1. Introduction
The development and social necessity of new technologies and the energy transition leads to an increasing consumption of metals, contributing to risks of scarcity of various metals (Mo, Co, Li, Zn, Cu, Sn, Ni, Au, etc.). The extraction of metals from primary geological sources, as well as waste and by products, have to adapt to meet the growing demand. Supply risk of critical raw materials can harm a country's economy. Different techniques have been used to perform metal recovery. Pyrometallurgy process is one of most used. High purity alloys can be recovered with this process, however this process requires high temperatures, being an energy-intensive process (Dias et al., 2022). In addition, metal losses can be produced in the slag with lower recovery rates than with other techniques. Hydrometallurgical recovery is also widely used for separation of metals in aqueous medium (F. J. Alguacil et al., 2003). With this process, higher metal recovery rates are achieved with lower energy consumption, but more research is needed to reduce the environmental impact of hydrometallurgical processes (F. J. Alguacil et al., 2019) DESs are a potential tool that can contribute to the design of cleaner processes, due to good thermal and chemical stability, low melting point, easy synthesis, low vapour pressureand low or practically negligible toxicity. Most of them can be considered biodegradable solvents, showing themselves to be excellent “green” solvents. The aim of this review article collects the publications and highlights the relevance of the use of these solvents in metal recovery from industrial waste and by-products by hydrometallurgical methods. It is necessary to present a summary of the main properties, because them have a great influence on the use of DES in hydrometallurgical processes. To complete the review types, classification and preparation methods of these solvents, as well as the advantages and disadvantages (or limitations) of their use are also included.
2. Definition and history of DESs
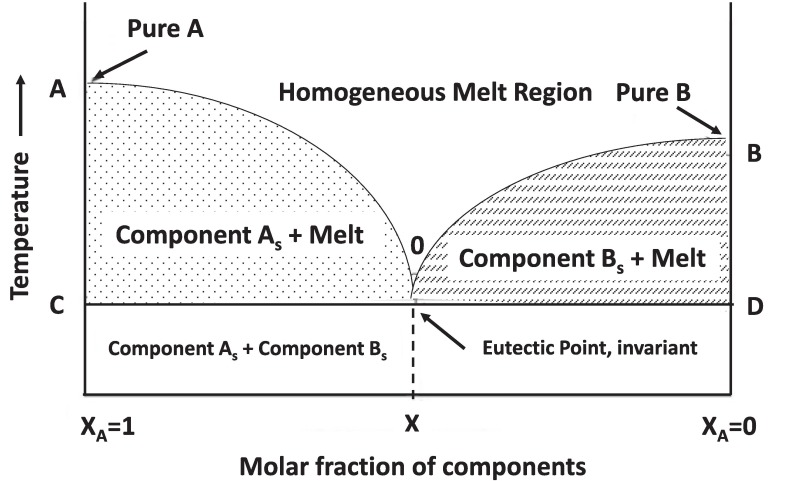
The term eutectic has its origin in the Greek word “eutēktos” which means easy to melt, and which is interpreted as reducing the melting point of the mixture (D. Yu et al., 2021). An eutectic mixture is “an approximately reversible isothermal mixture which does not react between different components during the cooling of a liquid system, causing the system’s freezing point to decrease compared to the melting points of the pure components” (Singh et al., 2021) as shown in Fig. 1.
 Fig. 1. Phase diagram of the two component system based on (Singh et al., 2021).
Fig. 1. Phase diagram of the two component system based on (Singh et al., 2021).This “deep” nature was first explained by Abbot et al. (Abbott et al., 2002),(Abbott, Capper, et al., 2004a), prepared different melts using metal chlorides (MCl2, M = Zn y/o Sn) with quaternary ammonium salts of formula [Me3NC2H4Y]Cl (Y = OH, Cl, OC(O)Me, OC(O)Ph) and abbreviated as “liquid ionic Lewis acids”. These authors indicated that the ChCl-MCl2 (1:2) (M = Zn o Sn) are a suitable medium for Diels-Alder reactions to take place, the main accelerating effect is associated with the Lewis acidity of the ionic liquid, these are facially recyclable by decanting and washing with hexane, have a reusability of five times (Abbott et al., 2002),(Abbott, Capper, et al., 2004a). This same idea was extended to other hydrated metal salts (CaCl26H2O, LaCl36H2O, CoCl26H2O, LiNO34H2O y Zn(NO3)24H2O) and more specifically for the system ChCl/CrCl36H2O (Abbott, Capper, et al., 2004b).
Abbot et al. (Abbott et al., 2003) studied a mixture formed from choline chloride and urea in a 1:2 ratio and observed that they had unusual properties with a eutectic point of 285 K, this mixture is currently known as reline. The melting point of the mixture is less than each of its individual components corresponding to 575 K and 406 K respectively; the decrease in the freezing point may be due to the interaction between urea molecules and chloride ions. This depression at the melting point gave rise to the term “Deep”, these compounds being known as Deep Eutectic Solvent, DES (Abbott et al., 2003).
DESs are obtained from the mixture of two or three substances with a given composition where the melting points of each of the individual components are higher than that of the mixture, consisting of the appropriate combination of hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBA). The decrease of the freezing point is due to the extensive interspecific hydrogen bonds and the offshoring of the charges (E. L. Smith et al., 2014), Fig. 2. Typical HBDs used are alcohols, carboxylic acids, amides and amino acids and HBAs are ChCl, tetraalkyl halides, tetrabutylphophonium bromide and hydrated metal halides/halides.Fig. 3..
 Fig. 2. Schematic illustration of the fabrication pathway of DESs based on (Taghizadeh et al., 2021).
Fig. 2. Schematic illustration of the fabrication pathway of DESs based on (Taghizadeh et al., 2021). Fig. 3. DESs classification.
Fig. 3. DESs classification.DESs are compounds with low vapour pressure, a relatively wide liquid range, low or negligible toxicity, low reactivity and non-flammability. These properties make them a new kind of emerging ecological solvent, which is currently in its infancy (Steudte et al., 2014).
Although DESs have physical properties similar to ionic liquids (IL), the terms DESs and ionic liquids are used to refer to different compounds. IL is usually defined as ionic compounds having melting temperatures below 373 K (F. J. Alguacil et al., 2019). The DESs are obtained from the combination of acids and bases of Lewis or BrØnsted, which differs from the IL formed by systems entirely of discrete ions. Other differences between IL and DES are that DES are easier to synthesize than IL, less expensive and are considered as ecological solvents (Q. Zhang et al., 2012).
3. Types and classification
According to the literature, DESs can be classified into five types according to their chemical structure. The general formula for the first type of DES is, Cat+X-x(MCln), where, X- and x refer to a Lewis base and the number of MCln in the DES unit, respectively. This type of compounds is achieved by the combination of non-hydrated metal chlorides (MCln) and quaternary ammonium salts (HBA), within this type of structures include choline chloride (ChCl), carboxylic acidsand amines. The metal elements normally used, represented by M are Ga, Sn, In, Zn, Al, and Fe. The magnitude of n in formulas is restricted to reach DES with a low melting point, ZnCl2, FeCl2, AgCl, CuCl2; CdCl2; LiCl, SnCl2 y SnCl4 (J. Wang et al., 2017)(Kalhor & Ghandi, 2019)(Ijardar et al., 2022). One way to solve this problem is to use hydrated halide metals, instead of anhydrous ones, which correspond to type II DES. Where the melting point is further reduced due to hydration water, further decreasing network energy (E. L. Smith et al., 2014).
The second type of DES is obtained from the same HBA but the metallic chloride is hydrated (MCln·zH2O), where z represents the number of water molecules in the unit cell of salt. The general formula for this type of DES is Cat+X-x(MCln)zH2O y M corresponds to metals such as Fe, Ni, Cu, Co, Cr.
Type III DES has been extensively studied. This type of DES is obtained from the combination of quaternary ammonium salts such as HBA and HBD (carboxylic acid, alcohols, amides and carbohydrates, etc.) (Abbott et al., 2003)(Abbott, Boothby, et al., 2004). Fig. 4 shows the HBAs normally used for the preparation of DESs. These DESs have great importance due to their ability to dissolve different transition metals (Plechkova & Seddon, 2008).
 Fig. 4. List of commonly used HBAs (a) and HBDs (b) in preparation of DESs based on (Ijardar et al., 2022).
Fig. 4. List of commonly used HBAs (a) and HBDs (b) in preparation of DESs based on (Ijardar et al., 2022).Type IV DES is obtained from metallic salts or hydrated metal salts, namely transition metal chlorides and HBD, such as ZnCl2:urea, these metal salts can also form DESs with compounds such as ethylene glycol, acetamide and 1,6 hexanodiol (Ijardar et al., 2022); (Abbott, Capper, et al., 2004b); (Gambino & Bros, 1988). Type V DES is a new type of DES, which has recently been described (Abbott et al., 2007). They consist of non-ionic molecular substances such as donors and acceptors of hydrogen bonds. They are non-ionic DESs formed by compounds such as thymol, menthol in a 1:2 M ratio. Although ionic contribution is not present in DESs type V have the characteristics of the DESs melting point. This could be due to the large number of hydrogen bonds present in these DESs (Abranches et al., 2019).
Natural eutectic solvents (NADES): those DESs derived from cellular metabolites such as alcohols, amino acids, organic acids and sugars (Xie et al., 2019). NADES have a fundamental role in cell metabolism and in many biological processes (germination, resistance to follow, dehydration, etc.) (Shaibuna et al., 2022). They also play an important role in the cryopreservation of organs in living organisms (Gertrudes et al., 2017). In addition, they are used in extraction, chromatography, biomass pre-treatment and enzymatic saccharification (Liu et al., 2018).
The DESs mentioned above are reviewed in this paper for the hydrometallurgical recovery of metals. However, in recent times another kind of DESs are gaining more attention. Therefore, it is considered necessary to mention in this review (Shaibuna et al., 2022).
Therapeutic DESs: those in which one of its active ingredients is a pharmaceutical compound (Duarte et al., 2017). This type of DES is used to reduce problems related to drug solubility, bioavailability, difficulty in manufacturing, handling and permeability (Aroso et al., 2016).
Polyuasi-eutectic solvents (PQESs): The term PEGylated was proposed by Jiang et al. (Jiang et al., 2017), called these systems as poly-quasi-eutectic solvents (PQESs) (Jiang et al., 2019). These are obtained from polymers such as polyethylene glycol (PEG) poly(ethylene glycol)-block-poly(propylene glycol)- block poly(ethylene glycol) (P123), poly(propylene glycol)bis(2- aminopropyl ether) (PPG-NH2), and poly(ethylene glycol) dimethyl ether (DMPEG) and donors of hydrogen bonds as carboxylic acids and amides (Jiang et al., 2019). Its application consists of the evolution reaction of oxygen and for the processing of metal oxides.
Deep eutectic polymeric solvents (PDESs) (Ren’ai et al., 2018): those DESs are considered to be the hydrogen donor part is polymerizable. They are obtained from various ammonium salts and acrylic/acrylic acids such as HBDs (Mota-Morales et al., 2013). Reactivity and frontal polymerization capability is based on the choice of ammonium salt the fully converted polymer can have medical applications for drug administration. PDESs have been successfully applied in nanotechnology (K. Zhang et al., 2020), gas separation (Isik et al., 2016) and catalysis (Ishaq et al., 2020).
4. Preparation methods
DESs synthesis methods are simple because they do not require multiple steps or separation methods, such as organic solvents. In addition, it should be noted that most DESs components are cheap and natural (Singh et al., 2021). DESs are obtained from the mixture of HBA and HBD in the appropriate proportions, do not involve chemical reactions and can therefore be called preparation methods and not synthesis methods (Farooq et al., 2020). There are different synthesis methods, including mixing components with mortar and stirring components with heating.
The grinding method was introduced to prepare DESs without heat. It consists of crushing the mixture of the compounds (HBA and HBD) at room temperature, with a mortar or a mortar hand, until the formation of a clear and homogeneous liquid, usually carried out under nitrogen atmosphere or in a glovebox. This method was introduced by Florindo et al. (Florindo et al., 2014) for the preparation of DESs based on choline chloride and carboxylic acids (Florindo, Oliveira, et al., 2017) (Florindo et al., 2018).
The most commonly used method for the preparation of DESs are the method of heating (between 323 and 373 K), under agitation of the mixture of the compounds until the formation of a homogeneous liquid (Abbott et al., 2001)(Shaibuna et al., 2022). The temperature is selected according to the melting point, the boiling point of the reagents and the stability of the reagents. High temperature may lead to potential DESs degradation due to esterification reactions (Abbott, Boothby, et al., 2004)(Abbott, Capper, et al., 2004b), it is essential to identify the appropriate temperature and preparation time.
Apart from these two more traditional methods of preparing DES, other methods based on lyophilization are also included in the literature, which involves the dissolution of the DESs components separately in the minimum amount of bi-distilled water the aqueous solutions were frozen between 77 and 253 K, subsequently freeze-dried obtaining viscous and clear liquids (Gutiérrez et al., 2010) (Nam et al., 2015).
The vacuum evaporation method was first used for the preparation of NADES. Dai et al. (Dai et al., 2013) (Dai et al., 2015) studied a method of DESs synthesis from evaporation methods, first dissolve the DESs components in water and then undergo evaporation at 323 K. Then the DESs is stored in a silica geldesiccator. This method uses relatively lower temperatures compared to the heating and stirring method and is frequently used for components with higher melting points. Considering the optimization of time and energy consumption Gómez et al. (Gomez et al., 2018) prepared Natural DESs from a more environmentally friendly method assisted by microwaves. With this objective Santana et al. (Santana et al., 2019) developed an unconventional NADES synthesis method assisted with an ultrasound bath, previously the mixtures were homogenized with a vortex.
The twin screw extrusion method is a preparation method that is used to overcome the limitations of heating and stirring methods in the preparation of DESs (Shaibuna et al., 2022)(Crawford et al., 2016). This method consists of two screws that rotate in the opposite direction, are housed in a stainless-steel barrel, where multiple transport and kneading sections are located. In the transport sections the materials are moved forward and the kneading section high shear and compression forces are applied on the material as it passes through. HBA and HBD are incorporated in the appropriate proportions after preheating of the double screw sections.
The method that is usually cheaper and faster for DESs production is the microwave irradiation method (Farooq et al., 2020). HBAs and HBDs are microwave irradiated for 20 s (Gomez et al., 2018)(Farooq et al., 2020). The application of this method requires careful optimization of heating time, power and component selection. Another method of preparation is the use of ultrasound (Calvo‐Flores & Mingorance‐Sánchez, 2021); (Farooq et al., 2020). Stoichiometric amounts of HBD and HBA are mixed in a glass vial and sealed and introduced into an ultrasound bath. The time and temperature for DES formation is based on pure constituents.
Solvent addition is not necessary in preparation methods, therefore no purification steps are necessary, increasing their potential as economic substitutes for traditional organic solvents (Hansen et al., 2021).
5. DESs properties in relation with hydrometallurgy process
The physical properties of DES, such as density, viscosity, surface tension and conductivity are essential to determine their applications. Its application in the different industrial sectors depends on these properties, being interesting that they present a low density, viscosity and surface tension (Qin et al., 2020).
The physico-chemical characteristics of DESs depend among other factors on the nature of the hydrogen bond acceptor and donor components, which form the eutectic mixture. It is possible to adjust the physico-chemical characteristics by modifying the molar ratio and the anion size of HBA and HBD (Omar & Sadeghi, 2021). The following sections will analyse the main properties of interest to the DESs.
5.1. Melting point
Deep eutectic solvents can be identified by their depression at the melting point (Tm), Fig. 1. As noted above, DESs have melting points lower than their pure components. To date, specific data on eutectic DESs compositions and accompanying binary phase diagrams have been virtually non-existent. This further underlines the importance of obtaining phase diagrams of all DESs under investigation as they provide information on the temperature and composition range that can be expected from a liquid, which will help other researchers design DESs systems for their specific applications (Hansen et al., 2021). Most papers on DESs analyse the mixtures in their assumed eutectic compositions, but diagrams justifying the choice of composition are not provided.
The depression of the eutectic melting point is due to the strong interaction between the hydrogen bond acceptor (halide anion) and the hydrogen bond donor (E. L. Smith et al., 2014). DEs with melting points below 323 K are the most researched and of great interest due to their low cost and could be used as alternative solvents in a wide variety of industrial applications (Singh et al., 2021). The molar ratio of organic salts, the alkyl chain length and the hydrogen bond donor have a major impact on the melting point of DES, as shown in Table 1.
Table 1. Melting temperature values of different DESs based on (Hansen et al., 2021) (Omar & Sadeghi, 2022).
| HBA | HBD |
HBA:HBD (molar ratio) |
Tm (K) | Reference |
|---|---|---|---|---|
| ChCl | Urea | 1:2 | 285 | (Shah & Mjalli, 2014) |
| ChCl | Glycerol | 1:2 | 290 | (AlOmar et al., 2016) |
| ChCl | Glycerol | 1:3 | 274 | (Shahbaz et al., 2012a) |
| ChCl | Glycerlo | 1:4 | 275 | (Shahbaz et al., 2012b) |
| ChCl | EG | 1:2 | 237 | (Ibrahim et al., 2019a) |
| ChCl | Imidazole | 3:7 | 329 | (Hou et al., 2008) |
| ChCl | Acrylic acid | 1:1.6 | 269 | (Mota-Morales et al., 2011) |
| ChCl | Pyrogallol | 1:1 | −346 | (Omar & Sadeghi, 2021) |
| ChCl | Pyrogallol | 1:2 | 236 | (Omar & Sadeghi, 2021) |
| ChCl | Oxalic, pheny acetic, phenyl propionic, tricarballyclic, succinic and acid citric | 1:1 or 1:2 | 283 (malonic), 293 (phenylpropionic) − 363 (tricarballylic) | (Abbott et al., 2004) |
| ChCl | levulinic acid, itaconic acid, xylitol, D-sorbitol, L-(+)-tartaric acid, D-isosorbide, 4-hydroxybenzoic acid, caffeic acid, p-coumaric acid, trans-cinnamic acid, suberic acid, gallic acid | Varies (1:05. 1:1 or 1:2) | RT* (levulinic) − 366 (suberic) | (Maugeri & Domínguez de María, 2012) |
| ChCl | Phenol, o-cresol, 2, xylenol | 1:3 | 249 (o-cresol) − 290 (2,3-xylenol) | (Guo et al., 2013) |
| Triphenylphosphunium bromide | Etilen glycol | 1:3 | 227 | (Shahbaz et al., 2011b) |
| Triphenylphosphunium bromide | Etilen glycol | 1:4 | 223 | (Shahbaz et al., 2011a) |
| Triphenylphosphunium bromide | Etilen glycol | 1:5 | 265 | (Shahbaz et al., 2011a) |
| Tetraethylammonium bromide | Benzilic acid | 1:1 | 162 | (Omar & Sadeghi, 2020a) |
| Tetraethylammonium bromide | Benzilic acid | 1:1 | 265 | (Omar & Sadeghi, 2020b) |
| Tetrabutylammonium hydrogen sulfate | Benzilic acid | 1:1 | 254 | (Omar & Sadeghi, 2020b) |
| ZnCl2 | EG, urea | 1:4 (1:3.5 urea) | 243 (EG) | (Abbott et al., 2007) |
| Halide salts: MTPB, TBAB, BTPC, DEAC | EG, glycerol | (1:1–1:4) | 242 (EG)-276(MTPB:glycerol) | (Ibrahim et al., 2019b)(Shahbaz et al., 2012b) |
* = observed to be liquid at room temperature.
Bibliography.
Abbott, A. P., Barron, J. C., Ryder, K. S., & Wilson, D. (2007). Eutectic-Based Ionic Liquids with Metal-Containing Anions and Cations. Chemistry - A European Journal, 13(22), 6495–6501. https://doi.org/10.1002/chem.200601738.
Abbott, A. P., Boothby, D., Capper, G., Davies, D. L., & Rasheed, R. K. (2004). Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. Journal of the American Chemical Society, 126(29), 9142–9147. https://doi.org/10.1021/ja048266j.
AlOmar, M. K., Hayyan, M., Alsaadi, M. A., Akib, S., Hayyan, A., & Hashim, M. A. (2016). Glycerol-based deep eutectic solvents: Physical properties. Journal of Molecular Liquids, 215, 98–103. https://doi.org/10.1016/j.molliq.2015.11.032.
Guo, W., Hou, Y., Ren, S., Tian, S., & Wu, W. (2013). Formation of Deep Eutectic Solvents by Phenols and Choline Chloride and Their Physical Properties. Journal of Chemical & Engineering Data, 58(4), 866–872. https://doi.org/10.1021/je300997v.
Hansen, B. B., Spittle, S., Chen, B., Poe, D., Zhang, Y., Klein, J. M., Horton, A., Adhikari, L., Zelovich, T., Doherty, B. W., Gurkan, B., Maginn, E. J., Ragauskas, A., Dadmun, M., Zawodzinski, T. A., Baker, G. A., Tuckerman, M. E., Savinell, R. F., & Sangoro, J. R. (2021). Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chemical Reviews, 121(3), 1232–1285. https://doi.org/10.1021/acs.chemrev.0c00385.
Hou, Y., Gu, Y., Zhang, S., Yang, F., Ding, H., & Shan, Y. (2008). Novel binary eutectic mixtures based on imidazole. Journal of Molecular Liquids, 143(2–3), 154–159. https://doi.org/10.1016/j.molliq.2008.07.009.
Ibrahim, R. K., Hayyan, M., AlSaadi, M. A., Ibrahim, S., Hayyan, A., & Hashim, M. A. (2019a). Physical properties of ethylene glycol-based deep eutectic solvents. Journal of Molecular Liquids, 276, 794–800. https://doi.org/10.1016/j.molliq.2018.12.032.
Ibrahim, R. K., Hayyan, M., AlSaadi, M. A., Ibrahim, S., Hayyan, A., & Hashim, M. A. (2019b). Physical properties of ethylene glycol-based deep eutectic solvents. Journal of Molecular Liquids, 276, 794–800. https://doi.org/10.1016/j.molliq.2018.12.032.
Maugeri, Z., & Domínguez de María, P. (2012). Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: levulinic acid and sugar-based polyols. RSC Adv., 2(2), 421–425. https://doi.org/10.1039/C1RA00630D.
Mota-Morales, J. D., Gutiérrez, M. C., Sanchez, I. C., Luna-Bárcenas, G., & del Monte, F. (2011). Frontal polymerizations carried out in deep-eutectic mixtures providing both the monomers and the polymerization medium. Chemical Communications, 47(18), 5328. https://doi.org/10.1039/c1cc10391a.
Omar, K. A., & Sadeghi, R. (2020a). Novel benzilic acid-based deep-eutectic-solvents: Preparation and physicochemical properties determination. Fluid Phase Equilibria, 522, 112752. https://doi.org/10.1016/j.fluid.2020.112752.
Omar, K. A., & Sadeghi, R. (2020b). Novel benzilic acid-based deep-eutectic-solvents: Preparation and physicochemical properties determination. Fluid Phase Equilibria, 522, 112752. https://doi.org/10.1016/j.fluid.2020.112752.
Omar, K. A., & Sadeghi, R. (2021). Novel Deep Eutectic Solvents Based on Pyrogallol: Synthesis and Characterizations. Journal of Chemical & Engineering Data, 66(5), 2088–2095. https://doi.org/10.1021/acs.jced.1c00023.
Omar, K. A., & Sadeghi, R. (2022). Physicochemical properties of deep eutectic solvents: A review. Journal of Molecular Liquids, 360, 119524. https://doi.org/10.1016/j.molliq.2022.119524.
Shah, D., & Mjalli, F. S. (2014). Effect of water on the thermo-physical properties of Reline: An experimental and molecular simulation based approach. Phys. Chem. Chem. Phys., 16(43), 23900–23907. https://doi.org/10.1039/C4CP02600D.
Shahbaz, K., Baroutian, S., Mjalli, F. S., Hashim, M. A., & AlNashef, I. M. (2012a). Densities of ammonium and phosphonium based deep eutectic solvents: Prediction using artificial intelligence and group contribution techniques. Thermochimica Acta, 527, 59–66. https://doi.org/10.1016/j.tca.2011.10.010.
Shahbaz, K., Baroutian, S., Mjalli, F. S., Hashim, M. A., & AlNashef, I. M. (2012b). Densities of ammonium and phosphonium based deep eutectic solvents: Prediction using artificial intelligence and group contribution techniques. Thermochimica Acta, 527, 59–66. https://doi.org/10.1016/j.tca.2011.10.010.
Shahbaz, K., Mjalli, F. S., Hashim, M. A., & AlNashef, I. M. (2011a). Using Deep Eutectic Solvents Based on Methyl Triphenyl Phosphunium Bromide for the Removal of Glycerol from Palm-Oil-Based Biodiesel. Energy & Fuels, 25(6), 2671–2678. https://doi.org/10.1021/ef2004943.
Shahbaz, K., Mjalli, F. S., Hashim, M. A., & AlNashef, I. M. (2011b). Using Deep Eutectic Solvents Based on Methyl Triphenyl Phosphunium Bromide for the Removal of Glycerol from Palm-Oil-Based Biodiesel. Energy & Fuels, 25(6), 2671–2678. https://doi.org/10.1021/ef2004943.
Anions also have a significant impact on the DESs melting point. Therefore, the DESs formed from choline and urea salt decreased in the order F > NO3 > Cl > BF4, which indicates that there is a strong correlation with the strength of the hydrogen bond as shown in Table 2. According to the above the melting points of eutectic mixtures depends on the way the salt anion interacts with HBDs, the grid energy and the change of entropy in the result of the formation of the liquid phase.
Table 2. Effect of the anion salt on the melting points of the DESs based on (Omar & Sadeghi, 2022).
| HBA | HBD | HBA:HBD(molar ratio) | Tm (K) | Reference |
|---|---|---|---|---|
|
|
Urea | 1:2 | 273 | (Abbott et al., 2003) |
|
|
Urea | 1:2 | 277 | (Abbott et al., 2003) |
 |
Urea | 1:2 | 285 | (Abbott et al., 2003) |
 |
Urea | 1:2 | 340 | (Abbott et al., 2003) |
Bibliography.
Abbott, A. P., Capper, G., Davies, D. L., Rasheed, R. K., & Tambyrajah, V. (2003). Novel solvent properties of choline chloride/urea mixturesElectronic supplementary information (ESI) available: spectroscopic data. See https://www.rsc.org/suppdata/cc/b2/b210714g/. Chemical Communications, 1, 70–71. https://doi.org/10.1039/b210714g.
Omar, K. A., & Sadeghi, R. (2022). Physicochemical properties of deep eutectic solvents: A review. Journal of Molecular Liquids, 360, 119524. https://doi.org/10.1016/j.molliq.2022.119524.
5.2. Density
Density is one of the fundamental properties of DESs to consider in the selection of the solvent and separation performance in the hydrometallurgyprocesses considering the biphasic nature of this methods. In general the density of DESs are higher than that of water, the values oscillate between 1.0 and 1.3 g cm−3 a 298 K, DESs based on metallic salts have a slightly higher density in the range of 1.3–1.6 g cm−3 (Tang & Row, 2013). For example, ethaline has a density of 1.14 g cm−3 and glycine from 1.19 g cm−3 a 293 K. An exception is the case of some hydrophobic DESs, where its density is lower than that of water (Florindo et al., 2019). Table 3 shows the different densities of these compounds.
Table 3. DESs densities at 298 K based on (Hansen et al., 2021)(Ijardar et al., 2022).
| HBA | HBD | HBA:HBD(molar ratio) | Density values (g /ml) at 298 K | Reference |
|---|---|---|---|---|
| ChCl | Urea | 1:2 | 1.21 | (Mjalli & Abdel Jabbar, 2014) |
| ChCl | Urea | 1:2 | 1.25 | (Abbott et al., 2006) |
| ChCl | glycerol | 1:1 | 1.16 | (Abbott et al., 2006) |
| ChCl | glycerol | 1:2 | 1.18 | (AlOmar et al., 2016) |
| ChCl | glycerol | 1:3 | 1.20 | (Abbott, Harris, et al., 2007) |
| ChCl | EG | 1:2 | 1.12 | (Zhang et al., 2020) |
| ChCl | EG | 1.3 | 1.12 | (Abbott, Harris, et al., 2007) |
| ChCl | Malic acid | 1:1 | 1.185 (293 K) | (Yadav et al., 2015) |
| ChCl | Acetic acid | 1:1 | 1.12 | (Zhu et al., 2016) |
| ChCl | D-(+)-glucose, citric acid, D-(+)-sucrose; L-(+)-tartaric acid, D-(+)-xylose | 1:1 | 1.22 (sucrose) − 1.27 (glucose) | (Craveiro et al., 2016) |
| ChCl | Oxalic acid | 1:1 | 1.15 | (Florindo et al., 2014) |
| ChCl | amines: MEA, DEA, MDEA | 1:6 | 1.056–1.102 (293 K) | (Adeyemi et al., 2018) |
| ChCl | phenol, o-cresol, 2,3-xylenol | 1:3 | 1.071 (cresol), 1.095 (phenol) | (Guo et al., 2013) |
| ChCl | Glycolic acid | 1:1 | 1.259 | (Florindo et al., 2014) |
| ChCl | Malonic acid | 1:1 | 1.231 | (Florindo et al., 2014) |
| ChCl | 1-(trifluoromethyl) urea | 1:1.15 | 1.324 | (Abbott et al., 2006) |
| ChCl | Glucose | 2:1 | 1.2423 | (Mjalli & Ahmad, 2017) |
| ChAc | Urea | 1:2 | 1.206 | (Abbott et al., 2006) |
| ZnCl2 | Acetamide | 1:4 | 1.36 | (Abbott et al., 2004) |
| ZnCl2 | EG | 1:4 | 1.45 | (Abbott, Barron, et al., 2007) |
| ZnCl2 | Urea | 1:3.5 | 1.63 | (Abbott, Barron, et al., 2007) |
| MTPB, BTPC, DEAC, TBAB | EG | 1:2 (1:11 BTPC) | 1.07 (TBAB) − 1.24 (MTPB) | (AlOmar et al., 2016) |
| MTPB, BTPC, ATPB, DEAC, TBAB | Glycerol | 1:3 (MTPB), 1:4 (TBAB), 1:16 (BTPC) | 1.17 (TBAB) − 1.30 (MTPB) | (AlOmar et al., 2016) |
| Decanoic acdi | lidocaine, atropine, menthol | 2:1 (1:1 menthol) | 0.899 (menthol) − 1.026 (atropine) | (van Osch et al., 2019b) |
| dodecanoic acid | lidocaine, atropine | 2:1 | 0.949 (lidocaine) − 1.008 (atropine) | (van Osch et al., 2019a) |
| Menthol | lidocaine | 2:1 | 0.939 | (van Osch et al., 2019a) |
| Thymol | lidocaine, coumarin, menthol | 1:1 | 0.936 (menthol) − 1.091 (coumarin) | (van Osch et al., 2019a) |
Bibliography.
Abbott, A. P., Barron, J. C., Ryder, K. S., & Wilson, D. (2007). Eutectic-Based Ionic Liquids with Metal-Containing Anions and Cations. Chemistry - A European Journal, 13(22), 6495–6501. https://doi.org/10.1002/chem.200601738.
Abbott, A. P., Capper, G., Davies, D. L., & Rasheed, R. K. (2004). Ionic Liquid Analogues Formed from Hydrated Metal Salts. Chemistry - A European Journal, 10(15), 3769–3774. https://doi.org/10.1002/chem.200400127.
Abbott, A. P., Capper, G., & Gray, S. (2006). Design of Improved Deep Eutectic Solvents Using Hole Theory. ChemPhysChem, 7(4), 803–806. https://doi.org/10.1002/cphc.200500489.
Abbott, A. P., Harris, R. C., & Ryder, K. S. (2007). Application of Hole Theory to Define Ionic Liquids by their Transport Properties. The Journal of Physical Chemistry B, 111(18), 4910–4913. https://doi.org/10.1021/jp0671998.
Adeyemi, I., Abu-Zahra, M. R. M., & AlNashef, I. M. (2018). Physicochemical properties of alkanolamine-choline chloride deep eutectic solvents: Measurements, group contribution and artificial intelligence prediction techniques. Journal of Molecular Liquids, 256, 581–590. https://doi.org/10.1016/j.molliq.2018.02.085.
AlOmar, M. K., Hayyan, M., Alsaadi, M. A., Akib, S., Hayyan, A., & Hashim, M. A. (2016). Glycerol-based deep eutectic solvents: Physical properties. Journal of Molecular Liquids, 215, 98–103. https://doi.org/10.1016/j.molliq.2015.11.032.
Craveiro, R., Aroso, I., Flammia, V., Carvalho, T., Viciosa, M. T., Dionísio, M., Barreiros, S., Reis, R. L., Duarte, A. R. C., & Paiva, A. (2016). Properties and thermal behavior of natural deep eutectic solvents. Journal of Molecular Liquids, 215, 534–540. https://doi.org/10.1016/j.molliq.2016.01.038.
Florindo, C., Oliveira, F. S., Rebelo, L. P. N., Fernandes, A. M., & Marrucho, I. M. (2014). Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustainable Chemistry & Engineering, 2(10), 2416–2425. https://doi.org/10.1021/sc500439w.
Guo, W., Hou, Y., Ren, S., Tian, S., & Wu, W. (2013). Formation of Deep Eutectic Solvents by Phenols and Choline Chloride and Their Physical Properties. Journal of Chemical & Engineering Data, 58(4), 866–872. https://doi.org/10.1021/je300997v.
Hansen, B. B., Spittle, S., Chen, B., Poe, D., Zhang, Y., Klein, J. M., Horton, A., Adhikari, L., Zelovich, T., Doherty, B. W., Gurkan, B., Maginn, E. J., Ragauskas, A., Dadmun, M., Zawodzinski, T. A., Baker, G. A., Tuckerman, M. E., Savinell, R. F., & Sangoro, J. R. (2021). Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chemical Reviews, 121(3), 1232–1285. https://doi.org/10.1021/acs.chemrev.0c00385.
Ijardar, S. P., Singh, V., & Gardas, R. L. (2022). Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents. Molecules, 27(4), 1368. https://doi.org/10.3390/molecules27041368.
Mjalli, F. S., & Abdel Jabbar, N. M. (2014). Acoustic investigation of choline chloride based ionic liquids analogs. Fluid Phase Equilibria, 381, 71–76. https://doi.org/10.1016/j.fluid.2014.08.017.
Mjalli, F. S., & Ahmad, O. (2017). Density of aqueous choline chloride-based ionic liquids analogues. Thermochimica Acta, 647, 8–14. https://doi.org/10.1016/j.tca.2016.11.008.
van Osch, D. J. G. P., Dietz, C. H. J. T., van Spronsen, J., Kroon, M. C., Gallucci, F., van Sint Annaland, M., & Tuinier, R. (2019a). A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustainable Chemistry & Engineering, 7(3), 2933–2942. https://doi.org/10.1021/acssuschemeng.8b03520.
van Osch, D. J. G. P., Dietz, C. H. J. T., van Spronsen, J., Kroon, M. C., Gallucci, F., van Sint Annaland, M., & Tuinier, R. (2019b). A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustainable Chemistry & Engineering, 7(3), 2933–2942. https://doi.org/10.1021/acssuschemeng.8b03520.
Yadav, A., Kar, J. R., Verma, M., Naqvi, S., & Pandey, S. (2015). Densities of aqueous mixtures of (choline chloride + ethylene glycol) and (choline chloride + malonic acid) deep eutectic solvents in temperature range 283.15–363.15 K. Thermochimica Acta, 600, 95–101. https://doi.org/10.1016/j.tca.2014.11.028.
Zhang, Y., Poe, D., Heroux, L., Squire, H., Doherty, B. W., Long, Z., Dadmun, M., Gurkan, B., Tuckerman, M. E., & Maginn, E. J. (2020). Liquid Structure and Transport Properties of the Deep Eutectic Solvent Ethaline. The Journal of Physical Chemistry B, 124(25), 5251–5264. https://doi.org/10.1021/acs.jpcb.0c04058.
Zhu, S., Li, H., Zhu, W., Jiang, W., Wang, C., Wu, P., Zhang, Q., & Li, H. (2016). Vibrational analysis and formation mechanism of typical deep eutectic solvents: An experimental and theoretical study. Journal of Molecular Graphics and Modelling, 68, 158–175. https://doi.org/10.1016/j.jmgm.2016.05.003.
The density of DESs depends on the organization and molecular packaging, it is affected by the existence of gaps and vacancies within liquid DESs. That is why the density of DESs urea/choline chloride is higher than that of the urea/acetylcholine chloride system due to the presence of a large hole in acetylcholine chloride (Vigier et al., 2012)(Ul Haq et al., 2022)(Abbott et al., 2007). Another parameter that affects the density of DESs is the molar ratio HBA and HBD.
Abbot et al. (Abbott, Capper, & Gray, 2006) indicated that the addition of choline chloride to glycerol reduces the density of DESs due to increased free volume. An increase in the length of the alkyl cation chain leads to a decrease in the density of DESs, as follows: tetraethyl ammonium bromide > tetra propyl ammonium bromide > tetra butyl ammonium bromide. This indicates that the increase that occurs in the free volume is due to the elongation of alkyl chain length (García et al., 2015)(Montalbán et al., 2015).
Basaiahgari et al. (Basaiahgari et al., 2018) measured the density of ethylene, diethylene and triethylene glycol and glycerol as HBD and benzyl ammonium chloride salts as HBA. The results showed that the DESs obtained from ethylene glycol has a lower density than the DESs obtained from glycerol. According to the authors this increase in density by replacing ethylene glycol with diethylene glycol, triethylene glycol and glycerol means that the increase in the number of OH functional groups in HBD increases the formation of more H bonds, resulting in a decrease in the average available volume.
The composition and molar ratio between HBA and HBD are ways of modifying the density of eutectic mixtures. DESs based on ChCl and citric acid were studied by Shafie et al. (Shafie et al., 2019), observed that as the amount of ChCl increases in relation to citric acid, density decreases and vice versa, an increase in citric acid means an increase in viscosity.
The density of the deep eutectic solvent shows a temperature-dependent behaviour, decreasing linearly as the temperature increases, due to the thermal expansion of DES (Cui et al., 2017)(Ibrahim et al., 2019)(Florindo et al., 2014)(Shahbaz et al., 2012). According to Hole theory thermal energy can generate fluctuations in local densities, which leads to the increase of space between the HBA and HBD of the liquid DESs system (Omar & Sadeghi, 2022). The effect of the temperature on the density of DESs could be expressed in terms of isobaric thermal expansion coefficients, Eq. (1), which defines the available volume of free DES (Ijardar et al., 2022). The calculation of this expansion coefficient may be useful for understanding the compressible behaviour of DES.(1)
So, the linear decrease in the density of the DESs is observed with an increase of the temperature, resulting in the availability more free space, the available space in DESs is related to change in α values. The value of α in DESs are minimal compared with the value of common solvents therefore the temperature showed a little effect on density and the isobaric thermal coefficient. DESS expanded or compressed less in comparison to ILs and other organic solvents.
5.3. Viscosity
Viscosity is another important factor of DESs due to its influence in separation application, such as leaching or liquid–liquid extraction. The DESs viscosity has extensively studied Table 4. Viscosity can be defined as the resistance of a fluid in response to a deformation at a given shear speed. This indicate that fluids with low viscosities flow easily, however, liquids with higher viscosities have a slower flow. Most DESs are recognized as viscous liquids at room temperature, with a viscosity ƞ>100 mPaּS (El Achkar et al., 2021), this makes it difficult to use in commercial applications such as catalysis, synthesis etc. compared to other types of compounds (Yadav & Pandey, 2014). The viscosity of DESs are higher than that of water, but comparable to that of ionic liquids. This increased viscosity is attributed to the presence of an extensive network of hydrogen bonds along with other interactions such as Van de Waals forces and electrostatic interactions between donor and acceptor of hydrogen bonds of DES components, which leads to high viscosity and lower ionic mobility in the small empty volume within the liquid DES (Omar & Sadeghi, 2022).