1. Introduction
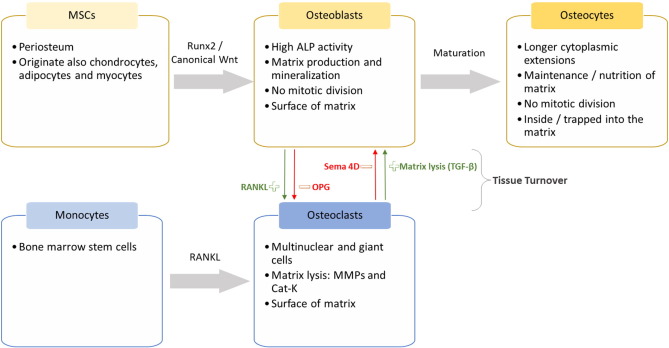
Bone is a mineralized conjunctive tissue, with a unique trauma healing capability [1]. Osteoblasts, osteocytes and osteoclasts are the specialized bone cells (Fig. 1).

Fig. 1. The specialized bone tissue cells: origin and characteristics.
Abbreviations: alkaline phosphatase – ALP, cathepsin K – Cat-k, matrix metalloproteinase – MMP, mesenchymal stem cell – MSCs, osteoprotegerin – OPG, receptor activator of nuclear factor κB ligand – RANKL, Runt-related transcription factor 2 - RUNX2, semaphorin-4D – SEMA 4D and transforming growth factor beta - TGF-β.
When a bone defect occurs due to injury, cells are supplied from the periosteum and the regeneration occurs [1], [2]. However, satisfactory regeneration becomes more difficult the wider the bone defect. That is why even with this property of healing in many situations bone graft is necessary [3]. Orthopedic procedures, like pseudo-arthrosis chirurgical treatment, hip or knee arthroplasty, arthrodesis, and tumor removal commonly require bone graft [4]. Furthermore, oral and maxillofacial procedures often demands replacing the missing bone (Fig. 2). For example, to allow the correct implant insertion, based on the prosthesis ideal position when the residual bone is not enough, to optimize functional/biomechanics results and to improve gingival and facial aesthetic [3].
 Fig. 2. Example of bone draft demand in oral surgery. Maxillary sinus pneumatization caused by loss of tooth, where the installation of dental implant is not possible by the lack of bone height. Radiograph assigned by Victor Z Martin DDs.
Fig. 2. Example of bone draft demand in oral surgery. Maxillary sinus pneumatization caused by loss of tooth, where the installation of dental implant is not possible by the lack of bone height. Radiograph assigned by Victor Z Martin DDs.Also important is the expected population aging along with the increasing on chronic diseases as obesity and diabetes, meaning that the number of patients undergoing orthopedic and/or dental procedures will raise and, consequently, the need for optimal bone grafts will greatly increase.
To date, the main approach for replacing missing bone is by using grafts. Bone grafts may be autogenous, homogenous, heterogeneous or synthetic. Autogenous means that the bone is removed from the patient's own body, often from the iliac crest, skullcap, mandible or tibia. To the present it is considered the gold standard (Fig. 3) once it contains growth factors for osteoinduction (i.e.to promote the differentiation of cells into active osteoblasts), cells for the osteogenesis and the framework for osteoconduction (meaning bone growth on the surface) [5]. However, it presents fast remodelation and limited source, once a donator area is required. In addition, autogenous grafts are often associated with high surgical risks and morbidity [3].

Fig. 3. The most used current therapies for bone repair and regeneration. Big gap between the gold standard and the current graft alternatives, presenting only one of three capabilities of the autogenous type. Osteoinduction is the stimulation of osteoprogenitor cells to differentiate into osteoblasts; Osteoconduction is the capability of the material of serving as a scaffold for the cells and blood capillary; Osteogenesis is vital osteoblasts that contribute to the growth of new bone along with bone formation.
Abbreviations: Bone morphogenetic protein 2 – BMP-2, bone marrow aspirate – BMA and platelet rich plasma – PRP.
The second most common type of bone grafts is homogenous or allograft, which is a graft removed from human cadavers and it is available in bone banks [5]. Heterogeneous or xenograft is a graft obtained from species other than human, as bovine bone [3]. These types of bone grafts often requires sterilization and deactivation of proteins, remaining only the mineral matrix. Homogenous or heterogeneous grafts are only osteoconductive, meaning that these grafts are often combined with patient's own stem cells (also termed mesenchymal stem cells) or growth factors [6]. That is, osteogenic as bone marrow aspirate (BMA) [7] or osteoinductive compounds as bone morphogenetic proteins (BMPs) or platelet rich plasma (PRP) [8], [9] must be also used in the procedures (Fig. 3).
Alternatively, research has been focusing on finding, safer, less expensive and easier to use synthetic bone grafts. These bone substitutes can be created from biomaterials as hydroxyapatite (HA), tricalcium phosphate (TCP), bioactive glass, ceramics and polymers [5]. To date, those types of grafts can provide only osteoconduction, limiting its usage in bone reconstruction [3].
To improve biomaterials clinical outcome as bone grafts, researchers are trying to combine different compounds as hormones, growth factors and drugs with the materials. Synthetic scaffolds are being developed to deliver different compounds in the target area, aiming to achieve a high local concentration with minimum side effects (Fig. 4), besides maintaining the space, so that defects can be adequately replaced by newly formed bone. These grafts can facilitate the chirurgical procedure, reduce the time of the surgery, as well as the morbidity and provide an unlimited source.
 Fig. 4. Biomaterials as local drug delivery systems. The released compounds from scaffolds are in close contact with the osteoblasts.
Fig. 4. Biomaterials as local drug delivery systems. The released compounds from scaffolds are in close contact with the osteoblasts.With the ongoing intensive research in the field, there is a need to review and update the current advances on the novel approaches in terms of biological and synthetic compounds release by local scaffolds, designed to provide osteoinduction in osteoconductive materials, favoring the enhancement of bone regeneration, in the attempt of replacing the autologous grafts.
Up to date, published reviews are mainly focused on the biomaterial as a bone graft or growth factors role, either without exploring or comparing the enormous potential of local delivery systems in the context of bone regeneration. With that in mind, the present review aims to emphasize the current strategies, including their opportunities and limitations, which explore the role of biomaterials as local delivery systems to improve bone regeneration.
2. Current strategies and research on synthetic grafts as local delivery systems
Current studies exploring different types of biomaterials (metallic, polymeric, ceramic or composites) loaded with biological compounds (growth factors, platelet lysates, hormones, phytohormones) or different types of drugs (antibiotics, alendronate, simvastatin, raloxifene) will be next detailed.
2.1. Growth factors
BMP is a generic name given for specific proteins extracted from bone matrix [3]. These proteins are members of the transforming growth factor-beta (TGF-β) superfamily and the most studied are BMP-2, BMP-3, BMP-4 and BMP-7 [11]. As function mechanism, BMP-2 binds to BMP receptor (BMPr) and participates in the regulation of Runx2 gene expression resulting in osteoblastogenesis activity [12]. Other independent pathways are also reported, involving P38/MAPK pathway and miRNAs, as the enhancement of alkaline phosphatase (ALP) activity [13], as illustrated in Fig. 5. However, BMP-2 usage is associated with severe edemas, undesired ectopic bone formation, delayed bone formation and possibly increased cancer risk [14]. One of the possible reasons for these undesirable side effects is the overdoses of the compound or a burst release, related with the loaded vehicle, as observed with an absorbable collagen sponge (ACS) carrier [15]. Current studies are trying to reach a minimal dosage and a controlled delivery system capable of avoiding the initial high release related to the overdoses side effects (Table 1).

Fig. 5. Simplified BMP-2 signaling pathways, via SMADs and p38. miRNAs can be associated with the BMP signaling.
Abbreviations: Bone morphogenic protein – BMP, Bone morphogenic.protein receptor – BMPr, distal-less homeobox 5 – DLX-5, mitogen activated protein kinase – MKK/ERK, Osterix – OSX, Runt-related transcription factor 2 – Runx2, phosphorylation – Pi, similar Mothers against Decapentaplegic – Smad, TGF beta activated kinase – Tab/tak.
Table 1. Overview of growth factors compound for bone regeneration.
| Type | Active compound | Carrier | Signaling | Limitations/systemic side effects | Reference |
|---|---|---|---|---|---|
| Growth factors | BMP-2 |
Scaffolds: BCPC/BCP honeycomb + β-TCP PLC + HA + Silk PPF + PLGA Graft: BCP Sponge: DSPC + ACS Nanocomplexes: HTCC |
Smads and P38/MAPK pathways |
- High cost - Edema - Cancer risks |
[10] [16] [19] [18] [17] [13] [22] |
| BMP-7 | Microparticles: Chitosan | - Less effective than BMP-2 with the same side effects | [21] | ||
| TGF-β | Scaffold: PLC + HA + Silk |
- Inflammation - ROS - Cancer risks |
[19] | ||
| VEGF | Scaffold: β-TCP + PLGA | VEGF and BMP-2 pathways |
- Unwanted vascularization - Tumor growth |
[25] | |
| PIGF-2 | Nanocomplexes: HTCC | VEGF and Hypoxia pathways |
- Expressed by cancer cells - Osteoclast recruitment and activation |
[22] |
Abbreviations:1,2-distearoyl-sn-glycero-3-phosphocholine – DSPC, Absorbable collagen sponge – ACS, Biphasic calcium phosphate – BCP, Biphasic calcium phosphate composite – BCPC, Bone morphogenetic protein – BMP, hydroxyapatite – HA, Hyaluronic acid – HAc, N-(2-hydroxyl)propyl-3-trimethyl ammonium chitosan chloride – HTCC, Placental growth factor – PlGF, platelet-derived growth factor – PDGF, poly-caprolactone – PLC, poly(propylene fumarate) – PPF, poly(lactic-co-glycolic acid) - PLGA, reactive oxygen species – ROS, Transforming growth factor beta - TGF-β, Tricalcium phosphate – TCP and vascular endothelial growth factor – VEGF.
Various calcium phosphate based scaffolds are being tested as carriers of BMP-2. For example, in the study of Lee et al. [10] it was compared the bone regeneration of a scaffold composed with collagen based biphasic calcium phosphate composite (BCPC) + BMP-2 and biphasic calcium phosphate (BCP) + BMP-2. In vitro results showed that the release of BMP-2 was lower and more constant in the BCPC sample, a desirable effect to avoid the burst release and to improve bone regeneration. The in vivo study showed that the BCPC with BMP-2 presented more bone formation and more osteoblasts, including in the center of the defect, when compared with the other groups in a two and eight weeks period. It was also noticed that BCPC samples are clinically easier to use, present more moldability and adaptability in comparison with BCP samples.
Watanabe et al. [16] developed one scaffold made of honeycomb and β-tricalcium phosphate (β-TCP) that could locally deliver BMP-2. The in vivoresults showed that the scaffolds containing porous between 300 μm and 500 μm with 1000 ng of BMP-2 presented bone formation up to the center of the scaffold and numerous osteoblasts were arrayed, with large amount of capillaries. Additionally infiltration of inflammatory cells was not observed in the scaffolds, showing high biocompatibility of the material.
Other approaches, like particulate BCP loaded with low concentration of BMP-2 (0.005 mg/mL) were tested in a sinus lift procedure [17]. The results showed superior mineralization and the formation of an area with new bone. It was shown that, even in a very low concentration, BMP-2 able to increase bone regeneration with an osteoconductive material in a non-scaffold shape. It also showed that procedures like sinus lift, can present acceptable healing results even when only an osteoconductive material is use in the reconstructive procedure.
Strategies using other type of carriers as liposomes are also being tested. In the study of Crasto et al. [13] a liposome-based carrier system composed by cholesterol, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and polyethylene glycol loaded with BMP-2 was developed. The liposomes were formulated to present sonodisruptable release capability. The in vivo assay compared the effects of an absorbable collagen sponge (ACS) loaded with the regular BMP-2 product and the ACS loaded with the liposomes into biceps femoris muscle pouches in rats. The results between the groups were comparable, suggesting that the liposomes can substitute the regular product, in a more secure way to apply the BMP-2 with a trigger activation control. Unfortunately, this system cannot provide the continuous delivery of the product like the scaffolds, and, in a bone defect, it might be more difficult to activate the liposomes by ultrasound, once these defects could be more profound than an intramuscular wound. Recently, poly-propylene fumarate (PPF) scaffolds were loaded with different amounts of poly(lactic-co-glycolic acid) (PLGA) microspheres used as delivery vehicles of BMP-2 (18). Results showed a sustained release over a period of 14 weeks and ectopic bone formation in a subcutaneous rat model. Interestingly, differences in the release kinetics did not correspond to changes in the osteogenic efficacy of the several tested formulations. More biomaterials should be tested in order to obtain a better understanding of the influence of BMP-2 release on bone formation.
Another possibility of working with BMP-2 in a lower dose exposure is the combination with other growth factors. One of these experiences was performed by Bhattacharjee et al. [19], combining BMP-2 and transforming growth factor beta (TGF-β), which is a multifunctional cytokine responsible for regulating the transcription of different target genes, related with inflammation, proliferation and differentiation of cells [20]. A scaffold composed of poly (ε-caprolactone), nano-hydroxyapatite (25%) and non-mulberry silk fibroin was tested for the release of 100 ng BMP-2 and/or 4 ng TGF-β in vitro. Results showed that the combination of both GF presented superior cell viability, cell proliferation, osteoblastogenesis gene expression and higher level of calcium deposits without presenting a cytotoxic effect. Importantly, results suggested that TGF-β could enhance the BMP-2 effects even in a very low dose delivery system.
Following this line of thinking, Liu et al. [22] studied the association of BMP-2 and placental growth factor (PlGF), which is an angiogenic growth factor [23]. Regarding bone regeneration, PIGF is also important, enhancing markers of bone regeneration in osteoblasts and enhancing osteoclast migration and differentiation. While relatively high concentrations of PlGF enhance angiogenesis and osteoclast recruitment and activation, low concentrations exhibit a direct osteogenic effect, related to the activation of hypoxia-mediated pathways [24]. The performed research compared the effect of local delivery of 1 μg of BMP-2 and 0.5 μg of PIGF-2 by nanocomplexes made with N-(2-hydroxyl)propyl-3-trimethyl ammonium chitosan chloride (HTCC) and heparin in vitro. Results showed that the association of growth factors presented an enhancement of cell proliferation, ALP activity and mineral deposition. As the association with BMP-2 and TGF-β, it can be the solution for the side effects problems of higher dose of the BMP-2 alone, if in vivo assays confirm those advantages.
Other growth factor explored recently is the vascular endothelial growth factor (VEGF), responsible for the promotion of vascular endothelial cells mitosis and the increase of vessels permeability [25]. It is also known that VEGF can lead to an upregulation of BMP-2. Khojasteh [25] fabricated a high porous β-TCP scaffold with controlled releasing of VEGF encapsulated in PLGA on surface, and tested in vitro. Results showed an increase of cell proliferation and matrix production, as well as the upregulation of COL1 and Runx2 gene expression. One concern regarding VEGF use is that it can promote vascularization in non-target sites and enhance the risk of tumor growth in distant areas. In vivostudies should be performed to evaluate the risks of this growth factor [25].
2.2. Platelet lysate
Platelet Lysate (PL) is obtained by the activation of different batches of platelet concentrate produced by plasma apheresis, buffy coat or PRP with three cycles of freezing and thawing (Fig. 6) [26], [6]. In other words, platelets suffer lysis and release growth factors. In the study of Babo et al. [26] an injectable calcium phosphate (CP) cement system incorporating hyaluronic acid (HAc) micro particles loaded with PL or PL directly incorporated in calcium phosphate were developed. The results showed that the incorporation of HAc and PL or PL alone in the CP system increased the porosity and the solubility of the cement. The compressive strength was severely reduced in HAc samples, however no significant changes in PL only sample were observed. Enhanced cell proliferation and expression of osteoblastogenesis genes were present in both samples. The CP with PL resented the best results, without sacrificing the mechanical properties of the cement. Future in vivo studies need to be performed to prove those benefits.

Fig. 6. Platelet lysate preparation methods. Adapted from [6].
Abbreviations: PRP: Platelet rich plasma; PL: platelet lysate.
At present, although PL represents a valuable opportunity as a coating agent for osteoconductive scaffolds as it contains many bioactive factors essential for bone defect repair, some major limitations still persist as reviewed in detail by Altaie et al. [6]. For example, the exact amounts and ratios between the bioactive molecules remain to be established in order to achieve the desired and timely therapeutic effect of bone repair; it is mandatory to establish standard protocols and quality control procedures for PL production and clinical studies investigating the benefits of PL-based products for improving bone regeneration are still lacking.
2.3. Hormones and phytohormones
One of the several functions of hormones in the organism is control the blood levels of calcium and its homeostasis. Estrogens, growth hormones and thyroid hormones, as calcitonin and parathyroid hormone (PTH) are some examples of hormones directly linked to bone metabolism. Current studies are trying to use some of these hormones to improve local bone regeneration (Table 2).
Table 2. Overview of hormones with interest in local delivery applications.
| Type | Active compound | Carrier | Signaling | Limitations/Systemic side effects | Reference |
|---|---|---|---|---|---|
| Hormones | PTH | Scaffold: PLLA + PA | Wnt signaling, VEGF Pathways and PTHr |
- High blood calcium levels - Inhibition of bone regeneration - Cancer risks |
[29] |
| Resveratrol |
Scaffold: PLC + COOH PAA PLC + ALB Systemic: Subcutaneous injection |
ER, Wnt and Sirtuin 1 signaling |
- Low solubility - Short plasma half-life - Low bioavailability |
[38] [40] [39] [35] |
Abbreviations: albumin – ALB, carboxylic acid – COOH, estrogen receptor – ER, Parathyroid hormone – PTH, poly(l-lactic acid) – PLLA, poly-acrylic acid – PAA, polyanhydride – PA, poly-caprolactone – PLC, and vascular endothelial growth factor – VEGF.
PTH is well known to stimulate bone remodeling; its usage is approved by FDA in the treatment of osteoporosis since 2002. Intermittent administration can lead to bone formation, with an increase of osteoblasts activity by stimulating Wnt signaling pathway and the production of pro-angiogenesis growth factors like VEGF and angiopoetin1 [27]. However, in a continuous exposure, increases the osteoclasts activity [28]. In the study of Dang et al. [29], PTH was tested by an in vivo mice model, for systemic and local use, with a local calvarial defect regeneration model and a biodegradable scaffold made with poly(l-lactic acid) and a surface erosion polyanhydride (PA). The obtained results showed that the local intermittent delivery of PTH presented the best regeneration in bone volume and bone area, without important systemic alterations and side effects. Subcutaneous PTH injection presented good bone regeneration results, but also presented systemic side effects and inferior results compared with local intermittent exposure in local bone regeneration.
Estrogens, especially 17β-estradiol (E2), are responsible for the maintenance of bone homeostasis in mammals. For instance, lack of E2 results in bone resorption and the increase of fat tissue in bone marrow. E2, via membrane associated estrogen receptors (ERs) can increase the differentiation of MSCs in osteoblasts instead of adipocytes, by activating MAPK and Erk1/2, increase their activity and inhibits osteoclasts resorption function [30]. However, estrogens were related with increased risks of breast cancer, cardiovascular diseases and thromboembolisms [31].
Resveratrol is a phytoestrogen extracted from grapevine, peanut and other plants when in contact with certain microorganisms. It has been studied intensely for different purposes, like cancer treatment, anti-oxidation, cardio protection, anti-inflammatory and bone regeneration without toxic effects even with long-term use [32]. In MSCs, resveratrol can increase the osteogenic differentiation, the matrix mineralization and decrease the adipogenic transcription factors in different pathways, including ERs independent pathways, such as Wnt signaling and Sirtuin 1 signaling [33]. Furthermore, resveratrol can stimulate BMP-2 production by osteoblasts through Src kinase-dependent ER activation [34]. Regarding the prevention of alveolar bone loss, it is known that resveratrol can inhibit the loss of this type of bone, reducing interleukin-17 in gingival tissue, activating Nrf2 pathway and decreasing oxidative stress and pro-inflammatory cytokine production [35]. In osteoblasts, it is known that resveratrol dose-dependently increased the DNA synthesis and ALP activity [36]. However, resveratrol has low solubility and it is quickly metabolized in human body, presenting an extremely short plasma half-life and low bioavailability [37]. The systemic usage of resveratrol can avoid alveolar bone loss and help in local bone regeneration [35].
Regarding studies focusing on local resveratrol use, the study of Li et al. [38]evaluated a porous scaffold made with acrylic acid, poly-ϵ-caprolactone (PLC), carboxylic acid and resveratrol, covalently bounded with the carboxylic acid and tested in vitro and in vivo. The results showed that the resveratrol scaffold could increase the ALP activity during 21 days showed more proteinaceous matrix and mineralized nodules. No significant difference was found regarding defect size when compared with the control, however, the loaded scaffold presented significantly higher X-ray density and greater area of bone regeneration, with bone-like only in the resveratrol scaffold.
Kamath et al. [39] performed another attempt, with a 3D porous polycaprolactone (PCL) scaffold loaded with resveratrol albumin nanoparticles. The results showed that the samples presented adequate release properties. Cell viability, ALP activity and mineralization were higher in the resveratrol samples, even when compared with the osteogenic medium.
Regarding the potential of resveratrol in reducing inflammation, Wang et al. [40] tested a scaffold made of poly-acrylic acid (PAA) with resveratrol in vivo. The results showed that cell viability increased and the mRNA expression of IL-1β, MMP-13 and COX-2 was reduced, which means reduction of inflammation. Furthermore, the levels of mRNA of SOX-9, Coll II and Coll I were up regulated in the loaded scaffold.
2.4. Antibiotics
Interestingly some antibiotics may have an effect on bone metabolism along with their antimicrobial activity (Table 3). An example is the tetracyclines (TCs) and their derivatives. The effects include the direct interaction with matrix metalloproteinase enzymes (MMPs), tissue inhibitors of MMPs, growth factors and cytokine [41], [42]. Tetracyclines bone effects are also related with the increase of collagen type 1 synthesis, preventing bone loss and increasing bone formation [43].
Table 3. Overview of antibiotics compounds with interest in local delivery applications.