite are affected by its impurity content. For example, while using high-grade natural pyrite for experimentation, Pridmore and Shuey (1976) reported no significant differences in the resistivity of natural pyrite samples with varying compositions. Similarly, Doyle and Mirza, 1996, Osborne, 2013, while measuring the conductivity, resistivity and rest potential of several pyrite specimens, concluded that the presence of impurities in their structure did not significantly affect its electrochemical properties. Likewise, Liu et al. (2008), while studying the oxidative dissolution of pyrites with variable impurity content, concluded that there was no direct link between As content and pyrite reactivity.
In contrast, Lehner et al. (2007) studied the electrochemical reactivity of synthetic pyrite doped with As, Ni and Co, using AC and cyclic voltammetry measurements, finding that the presence of impurities (mainly arsenic), increased the reactivity of the pyrite. Similarly, Nourmohamadi et al. (2022), carrying out theoretical density functional theory (DFT) calculations for pure and As-doped pyrite, determined that the presence of As increased the pyrite surface reactivity. Further studies on natural pyrite under alkaline conditions found that the presence of impurities, particularly As, affected pyrite's electrochemical properties and flotation behaviour (Forbes et al., 2018, John, 2017). Xian et al. (2012) found that As-substituted pyrite was easier to be depressed by intensive oxidation compared to natural pyrite and Co-substituted pyrite. The differences in the studies using natural and synthetic pyrite could be attributed to the difference in purity of the pyrite sample used for experimentation. The presence of other minerals in the pyrite sample can affect the reactivity of the pyrite (Tao et al., 2003). Additional differences in the electrochemical measurements could be attributed to differences in pH, temperature and the methods of pyrite surface preparation adopted by each researcher (Schoonen et al., 2000, Tao et al., 2003).
Fig. 10 shows the natural pyrite rest potential variations with impurity content identified in this literature review. A decrease in pyrite rest potential with increased Cu and As content can be observed across studies, seemingly confirming that pyrite impurity content increases its reactivity. The interaction of pyrite with Cu ions is significant in mineral processing. Copper-containing minerals can release Cu ions during grinding and flotation, which have been shown to activate the pyrite surface affecting its flotation behaviour (Moslemi and Gharabaghi, 2017, Peng et al., 2003, Peng et al., 2012). Additionally, the flotation recovery of Cu-activated pyrite is significantly affected by the electrochemical potential during grinding and flotation (He et al., 2005, Peng et al., 2012). However, the variation in rest potential across studies can be attributed to variations in pH during experimentation, as shown in Fig. 11. A significant decrease in pyrite rest potential with increasing pH was also reported by Moslemi et al. (2012).
 Fig. 10. Summary of pyrite rest potential measurements versus pyrite impurity content, copper, and arsenic content. Impurities refer to the sum of reported Cu, Pb, Zn, Ni, Sb, Co and As contents. Based on the work of Ahlberg et al., 1990, Cruz et al., 2001, Doyle and Mirza, 1996, Forbes et al., 2018, Moslemi et al., 2012, Mu et al., 2015.
Fig. 10. Summary of pyrite rest potential measurements versus pyrite impurity content, copper, and arsenic content. Impurities refer to the sum of reported Cu, Pb, Zn, Ni, Sb, Co and As contents. Based on the work of Ahlberg et al., 1990, Cruz et al., 2001, Doyle and Mirza, 1996, Forbes et al., 2018, Moslemi et al., 2012, Mu et al., 2015. Fig. 11. Variation of pyrite rest potential by pH. Based on the work of Ahlberg et al., 1990, Cruz et al., 2001, Doyle and Mirza, 1996, Forbes et al., 2018, Moslemi et al., 2012, Mu et al., 2015.
Fig. 11. Variation of pyrite rest potential by pH. Based on the work of Ahlberg et al., 1990, Cruz et al., 2001, Doyle and Mirza, 1996, Forbes et al., 2018, Moslemi et al., 2012, Mu et al., 2015.There is not enough information to determine if the presence of impurities on pyrite reactivity becomes more predominant at higher pH. It is important to mention that no variation in texture was considered in the cited studies on natural pyrites; pyrites were only referenced as large and massive specimens based on their origin and deposit type.
4.2. Effect of texture on pyrite electrochemical properties
Different pyrite textures have been shown to exhibit differentiated electrochemical properties (Liu et al., 2008, Rimstidt and Vaughan, 2003). Based on its formation and characteristics, poorly crystallized pyrite, fine pyrite and framboidal pyrite are expected to have a higher degree of reactivity than coarse-grained pyrite (Lottermoser, 2010). Mineralogical investigations have indicated that in acid generation studies, samples with framboidal pyrite present accelerated oxidation compared to coarse grain pyrite (Weisener and Weber, 2010). The microcrystal arrangement and secondary growth of pyrite infilling framboidal voids are suggested to facilitate oxidative pathways enhancing surface electrochemical properties (Ohfuji et al., 2002).
Furthermore, increased surface area, morphology and crystallographic defects of framboidal cores are expected to make them more susceptible to oxidation than coarse-grained pyrite (Parbhakar-Fox et al., 2011, Weisener and Weber, 2010). Pugh et al. (1984) compared the oxidation rate of natural framboidal pyrite and coarse grain pyrite and reported framboidal pyrite as more reactive than massive coarse pyrite and attributed the results to the difference in morphology and surface area. The framboidal pyrite sample, although not completely pure as it contained some coarse-grained pyrite, presented a ten times higher surface area than the massive coarse pyrite sample. Du et al. (2021) suggested that morphology rather than grain size and specific surface area control the reactivity of framboidal pyrite while measuring the oxidation rate of synthetic framboidal pyrite.
4.3. Effects of texture on flotation
Limited studies exist on the effect of specific pyrite textures, particularly complex disseminated and framboidal pyrite textures, on flotation performance. Barker et al., 2014, Can et al., 2021, Medina, 2012, while studying the flotation performance of ore samples with varying pyrite textures, found that the higher the surface area of the pyrite in the sample, the higher the pyrite flotation rates and recoveries. Barker et al. (2014) suggest that the increased surface area of framboidal pyrite increases galvanic interaction with other minerals and their floatability.
Can et al. (2021) found that in copper sulphides ore samples containing framboidal pyrite lower copper recoveries were obtained, and Fe was harder to depress than in samples with no reported framboidal pyrite. Similarly, Barker et al. (2014), while evaluating the flotation behaviour of lead sulphide samples containing framboidal pyrite, found that the presence of framboidal pyrite in the feed negatively impacted lead recoveries. These studies suggest that in mixed sulphide systems, pyrite textures affect its flotation performance and the flotation recoveries of other sulphides. It is important to note that in the mentioned studies, the prevalence of each pyrite texture in the test samples was not quantified.
The presence of framboidal and fine-grain pyrite at Mount Isa has been documented for the Zn/Pb deposit since 1937 (Grondijs and Schouten, 1937). Based on the publicly available studies, Mount Isa concentrators have experience difficulties during processing due to the presence of naturally floatable pyrite in the feed. For example, Davey and Slaughter (1970) reported that since 1956, the Zn/Pb concentrator experienced non-selective flotation and high reagent consumption while processing ore from areas where carbonaceous or framboidal pyrite had been reported. The difficulties meeting recoveries and concentrate grade goals led to changes in the flotation flowsheet, which included a reduction in grind size and installing a pre-flotation to remove hydrophobic carbonaceous pyrite.
For the copper concentrator, commissioned in 1973, the presence of carbonaceous pyrite represented an ongoing challenge. Throughout the life of mine, several attempts have been made to control the flotation of naturally hydrophobic materials, mainly talc and carbonaceous pyrite. Mount Isa performed extensive metallurgical testwork to selectively depress hydrophobic gangue minerals, obtaining mixed results. The studies used cyanide and organic depressants, such as dextrin and naphthalene sulphonate, at different flotation stages (Grano et al., 1991, O’Donnell and Muller, 2018). In 1994 a pre-flotation stage was implemented at the Cu concentrator to remove hydrophobic carbonaceous pyrite and talc, repurposing already available flotation cells, and in 2000 Jameson cells were introduced for pre-flotation cleaning (Lawson et al., 2017).
4.4. Typical depression methods
Pyrite is often regarded as undesirable in base metal processing. Therefore, the general flowsheet approach is to depress the pyrite during flotation. The primary strategy for the depression of pyrite is the addition of modifying reagents in the flotation stage to control pH and ORP during flotation and control de interaction of collectors between individual minerals (Fuerstenau et al., 2007).
Pyrite is typically hydrophilic in alkaline pH, with its floatability increasing at acidic pH (Fuerstenau et al., 2007). At alkaline conditions, hydroxylation of metal atoms most likely occurs on the pyrite surface, thus reducing its floatability (Fornasiero et al., 1992, Tao et al., 2018). Furthermore, the presence of iron hydroxyl species can hinder the adsorption of collectors (Li et al., 2012). Pyrite hydrophobicity is also affected by the pulp oxidation potential (Eh) during flotation. Extensive pyrite oxidation will occur at high Eh, promoting forming iron hydroxide/oxide films; thus, pyrite floatability decreases (He et al., 2005, Leja, 1982). Although the selective flotation of sulphide minerals by external electrochemical controls is an accepted concept, the industrial applications of such an approach for pyrite depression are not documented.
In an alkaline pH, secondary depressants such as organic compounds, sulphates, and cyanide are effectively used for pyrite depression. Cyanide is one of the best-known pyrite depressors in flotation (Bulatovic, 2007). However, it is associated with severe environmental and health issues (Wong-Chong et al., 2005). At low pH (<9.31) cyanide derived compounds volatilize, releasing toxic gas into the air (Lottermoser, 2010). Cyanide contaminates water sources when deposited in tailing dumps and, in high concentrations, can be harmful to humans and animals (Mehrabani et al., 2011, Ntemi, 2013).
In the last decades, the use of organic compounds for the depression of pyrite has been getting attention as an environmentally friendly alternative to inorganic depressants. Natural polysaccharides like dextrin, starch, and guar gum can depress pyrite by selectively adsorbing on the pyrite surface, effectively creating a hydrophobic coating (Bulatovic, 2007, López Valdivieso et al., 2004, Mu et al., 2016). Similarly, carboxymethyl cellulose (CMC) has been used for pyrite depression.
The reagents mentioned above have been proven effective for the depression of pyrite. However, it is essential to note there is no one fit all solution for selective depression of pyrite in flotation systems. Orebody characteristics will affect the pyrite behaviour in flotation, as explained in previous sections of this paper, and consequently, its response to standard depression methods employed. The information provided is a guideline on typical depression reagents; metallurgical testwork on ore samples representative of the deposit need to be carried out to identify a suitable processing flowsheet.
5. Identification and quantification of textures
The most widely used technology for automated mineral characterization, including textural analysis, are scanning electron microscope (SEM) based systems, Quantitative Evaluation of Mineralogy by Scanning Electron Microscope (QEMSCAN) developed by CSIRO (Grant et al., 1976) and Mineral Liberation Analyser (MLA) developed by JKMRC (Gu, 2003). Tescan Integrated Mineral Analyzer (TIMA) (Hrstka et al., 2018), Zeiss Mineralogic (Graham et al., 2015) and INCA Mineral (Muto et al., 2013) are other systems available.
SEM systems identify and characterize minerals using a combination of backscatter electron (BSE) and Energy-dispersive X-ray spectroscopy (EDX) analysis. The initial mineral grain delimitations are based on BSE brightness contrast. Minerals with higher atomic numbers appear brighter than those with lower ones. Detailed EDS on a grid pattern on individual particles or mineral grains can then be carried out for mineral identification (Fandrich et al., 2007, Madiba et al., 2019, Sylvester, 2012).
However, detecting mineral grain borders becomes problematic when fine-grained materials or complexly intergrown grain borders are present. While studying complex Sn fine-grained ores, Kern et al. (2018) found that the excitation volume can affect more than one mineral for narrow grid EDX step measurements in closely packed fine disseminated textures. These measurements can result in mixed X-ray spectra that cannot be recognized in the EDX spectra library and are reported as unknowns, as shown in Fig. 12 (Kern et al., 2018). The phenomenon is inferred to be more prevalent for ores with finer-grained and complex intergrown minerals. Similarly, grain border delimitation is not detected when mineral grains are finely agglomerated. In this case, the image map will show a coarse mineral grain rather than a multitude of smaller grains clumped together. Thus, the presence or prevalence of fine complex textures can be overlooked or under-represented (Fig. 13).
 Fig. 12. Outcomes of classifying an MLA measurement (GXMAP) with the conventional approach. Adapted from Kern et al. (2018).
Fig. 12. Outcomes of classifying an MLA measurement (GXMAP) with the conventional approach. Adapted from Kern et al. (2018). Fig. 13. Comparison of pyrite grains in BSE and mineral map images from mount Isa copper ore samples.
Fig. 13. Comparison of pyrite grains in BSE and mineral map images from mount Isa copper ore samples.As an alternative, image analysis approaches have been used to identify mineralogical and textural features mainly in texture-based analysis to predict liberation for breakage models or flotation performance predictions based on liberation (Bonnici, 2012, Evans, 2010, Evans et al., 2015, Hunt et al., 2011, Mariano, 2016). The studies have been carried out using high-resolution images from optical microscopy images (Pirard et al., 2008, Pirard et al., 2007, Ricardo et al., 2010), grey-scale BSE images obtained from MLA (Bonnici, 2012, Moen, 2008), X-ray radiographs (Mariano, 2016) and X-ray microcomputed tomography (μCT) images (Evans et al., 2012, Evans et al., 2015, Guntoro et al., 2019, Medina, 2012).
6. Concluding remarks
Based on the reviewed studies, natural pyrite is widely variable in terms of texture and elemental composition. This variability has been found to affect their electrochemical properties and flotation behaviour. A summary of the pyrites variations and their effect on pyrite flotation behaviour based on major texture variations is shown in Table 1. Furthermore, since more than one pyrite texture can be present within an orebody, their relative proportion in the ore could affect the overall flotation behaviour. However, this effect has not been evaluated or studied. The complex pyrite textures in an ore sample have not been systematically quantified in the reviewed studies.