1. Introduction
A perfect two-dimensional material named Graphene, consisting of a single atomic plane of carbon atoms and fundamental unit of almost all confined carbon based nanostructures has its widespread applications in various area [[1], [2], [3], [4]]. Graphene have extraordinary properties such as (i) large theoretical specific surface area of 2630 m2 g−1 [5] (ii) a room-temperature electron mobility of about 200,000 cm2 V−1 s−1 [6] (iii) a Young modulus of 1.0 TPa with 20% third-order elastic stiffness, a tensile strength of 130 GPa [7] (iv) 97.4% transmittance with as low as 125 Ω m−1 electrical sheet resistance [8] (v) a room-temperature thermal conduction of 5300 Wm−1 K−1 [9,10] etc. These interesting and exciting properties were experimentally verified by various researchers [[11], [12], [13], [14]]. Various methods and approaches have been developed so far, for the growth of high-quality, pure and high yield of grapheme. These includes mechanical exfoliation of graphite layers [15], graphene growth on SiC [16,17], large area graphene growth by chemical Vapor deposition on metal substrates [12,18,19] etc. Graphene is also a basic material which after folding in different ways or by aggregation of number of layers results in the carbon allotropes, like 0-D fullerene, 1-D carbon nanotubes, itself 2-D graphene and 3-D graphite [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29]].
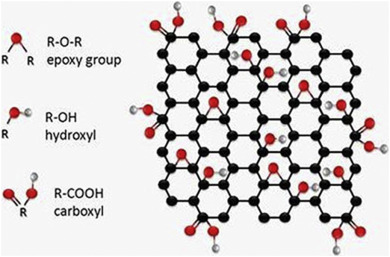
The oxidized form of graphene named as “Graphene oxide” (GO) are produced by the oxidation of bulk graphite powders via chemical oxidation processes [[30], [31], [32], [33]]. Graphene oxide have a mixed structure bearing variety of oxygen-containing various functional groups like epoxy (>O), hydroxyl ( OH), carbonyl (C
OH), carbonyl (C O) and carboxylic (
O) and carboxylic ( COOH) groups as shown in Fig. 1 [34,35]. These functional groups attached on both or either side of the GO sheet stabilizes the sheet on water [36]. These functional groups attached to GO hold great promise for potential applications in many technological aspects as photo catalyst [[37], [38], [39]], photonics [35,[40], [41], [42], [43], [44]], electronics [[45], [46], [47], [48], [49], [50]], composites [[51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61]], electron field emission [[62], [63], [64]] and energy storage devices [[65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75]] etc. The fine biocompatibility, easy and efficient transports into cells, protecting peptides or DNA from enzymatic cleavage, high fluorescence quenching efficiency [76,77], selective adsorption of nucleotides [78] makes GO, a suitable candidate for potential applications in various frontiers areas of research as mentioned earlier. Unlike the highly crystalline graphene, GO is a unique 2D structure having both crystalline and amorphous defect regions, with the presence of sp3 carbon and oxygen functional groups [79,80]. However, functionalization disrupts the electronic structure of graphene making GO an insulator rather than a semi-metal and is conceptually different from graphene.
COOH) groups as shown in Fig. 1 [34,35]. These functional groups attached on both or either side of the GO sheet stabilizes the sheet on water [36]. These functional groups attached to GO hold great promise for potential applications in many technological aspects as photo catalyst [[37], [38], [39]], photonics [35,[40], [41], [42], [43], [44]], electronics [[45], [46], [47], [48], [49], [50]], composites [[51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61]], electron field emission [[62], [63], [64]] and energy storage devices [[65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75]] etc. The fine biocompatibility, easy and efficient transports into cells, protecting peptides or DNA from enzymatic cleavage, high fluorescence quenching efficiency [76,77], selective adsorption of nucleotides [78] makes GO, a suitable candidate for potential applications in various frontiers areas of research as mentioned earlier. Unlike the highly crystalline graphene, GO is a unique 2D structure having both crystalline and amorphous defect regions, with the presence of sp3 carbon and oxygen functional groups [79,80]. However, functionalization disrupts the electronic structure of graphene making GO an insulator rather than a semi-metal and is conceptually different from graphene.

Fig. 1. GO structure containing various functional groups on surfaces and at edges [35].
Reprinted (adapted) with permission from Ref. [35]. Copyright (2013) InTech publisher.
GO and its derivatives have also been successfully tested in numerous applications in bio devices, nanomedicine, biomedical, drug delivery, biotechnology, bioengineering, geno-sensing, imaging of cells, antifungal activity, biosensors, electrochemical sensors and energy storage [[81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98]]. Bulk graphite oxides material which is simply an accumulation of GO flakes can be easily exfoliated into single layer GO sheets by the ultrasonication in water solution or even with simple swirling. The various functional groups on GO sheets like  OH and
OH and  COOH, really work well as a tool for the functionalization of graphene to tune its properties [4,[99], [100], [101]].The various oxygenated functional groups on GO surfaces play very significant and crucial roles towards the properties of GO and hence in the bio-application. The controlled oxidation of GO provides a way to tune its electronic properties, optical transparency, and mechanical characteristics etc. [102,103]. The oxygenated groups increases with increasing oxidation and its electrical properties becomes poorer as compared to highly reduced GO [101]. Thus, the influence of specific different oxygenated groups extremely affects the electrical properties of GO [31]. By taking advantages of this tool various techniques have been adopted to attach variety of molecules to graphene sheets, rendering graphene/GO more versatile precursors for a wide range of applications [[104], [105], [106], [107]]. Multifunctional hybrid nanomaterials with enhanced therapeutic efficiency at physiologically safe dosages for externally triggered, image-guided therapy are highly attractive for nanomedicine. Fig. 2 describes the various bio-applications of GO. The review will mainly focus to these recent bio-applications basically research stepped up till date and future scopes need to step into.
COOH, really work well as a tool for the functionalization of graphene to tune its properties [4,[99], [100], [101]].The various oxygenated functional groups on GO surfaces play very significant and crucial roles towards the properties of GO and hence in the bio-application. The controlled oxidation of GO provides a way to tune its electronic properties, optical transparency, and mechanical characteristics etc. [102,103]. The oxygenated groups increases with increasing oxidation and its electrical properties becomes poorer as compared to highly reduced GO [101]. Thus, the influence of specific different oxygenated groups extremely affects the electrical properties of GO [31]. By taking advantages of this tool various techniques have been adopted to attach variety of molecules to graphene sheets, rendering graphene/GO more versatile precursors for a wide range of applications [[104], [105], [106], [107]]. Multifunctional hybrid nanomaterials with enhanced therapeutic efficiency at physiologically safe dosages for externally triggered, image-guided therapy are highly attractive for nanomedicine. Fig. 2 describes the various bio-applications of GO. The review will mainly focus to these recent bio-applications basically research stepped up till date and future scopes need to step into.

Fig. 2. Graphene-conjugate schematic for the novel multifunctional application of GO in different research field of biotechnological/biomedical.
(Images taken from google: https://dietdietsihat.com/20-tanda-awal-kanser/).2. Graphene oxide based biosensors
Biosensors with the ability to detect biologically active molecules are of critical importance from both biomedical, environmental, and security point of view [99,[108], [109], [110]]. GO, due to coexistence of both hydrophobic and hydrophilic nature due to the presence of pristine graphite structure and oxygen containing functional groups, is among one of the most attributed materials having new possibilities to develop next, new and advanced level of biosensors [46,[111], [112], [113], [114], [115], [116]]. It exhibits not only good water dispersibility, biocompatibility, but high affinity for specific biomolecules [117,118]. These specific and versatile properties of GO provide a lot of opportunities for the development of novel biological sensing platforms, including fluorescence resonance energy transfer (FRET) based biosensors, surface-enhanced Raman spectroscopy (SERS), electrochemical detection and laser desorption/ionization mass spectrometry (LDI-MS), [[119], [120], [121]].
2.1. Fluorescent sensors for DNA/RNA detection
The surface interaction and their understandings between DNA (containing a negative charge due to phosphate backbone) and GO (containing a various functional groups of  OH, C
OH, C O and
O and  COOH groups at their surface and edges) can be of great interest for design and optimization of biosensors and biological devices for disease detections and cure [122]. Recently diverse studies on GO reported the adsorbtion of DNA effectively and hence simultaneously quenched the adsorbed fluorophores with DNA. These interesting behavior of surface adsorption of DNA and quenching of the adsorbed fluorophores opens a new scope to utilize GO as a suitable candidate for DNA based fluorescent biosensors. This DNA and GO interaction strongly depends on temperature, hydrophobic interactions, ion concentration, electrostatic repulsion, pH of the buffer etc.
COOH groups at their surface and edges) can be of great interest for design and optimization of biosensors and biological devices for disease detections and cure [122]. Recently diverse studies on GO reported the adsorbtion of DNA effectively and hence simultaneously quenched the adsorbed fluorophores with DNA. These interesting behavior of surface adsorption of DNA and quenching of the adsorbed fluorophores opens a new scope to utilize GO as a suitable candidate for DNA based fluorescent biosensors. This DNA and GO interaction strongly depends on temperature, hydrophobic interactions, ion concentration, electrostatic repulsion, pH of the buffer etc.
2.1.1. DNA detection
High sensitivity and selectivity for specific DNA sequence is of immense signification in clinical diagnostics, forensic science, environmental monitoring, and a variety of other biomedical applications [[123], [124], [125], [126], [127], [128], [129]]. In DNA detection in solution, GO act as an excellent acceptor of FRET to quench the fluorescence in dye labeled DNA sequences [130]. A fluorophore-labeled single-stranded DNA adsorbed on GO serves as a sensor because subsequent desorption of it in the presence of complementary target DNA enhances the fluorescence. Wu et al. [131] used different length single stranded DNAs 12-, 18-, 24-, and 36-mer for the adsorption on GO. Several factors like organic solvent, pH and cations affected the adsorption and binding of DNA on GO. Rapid absorption and tighter binding on graphene was observed for shorter DNAs as well as lower pH and higher ionic strength favored it. The ionic strength and hence the adsorption was controlled by the ethanol concentration. When the cDNA was added almost 100% desorption was achieved. Temperature dependent desorption was not effective which indicates the high binding affinity between graphene and DNA. The adsorbed DNA was easily exchanged by free DNA in solution which signifies that GO bonded DNAs are reversible and stable. Fig. 3a shows the schematic representation of FAM-labeled DNA adsorption and desorption on GO. An AFM image (Fig. 3b) showing GO sheets deposited on a silicon wafer with height profile (Fig. 3c) of the line in (b) shows the sheets to be ~1.5 nm in thickness. The fluorescence spectra of 100 nM 18-mer DNA in 25 mM HEPES, 100 mM NaCl, and 5 mM MgCl2 in the presence and absence of 50 μg/mL of GO has been shown in Fig. 3d.

Fig. 3. Schematic presentation of FAM-labeled DNA adsorption and desorption on GO.
Reprinted (adapted) with permission from Ref. [131]. Copyright (2011) American Chemical Society.Instead of using monomer only, single- and double-stranded DNAs were used to investigate the interaction with GO [132]. Besides, monovalent and divalent salts were also used to see the effect of binding of nucleic acid to GO. Since divalent ions are efficient to screen the negative charge that is why these ions were much effective than monovalent ions in the adsorption and desorption of ssDNA (single-stranded DNA). The base recognition between the incoming ssDNA and the probe F-ssDNA (FITC-labeled ssDNA; FITC: Fluorescein isothiocyanate) pre-adsorbed on GO was examined by monitoring the increase in fluorescence during the dissociation of probe F-ssDNA from the GO surface. The base complementarity of dsDNA (double-stranded DNA) was analyzed by adding ssDNA (c-ssDNA: complementary-ssDNA or non- c-ssDNA: noncomplementary ssDNA) to the F-dsDNA−GO complex. The results showed that base pairing in dsDNA was exposed to the incoming complementary strand, indicating that hybridization with incoming c-ssDNA on the GO surface was not inhibited. According to the law of mass action, when the GO surface was almost saturated with adsorbed DNA, the incoming DNA molecules simply displace the pre adsorbed DNA molecules on the GO surface.
To understand the mechanism of DNA sensing with GO, a mechanism involving nonspecific probe displacement followed by hybridization in solution phase was reported [133]. Two possible mechanism for the adsorption and desorption of DNA was proposed as shown in Fig. 4. First, Langmuir-Hinshelwood mechanism in which the target DNA is adsorbed on the GO and gets hybridized with probe DNA and then desorbs in the solution producing fluorescence (Fig. 4a). Second is Eley−Rideal mechanism where the adsorbed probe DNA directly reacts with target DNA (they do not need to be adsorbed) dissolved in the solution phase. It directly hybridizes with probe DNA and desorbs in the solution (Fig. 4b). A slight other possibility may be that during the competition for nonspecific surface binding sites between cDNA and probe DNA some probe DNA are displaced by the cDNA into the solution and hybridized with the free cDNA (Fig. 4c). Based on the experiments, it was predicted that the displaced probe DNA was hybridized in the solution. It was found that very low percentage (∼15%) of cDNA was employed for signal generation even after the optimal condition of low-affinity probe and high-affinity cDNA was maintained. It was considered that fluorescence of the fluorophore-labeled probe DNA was quenched by the adsorption of DNA on graphene and hence produced a dark background. Addition of cDNA enhanced the fluorescence due to adsorption of at least one DNA by GO during the hybridization reaction.

Fig. 4. Different possible mechanisms of hybridization between a probe DNA adsorbed by GO and its cDNA (target DNA). (a) Langmuir−Hinshelwood mechanism. (b) Eley−Rideal mechanism. (c) Displacement mechanism.
Reprinted (adapted) with permission from Ref. [133]. Copyright (2013) American Chemical Society.The size effect of GO on the DNA-GO interaction was investigated and found that the larger GO had relatively stronger quenching ability for fluorophore-modified DNAs [134]. Most reported fluorescent sensors relied on DNA probes were based on physiosorbtion by GO, which may suffer from nonspecific probe displacement and false positive signal. Huang et al. [135] reported a molecular beacon (MB) [136] kind of structure comprised of a fluorophore and a quencher joined by DNA for the uniform detection of DNA. GO which served as a quencher was covalently attached to an amino and FAM (6-carboxy fluorescein) dual labeled DNA. A significant challenge was to get rid of non-covalently bounded probes owing to sturdy DNA sorption by GO. In general, high pH, high temperature, low salt concentration, organic salts etc. play important role to for DNA desorption. These parameters were applied to cDNA to have complete desorption of non-covalently linked DNA probes. On the other hand, the covalent probe was highly resistant to nonspecific probe displacement. Zhang et al. [137] fabricated an efficient “molecular beacon” based on Hoechst dyes (HMB) which acted as a fluorescent “on/off” switch where HMB was utilized as signal indicator and GO as an energy acceptor to quench the fluorescence of HMB. The label easy fluorescent HMB was turned on by the detection of sequence-specific DNA and detection of exonuclease with sensitivity and selectivity turned it off. Recently, a series of nanomaterials which can act as efficient fluorescence quenchers were widely studied to build biosensing platforms [[138], [139], [140]]. Metal nanoparticles (Ag, Au etc.) attached to GO has a great capability to open a new avenue of research with significant opportunities in the biomedical field [[141], [142], [143]]. Fan et al. [144] reported a hybrid SERS probe based on GO attached to a popcorn-shaped Au nanoparticle which not only showed an ultrasensitive label-free sensing of HIV DNA (femto-molar level) and bacteria but also provided its chemical fingerprint. In the case of MRSA bacteria this label-free SERS detection limit was as low as 10 CFU/mL.
Determination of human gene-related diseases are a matter of great concern in the modern biomedical science. Huang et al. [145] developed a GO-based microfluidic biosensor for the detection of type, location of the single-base mismatch and influence of the length of the strands by DNA single-base mismatch study. By applying a 40-mer probe DNA (P1), both short (20-mer) and long (60-mer) targets exhibited lower fluorescence signals than the complementary target T1 that was of the same length as P1. The mismatched base type had negligible influence on the results. Seven mismatched strands with different locations were studied. The results indicated that when the dye was tagged at the 5′ end of the probe, the targets with mismatch locations near the 5′ produced higher fluorescence intensity than those near the 3′ end. Huang et al. [146] reported a sensing system for the detection of arbitrary DNA mutations by the probe ssDNA labeled by the fluorescent amidite, ethidium bromide and GO. To develop the sensing system first, the FAM was attached to ssDNA probes and EB molecules which exhibited different fluorescence for the matched, mismatched, and random DNA sequences, and then GO was attached to this probe. The as described sensor FAM-ssDNA probes-EB-GO did not show much difference in the fluorescent signal of the perfectly matched and random DNA whereas a significant change was detected for the mismatched DNA targets. The mutations from normal and random DNA sequences was clearly distinguished by the fluorescent signal ratio of before and after the addition of GO. Among various luminescent probes highly photostable iridium(III) complexes have attracted much attention in biomedical science due to its very high luminescence.
By utilizing the iridium(III) complex Zhao et al. [147] developed a GO based biosensor for target ssDNA detection. Carboxyl group functionalized and activated with N-hydroxysuccinimide, the iridium(III) complex was further tagged to the amino terminated ssDNA. GO, due to its very strong luminescence quenching efficiency for iridium(III) complexes was further combined to this probe to design the ultimate GO-Ir-ssDNA biosensor. This sensor led almost zero background signal whereas the desorption of Ir-ssDNA from GO nanosheets exhibited very strong luminescence. The isolated Ir-ssDNA with specific target formed double helix that produced very high signal-to-background ratio and after UV irradiation the luminescent intensities was much higher than traditional GO based carboxyl fluorescein (FAM) biosensor.
A DNA biosensor platform was prepared by Cristobal et al. [148] by exploiting the FRET pair formed between NaYF4:Yb,Er nanoparticles (UCNP) and GO. The UCNPs were synthesized and functionalized with ssDNA. In the absence of cDNA, the ssDNA-coated UPCNs were physisorbed on the surface of the GO, and their fluorescence was quenched. When the cDNA was present, the UCNPs were not able to physisorb onto the GO template, and thus the fluorescence ability remained unquenched. The fluorescence intensity was correlated to the concentration of cDNA and the detection limit was in the picomolar range. Xing et al. [149] reported that the FRET depends on the interactions and hence the addition order between a fluorescein (FAM)-labeled ssDNA (P), GO, and a cationic conjugated polymer, poly [(9,9-bis(6′-N,N,N-trimethylammonium)hexyl)-fluorenylene phenylene dibromide] (PFP). It was observed that fluorescence change in the system of P-GO-PFP was inefficient when PFP was added into P/GO complex whereas this change was very efficient when P was added to PFP/GO complex while having equal binding tendency of P and PFP to GO. Time-resolved fluorescence and fluorescence anisotropy produced different signals depending on the addition order. Further it was concluded that instead of conventional PFP-based DNA sensor, the GO combined PFP based DNA sensor produce enhanced sensitivity up to the very low limit of 40 pM for target DNA detection. Kumar et al. [150] reported fabrication of rGO-graphene double-layer electrode and its application in label-free detection of DNA. The good electron transfer activity is attributed to a combination of the large number of electroactive sites in rGO and the high conductivity nature of graphene. In the process first the probe ssDNA was immobilized onto the surface of the rGO-graphene double-layer electrode via π–π interaction and then hybridized with its target cDNA. This efficient sensor was able to detect the human immunodeficiency virus 1 gene with a very high detection limit of 1.58 × 10−13 M.
2.1.2. Fluorescent based micro RNA detection
MicroRNA (miRNA) is a small non-coding RNA molecule that regulates gene expression and is frequently dysregulated in various types of human cancers and diabetes [[151], [152], [153]]. It act as a biomarker and various genetic diseases are reported now a days that causes due to dysregulation and hence abnormal expression of miRNA that is closely related to the pathogenesis of most human malignancies [[154], [155], [156]]. Therefore, there is a strong need to develop biosensors or biomarker that could detect miRNA expression levels quantitatively. This kind of biosensor will not serve only for early diagnosis of the diseases but assessing the prognosis and monitoring the response to treatment. Keeping these requirement in view a nanosized GO based miRNA sensor was designed by Ryoo et al. [157] for the quantitative detection of target miRNA expression in living cells. First they utilized nanosized GO (NGO) for the binding with fluorescent dye based peptide nucleic acid (PNA) probes that resulted the significant fluorescent quenching of the dye. Secondly this fluorescence quenching was recovered by the addition of target miRNA. PNA offered various advantages as a probe for miRNA sensing like high sequence specificity, higher loading capacity on the NGO surface compared to DNA. This sensor allowed the detection of specific target miRNAs with the detection limit as low as ~1 pM and the simultaneous monitoring of three different miRNAs in a living cell. Fig. 5 shows the strategy for a miRNA sensor based on NGO and PNA, and emission spectra and fluorescence images obtained for multiplexed miRNA sensing in vitro.

Fig. 5. Schematic representation, emission spectra and fluorescence images obtained for multiplexed miRNA sensor based on NGO and PNA.
Reprinted (adapted) with permission from Ref. [157]. Copyright (2013) American Chemical Society.For the specific and sensitive detection of miRNA, Tu et al. [158] designed a ssDNA probe that provided a region for the interaction of FAM, 6-carboxyfluorescein labeled GO for fluorescence quenching and another sensing region for the recognition and hybridization of target to form duplex. Through the cleavage of RsaI endonuclease the duplex was isolated from the GO surface and hence the fluorescence was recovered. The sensor had a detected limit up to 3.0 fM for miR-126 and a very specific detection of even a single-base mismatch of miRNA sequences. A very high label of detection limit up to 0.4 pM for miRNA was achieved by Hong et al. [159], by utilizing rolling circle amplification (RCA), GO surface, and fluorescently labeled peptide nucleic acid (F-PNA). First the template for RCA was prepared by the selective binding of the padlock probe DNA complementary to a target miRNA to form circular DNA. Further F-PNAs (complementary to the target miRNA) with ssRCA were annealed to create multiple targets for miRNA. The final product as F-PNA/RCAP duplex showed very less adsorption at the GO surface and hence ensues significant reduction in the quenching of the fluorescence signal whereas without miRNA the free F-PNA was completely adsorbed onto the GO resulting fluorescence quenching. Song et al. [78] represented a strategy in which first fluorophore-labeled RNA aptamer was adsorbed on GO surface resulting the fluorescence quenching and further fluorescent signal was re-generated by the addition of complementary blocking DNA that hybridize to the 3′-end of the aptamer and kept apart aptamer to the GO surface. Adenosine-5′-triphosphate (ATP) and guanosine-5′-triphosphate (GTP) are the nucleotides that are the source of energy and signal transducer or an activator in metabolic reactions like protein synthesis, gluconeogenesis and so on. In order to understand the phenomenon and mechanism of production, transduction and consumption, a versatile and efficient sensor is required for the detection of ATP, GTP and their derivatives in living cells. Based on GO nanosheets and DNA/RNA aptamer, Wang et al. [160] fabricated an efficient, selective and sensitive probe for the visualization of multiple nucleoside triphosphates in living cells. Since GO–AuNPs hybrid exhibits better signal enhancement, this hybrid was utilized instead of GO surface only, for selective and sensitive detection of miRNA families by Wang et al. [161]. The GOAu-SPR biosensor showed the equivalent performance to the ones obtained by using qRT-PCR. Besides, it eliminated the requirement of expensive kit of toxic chemicals and special capture probe (e.g. LNA probe) which prove it as a favorable and effective tool in clinical application. It shows that the developed SPR biosensor was able to achieve a detection limit as low as 1 fM.
2.2. Aptamer based DNA detection by coupling mechanism
Aptamers (oligonucleotide or peptides molecules) combined with GO which serve as a functional matrix act as a molecular recognition element for the development of various fluorescent based biosensor. This kind of sensor is very useful for the detection of aptamers, DNAs and various other biomolecules such as mRNA, protein and lipids molecules. Integration of GO with nucleic acids for the sensing and logic gate activation was reported by Liu et al. [162]. The GO immobilized multi fluorophore probes and served as a matrix for the multiplexed analysis of DNA or aptamer substrate complexes and hence the fluorescence signal was quenched effectively. The probe desorption from the surface of the GO triggered the fluorescence associated with the respective analytes. By coupling exonuclease III, Exo III, to the system, the recycling of the target DNAs was demonstrated, and this led to the amplified detection of the DNA targets (detection limit 5 × 10−12 M). This sensing platform had the following advantages (i) The GO acted as single common substrate for the probes modified with fluorophore for various targets. (ii) It sensed the DNA and aptamer substrates both which allowed the fast design of sensing matrices for number of targets.
A nucleic-acid-stabilized silver nanoclusters (AgNCs) and GO functional matrix were utilized as a hybrid material for the fluorescence based detection of DNA or aptamer−substrate by Liu et al. [163] Two different sizes of sliver nanoparticles namely (i) red emitting 616 nm and (ii) near-infrared-emitting (775 nm) were utilized for the DNA protected AgNCs complex. The single-stranded nucleic acids were efficiently conjugated to the DNA protected AgNCs complex leading to significant adsorption of the complex to GO and hence effective fluorescence quenching. The environment of complementary target DNAs to the nucleic acid associated with GO, led to the desorption of the double helix DNA linked to the AgNCs from GO that produced fluorescence which could be utilized as a signal for the sensing activity. A variety of high sensitivity and label free bioprobes have been designed by utilizing the aggregation-induced emission-active molecules for the sensing of aptamers. However, the interaction and hence the binding between aptamer and AIE probe is nonspecific that make this kind of sensor non selective and limits the development. Li et al. [164] developed the AIE-based aptasensor for highly selective and sensitive sensing of targeted DNA and protein with the aid of GO. The AIE-active aptasensor can avoid the notorious ACQ effect and sophisticated fluorophore labeling process of fluorescent probe. GO mediated ssDNA aptamer sensor was developed for the specific detection of T-2 toxin by Chen et al. [165]. A highly efficient ssDNA was achieved after various rounds of selection against T-2 toxin, and this aptamers exhibited a low dissociation constant (Kd) of 20.8 ± 3.1 nM and excellent selectivity for T-2 toxin. By using a specific aptamer Seq.16 as a recognition element, a fluorescent based bioassay was developed for the detection of T-2 in beer samples. This bioassay showed a detection of T2 with a broad linear range from 0.5 to 37.5 μM (R2 = 0.988) and a limit of detection up to 0.4 μM.
Two-photon excitation achieved by utilizing near-infra-red photons as the excitation source have several advantages of lower tissue auto-fluorescence and self-absorption, higher spatial resolution, reduced photodamage/photobleaching and deeper penetration depth >500 μm. A highly selective and sensitive GO/aptamer-based two-photon fluorescent nanoprobe was developed by Yi et al. [77] for in vitro or in vivo detection of biomolecules. This GO/Aptamer-TPdye biosensor exhibited highly selective and sensitive, robust and in vitro efficient detection of ATP even under complex biological conditions. Besides, GO/Aptamer-TPdye conjugated prominently with HeLa cells and zebrafish in vivo, producing a “signal-on” type fluorescence for the specific and high contrast imaging of the infected cells or tissues. Bisphenol A (BPA) is an organic synthetic compound which is widely used to make the certain plastics and epoxy resins. Due to its estrogen-mimicking, hormone-like properties it is a matter of concern for the consumer products and food packaging. A GO based anti-BPA aptamer, biosensor was developed by Zhu et al. [166] to detect BPA. The BPA was conjugated to anti BPA aptamer and hence restricted the adsorption of aptamer on GO surface resulting the fluorescence quenching. The concentration of the BPA highly affected the intensity of the signal of fluorescently modified anti-BPA aptamer (FAM-ssDNA) and GO. This biosensor exhibited the detection limit up to 0.05 ng/mL in the range 0.1–10 ng/mL. In the presence of BPA, BPA preferentially binds to the aptamer because of strong affinity effects, sensing principle shown by a schematic in Fig. 6. The improved biosensing performance was because upon addition of BPA, the anti-BPA aptamer switched its configuration, preventing the aptamer from being adsorbed onto the surface of GO. Hence, at a certain concentration, BPA may couple with FAM-ssDNA, and upon BPA binding, the fluorescence value of FAM-ssDNA, which was very sensitive to the amount of FAM-ssDNA in the solution, changed accordingly compared to the solution without added BPA.

Fig. 6. BPA based biosensor on the target-induced conformational change of the anti-BPA aptamer and the interactions between the FAM-ssDNA probe and GO.
Reprinted (adapted) with permission from Ref. [166]. Copyright (2015) American Chemical Society.2.3. Glucose biosensors
Despite many technological advances research in biosensor, the worldwide increase attention in the prevalence of chronic disease (diabetes mellitus) has been the driving force for the development of glucose sensors [[167], [168], [169], [170]]. Diabetes, occurs due to malfunctioning of pancreatic β cells responsible for production of insulin that control glucose level in the blood [171]. Therefore requirement of an efficient and effective glucose sensor is demand of the time to prevent the leading causes of death and disability in the world [172]. An efficient enzyme electrode was fabricated by Liu et al. [173] by using covalent attachment between carboxyl acid groups of GO sheets and amines of glucose oxidase. The resulting biosensor exhibited a broad linear range up to 28 mM mm−2 glucose with a sensitivity of 8.045 mA cm−2 M−1. Wan et al. [174] developed an electrochemical route combined with GO sheet-mediated Ag enhancement for biological/chemical analyte detection. They detected the targeted bacteria at 1.8 × 102 to 1.8 × 108 cfu mL−1 concentration range without appreciable effort to optimize the sensor system for sensitivity.
The CVD derived Au nanoparticles-encapsulated few layered graphene nanohybrids were synthesized and further utilized as an electrocatalyst the highly sensitive non-enzymatic detection of glucose by Thanh et al. [175]. This highly durable electrode with negligible interference from ascorbic acid, 4-acetamidophenol and uric acid, exhibited an excellent electrocatalytic activity towards glucose oxidation with a wide linear detection range of 6 μM–28.5 μM, low detection limit of 1 μM and a sensitivity of 0.195 μA mM−1 cm−2 at operating potential of 0.0 V. An enzyme like activity of GO integrated with chitosan was demonstrated by Wang et al. [176] and used as an efficient biosensing system for glucose detection. The Chitosan-GO hybrid was demonstrated to be a good enzyme mimetic for oxidation of a typical substrate (TMB) under visible light (λ ≥ 400 nm) stimulation and was independent of destructive hydrogen peroxide. By taking advantage of the enzyme-mimicking activity of CS-GO and the competition interaction between glucose and CS for Con A, a facile, rapid, sensitive, and selective colorimetric method was developed to determine glucose.
2.4. Other types of biosensors
CuO/GO probe fabricated by Song et al. [177] with different size and loading amount of hydrothermally synthesized CuO nanoparticles at different temperature exhibited accurate measurement of glucose in real serum sample with long-term stability, reproducibility, excellent selectivity. The CuO/GO composites with saturated loading of the CuO NPs exhibited a sensitivity of 262.52 μA mM−1 cm−2 to glucose with a 0.69 μM detection limit (S/N = 3) and a linear range from 2.79 μM to 2.03 mM under a working potential of +0.7 V. Yagati et al. [178] demonstrated a methodology to detect insulin in serum at low levels based on Ag nanoflower (AgNF) decorated rGO (AgNF-rGO composite) modified micro-disk electrode arrays (MDEAs). The hybrid interface exhibited enhanced electrical conductivity when compared with its individual elements and had improved capturing ability for antibody–antigen binding towards insulin detection. In order to measure quantitatively the insulin concentration in PBS and human serum, the change in impedance (ΔZ) from electrochemical impedance spectroscopy has been analyzed for various concentrations of insulin in [Fe(CN)6]3−/4− redox couple. The electrode with adsorbed antibodies showed an increase in ΔZ for the addition of antigen concentrations over a working range of 1–1000 ng mL−1. The detection limits were 50 and 70 pg mL−1 in PBS and human serum, respectively.
By incorporating the well-known fluorophore 8-aminoquinoline into GO, Cheng et al. [179] prepared a turn-on fluorescent sensor capable of specific detection of d-glucosamine with a high selectivity and sensitivity which can be used for the design and development of highly selective and sensitive turn - on optical sensors for selective detection of aminosaccharides and many other biomolecules. Based on the various reports in the area of biosensing the role of GO always restricted to the fluorescent quenching and signal transducer due to its size inconsistencies. Chou et al. [180] overcame these issues by using nanoscale GOs (~20 nm) as artificial receptors. The probes by utilizing nanosize GO exhibited enhanced supramolecular response and biomacromolecular affinities with a detection limit of 100 and 10 nM concentrations of an unknown proteins. Huang et al. [181] reported a GO-based fluorescent sensor for the sensing of two hairpin probes and a helper DNA up to detection limit 0.3 nM, for Hg2+ in aqueous solutions by using hybridization chain reactions (HCRs). Without Hg2+, they were adsorbed by the GO and the fluorescence of one of the hairpin probes was quenched. In the presence of Hg2+, the HCRs between the two hairpin probes were initiated by Hg2+ with the aid of the helper DNA through T-Hg2+-T coordination chemistry. Frost et al. [182] investigated the interaction of GO sheets with supported lipid membranes to understand the interaction dependency on GO sheet size (three samples in the range of 90–5000 nm) and on small and large liposomes. The interaction processes were monitored by two complementary, real time, surface-sensitive analytical techniques: quartz crystal microbalance with dissipation monitoring (QCM-D, electroacoustic sensing and indirect nano plasmonic sensing. The results showed that the sizes of each of the two components, GO and liposomes, were important parameters affecting the resulting multilayer structures. Spontaneous liposome rupture onto GO was obtained for large lateral dimensions of the GO sheets.
3. Biomedical and nanomedicine applications of graphene oxide
Astounding GO has been continuously attracting with its potential applications in biomedical fields such as drug/gene delivery [[183], [184], [185], [186], [187], [188], [189], [190], [191], [192], [193], [194]], parasitology [87,195], tissue engineering [[196], [197], [198], [199], [200], [201], [202], [203], [204]], antibacterial [[205], [206], [207], [208], [209], [210]], cancer therapy [[211], [212], [213], [214], [215], [216], [217], [218]], and other biomedical imaging and diagnostics [[219], [220], [221], [222], [223], [224], [225], [226]]. The use of GO in clinical research filed demands essentiality and confirmation of its toxicity and bio-compatibility through extensive in vitro and in vivo studies using specific cell lines, theoretical and animal models [184,227,228]. However, the toxicity issue of GO and its effect on health is a matter of due course, many researchers have shown that its hybrid structures induce low cell toxicity.
3.1. Cancer and other disease detections
Cancer is the uncontrolled growth of cells which causes death, affecting millions of people every year all over the world. With one in nine women expecting to develop breast cancer during their lifetime and one in twenty-nine women dying from it and breast cancer is the most frequent invasive malignancy in women worldwide [[229], [230], [231], [232]]. The GO are used now a day for the development of fluorescent biosensor for the analysis of peptide-receptor interactions using GO and fluorescein isothiocyanate (FITC)-labeled octreotide (FOC) [233]. The advantage of using FOC is that it has a great binding affinity for GO that results a complete fluorescent quenching and further binding of antibody anti-octreotide with FOC lead to efficient recovery of fluorescent signal. This GO-FOC biosensor can be utilized to image somatostatin receptor subtype 2 overexpressed AR42J tumor cells, which demonstrates high promise for molecular imaging in cancer diagnosis. A proper mapping of cancerous cell through magnetic resonance imaging (MRI) and hence targeted drug delivery is a great issue these days in biomedical applications and demands efficient MRI contrast agents (CAs) to improve the quality of MRI-based diagnosis. Zhang et al. [234] developed a positive T1 MRI CA, based on GO-gadolinium (Gd) complexes by chemical conjugation of GO with diethylenetriaminepentaacetic acid (DTPA) followed by Gd(III) complexation, to form a T1 MRI CA (GO-DTPA-Gd). This system significantly improved MRI T1 relaxivity and led to a better cellular MRI contrast effect than Magnevist, a commercially used CA. Besides loading of doxorubicin (DOX) an anticancer drug on GO exhibited significant cytotoxicity to the cancer cells (HepG2). Porous platinum nanoparticles (PtNPs) on GO can catalyze the reaction of peroxidase substrate in the presence of hydrogen peroxide. On the basis of the peroxidase-like activity, Zhang et al. [235] used the PtNPs/GO as a signal transducer to develop a colorimetric assay for the direct detection of cancer cells. They chose Folic acid as a recognition element to functionalize PtNPs/GO as folate receptors are over expressed on the cell membranes and hence can effectively target many tumor cells including ovarian, endometrial, colorectal, breast, lung, renal cell carcinomas, brain metastases derived from epithelial cancers, and neuroendocrine carcinomas. The selective binding PtNPs/GO converted the recognition process into a quantitative colorimetric signal even by naked-eye observation, 125 cancer cells (MCF-7) can be distinguished. The working principle is illustrated in Fig. 7.

Fig. 7. Folic Acid Functionalized PtNPs/GO structure for colorimetric Direction of Cancer Cells (Schematic).
Reprinted (adapted) with permission from Ref. [235]. Copyright (2014) American Chemical Society.Huang et al. [236] developed a label-free, ultrasensitive GO based probe for the detection of oligonucleotides by laser desorption/ionization mass spectrometry (LDI-MS). Based on the electrostatic interactions of rhodamine 6G (R6G) and GO, the nanocomposite R6G-modified GO (R6G-GO) probe was prepared. The increase in the R6G signal from the R6G-GO based on the LDI-MS spectra with increasing concentrations of short-length DNA was mainly attributable to the ability of DNA to prevent R6G-GO aggregation and the probe detection of target oligonucleotides was efficient enough to the femtomolar (fM) level. The developed Exo III-pDNAmiRNA-34a/R6G-GO probe has shown efficiency in the analysis of miRNA-34a expression in human cells. Besides the Exo III-pDNASCA/R6G-GO probe was used for the detection of SNP in the Arg249Ser unit of the TP53 gene, which indicated its potential in studying genetic diseases and cancers. Tao et al. [237] reported a fluorescent sensing array based on nanodot-GO probe for the detection of healthy, cancerous, and metastatic human breast cells effectively 49 cells out of 50 breast samples with accuracy up to 98%. The sensing probe nanodot-GO identified breast cancer cells and distinguished between estrogen receptor positive, human epidermal growth factor receptor-2 positive, and triple negative phenotypes by disrupting the probe in the presence of breast cells giving out the luminescent signal. Nergiz et al. [238] demonstrated a novel class of multifunctional hybrid nanopatches comprised of GO and gold nanostars for enhanced photothermal effect and image-guided therapy into epithelial breast cancer cells. Apart from a remarkably improved photothermal effect compared to that of either of the components at very low dosages of the hybrids (10 μg/mL GO) and using a low laser power (0.75 W cm−2), the hybrid nanopatches exhibited strong Raman scattering, making them excellent candidates for bioimaging, diagnostics, and image-guided therapy applications.
An aptamer-gold nanoparticle-hybridized GO (Apt-AuNP-GO) probe was developed by Yang et al. [239] for the targeted treatment of tumor cells and to detect heat shock proteins (HSPs) expression as a therapeutic response in human breast cancer cells. The probe not only selectively targeted MUC1-positive human breast cancer cells (MCF-7) due to the specific interaction between the MUC1-binding-aptamer and the MUC1 (type I transmembrane mucin glycoprotein) on cell membrane, but also had a high light-to-heat conversion efficiency for photo absorption of NIR light which exerted therapeutic effects on MCF-7 cells even at an ultralow concentration without damaging the healthy cells. Initially the sensor Apt-AuNP-GO induced transient increased in HSP70 expression, and then decreased that may lead to irreversible damage to Apt-AuNP-GO-treated MCF-7 cell under NIR illumination.
Recently for the therapy of myocardial infarction (MI), GO based mesenchymal stem cell (MSC) implantation has emerged as an effective and prominent tool [240]. However, the poor survival of MSCs implantation due to reactive oxygen species (ROS) generated in the ischemic myocardium after the restoration of blood flow is a matter of great concern, that limits its efficacy. ROS primarily causes the death of implanted MSCs by inhibiting the adhesion of the MSCs to extracellular matrices at the lesion site (i.e., anoikis). For the protection of implanted MSCs from ROS-mediated death, Park et al. [241] proposed the use of GO flakes that can adsorb extracellular matrix (ECM) proteins and hence could improve the therapeutic efficacy. GO can adsorb extracellular matrix (ECM) proteins. The study showed that GO flakes could effectively prevent a series of adverse cell-signaling cascades that resulted in the anoikis of MSCs in response to ROS that were generated in the ischemia-damaged and re-perfused myocardium. This effect was due to the ability of GO flakes to provide a platform for MSC adhesion. The improved survival of MSCs following the implantation of MSC-GO into the infarcted and re-perfused myocardium resulted in the enhanced secretion of reparative paracrine factors and reduced apoptosis of cardiac tissue, which enhanced angiogenesis and improved cardiac function. The adsorption of ECM proteins on GO promoted cell adhesion and the attachment of GO to MSCs (MSC-GO) preserved the cell ECM interactions. While unmodified MSCs would undergo anoikis when implanted into the infarcted region due to hindered cell adhesion by ROS in ischemia and reperfusion injury, MSCs adhering to GO prior to implantation would be able to escape anoikis due to cell-ECM interactions between MSCs and the ECM proteins adsorbed on GO (Fig. 8). To test this first they investigated whether MSC adhesion to GO attenuates ROS-mediated deterioration in adhesion, viability, and paracrine factor secretion of MSCs in vitro. Next, whether the implantation of MSC-GO to the reperfused MI region improved the survival, growth factor secretion, and therapeutic efficacy of implanted MSCs by examining angiogenesis, cardiac repair, and heart functions.

Fig. 8. The effects of GO adhesion on the MSCs prior to MSC implantation on the therapeutic efficacy of the MSCs injected into the infarcted myocardium. The adhesion of MSCs to GO flakes prior to implantation provides the cell-ECM interaction between MSCs and the ECM adsorbed on GO flakes. This allows MSCs to escape anoikis when implanted into ROS-abundant injured heart tissue.
Reprinted (adapted) with permission from Ref. [241]. Copyright (2015) American Chemical Society.The selective, sensitive and effective detection of extremely rare circulating tumor cells (CTCs) in the billions of other blood cells is a great challenge for the early detection of cancer and their treatment and cure on time. Most of the patients die due to very late detection of cancer until it is in the last stage. Driven by this need, Shi et al. [242] developed a sensing probe for the separation and detection of Glypican-3 (GPC3)-expressed Hep G2 liver cancer tumor CTCs from infected blood by utilizing GO quantum dots (GOQD) coated with highly luminescent magnetic particles. This nanoplatform attached with an anti-GPC3-antibody was able to separate selectively 10 Hep G2 hepatocellular carcinoma tumor cells/mL from the 15 mL infected blood sample. The probe anti-GPC3-antibody-attached GOQDs-coated magnetic nanoplatform exhibited very intense and selective imaging of the Hep G2 tumor cell due to its extremely high two photon absorption cross section. Additional experiments with mixture of various other cancer cells like GPC3(-) and SK-BR-3 breast cancer cells with Hep G2 hepatocellular carcinoma tumor cells indicated that the probe was highly selective to Hep G2 cells as shown in Fig. 9.

Fig. 9. (a) Magnetic based selective separation of Hep G2 tumor cells from blood sample using a GPC3-specific monoclonal anti-GPC3-antibody-attached GOQDs-coated magnetic nanoplatform and (b) TPL Imaging of Hep G2 tumor cells Using 960 nm Light after magnetic separation.
Reprinted (adapted) with permission from Ref. [242]. Copyright (2015) American Chemical Society.Among several astounding properties of graphene derivatives, its capability to convert heat from the photon i.e. photothermal ability can be utilized efficiently for the treatment and cure of the cancer cells. In this regard, Mauro et al. [243] studied in vitro test of breast cancer cells (MCF-7) by rGO-induced probe hyperthermia for the effective killing via selective laser beam thermoablation and hyperthermia-triggered chemotherapy. First the biotinylated inulin-doxorubicin conjugate (CJ-PEGBT) was achieved by the linkage of pentynoic acid and citraconic acid to inulin and then was attached to rGO surface to get the final probe. Polyethylene glycol-functionalized nano GO (n-GO) was synthesized by Rubio et al. [244] from alkyne-modified n-GO, using solvent-free click-mechanochemistry, i.e., copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC). The modified n-GO was subsequently conjugated to a mucin 1 receptor immunoglobulin G antibody (anti-MUC1 IgG) via thiolene coupling reaction. Cell targeting was confirmed in vitro in MDA-MB-231 cells, either expressing or lacking MUC1 receptors, using flow cytometry, confocal laser scanning microscopy (CLSM) and multiphoton (MP) fluorescence microscopy.
The chemotherapy being the most effective treatment for the cancer has major drawback of drug resistance to anticancer treatment. To overcome with this problem various combinations of chemo-photothermal and chemo-chemo treatments have been reported so far. Tran et al. [245] proposed a joint therapy to fulfill both purposes of generating heat to kill cancer cells by NIR laser irradiation and simultaneously delivering anticancer drugs too by the use of a probe based on dual anticancer drug (doxorubicin and irinotecan)-loaded GO stabilized with poloxamer 188. This probe exhibited higher cytotoxicity especially in MDA-MB-231 resistant breast cancer cells than the use of bare drug or combination of drug and blank GO. The results indicated that this joint treatment synergistically demonstrate very high therapeutic efficacy than the bare chemotherapy or photothermal treatment. Kenry et al. [246] investigated the cellular interactions between the graphene material films and breast cancer cell lines, specifically the effects of these films on cellular proliferation, spreading area and cytotoxicity. The GO film selectively accelerated the proliferation of both metastatic (MDA-MB-231) and nonmetastatic (MCF-7) breast cancer cells, but not that of noncancer breast epithelial cells (MCF-10A) whereas this accelerated proliferation was not observed with the use of graphene (G) film. The observed phenomena originated from the synergistic effect resulted from the high loading capacity and conformational change of cellular attachment proteins on the GO film, and the high amount of oxygenated groups present in the material. Fig. 10 shows the cellular spreading of human breast cancer cells on different graphene material films.