1. Introduction
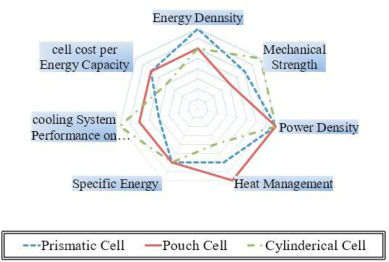
The importance of energy conversion and storage devices has increased mainly in today's world due to the demand for fixed and mobile power. In general, a large variety of energy storage systems, such as chemical, thermal, mechanical, and magnetic energy storage systems, are under development [1]- [2]. Nowadays chemical energy storage systems (i.e., electrochemical batteries) are considered among the most important types of energy storage and most used in several applications i.e. chemical energy storage allows technologies suitable for applications having high energy such as electric and hybrid vehicles and renewable and hybrid energy systems, and for those having low energy such as the portable electronic devices [[3], [4], [5]]. Among the electrochemical batteries, lithium-ion (Li-ion) batteries have attracted attention worldwide as a reliable source of energy as they offer high energy density, superior capacity, high efficiency, and long lifetime compared to other kinds of dry batteries [6,7]. As a result of advances in technology, Li-ion batteries have been extensively utilized as sources of electrical power for portable devices, wireless gadgets, and laptops since they were commercialized in 1991 [8]. Different designs of Li-ion cells are available in the market such as cylindrical, prismatic, and pouch designs to be suitable for a considerable number of applications [9]. A comparative summary of the popular design properties is represented in Fig. (1). The figure shows that the cylindrical Li-ion cell offers the features of low cost per unit and flexibility of system construction. In addition, due to having small size and thickness, the cylindrical design has easy temperature control; however, it is a small capacity per unit. The Prismatic design for Li-ion cells is the type that provides high energy content while it is similar in construction to cylindrical cells. The third design form is the pouch cell which uses a laminated flexible aluminum or polymer is used as a cell bag as opposed to the rigid metal housing in the case of the prismatic cell. Generally, repealing the rigid metal case decreases the cost, weight, and thickness of the cell, where the cell may suffer from the swelling problem, which reduces the lifetime, and capacity, and makes the cell less safe [[10], [11], [12], [13], [14]].
 Fig. 1. Comparison between properties of common lithium-ion cell designs [[10], [11], [12], [13], [14]].
Fig. 1. Comparison between properties of common lithium-ion cell designs [[10], [11], [12], [13], [14]].A typical Li-ion cell has two main parts; the negative terminal (a graphite anode) of the battery and the positive terminal (the cathode, lithium metal oxide) [15,16]. The charging/discharging process of Li-ion batteries is characterized by transferring lithium ions and electrons in what is called the ionization and oxidation process [17,18]. The other two parts of the Li-ion cell are the separator and the electrolyte. The separator lies between the electrodes to prevent electrical contact while allowing ions to pass through. The electrolyte material is used to facilitate ionic conduction [19]. Electrolytes of the Li-ion cells are non-aqueous solutions of lithium-containing salts, such as lithium hexafluorophosphate (LiPF6), melted in the solvent of organic liquid mixtures, e.g., ethylene carbonate, methyl carbonate, or propylene carbonate [20]. The electrode material provides electrons for the current collector which transports them to the external circuit and allows current flow [21].
Due to its demonstration of high energy quality and energy density, Li-ion batteries are preferable among other battery kinds such as nickel-cadmium and lead-acid [[22], [23],24]. In addition, Li-ion batteries have a long lifetime, fast charging capability, high capacity, and high discharge rate [16]. On the contrary, Li-ion batteries have some drawbacks such as degradation of the battery performance at high temperatures, and severe degradation with over-discharging and overcharging, accordingly, safety is likely the highest concern. Thermal runaway can be caused by extremely high temperatures, overcharging of the battery, or physical damage to the battery. As a consequence, systems such as mechanical separators and battery thermal management systems(BTMS) should be utilized for maintaining the battery in suitable operating conditions and preserving it in face of the over-discharge, overcharging, and abnormal thermal conditions [6].
In the literature, research on BTMS can be classified into two directions: the first one is the internal thermal management systems when the inside temperature of the Li-ion battery cell is required to be treated. While the second research direction is external thermal management, through which the surface temperature of the battery pack is targeted to be minimized. The abnormal increment of the internal temperature of the cell may be occurred because of one or more of the internal battery faults i.e., over-discharge, internal and external short circuits, overheating, accelerated degradation, and thermal runaway. The abnormal increment of the internal temperature of the cell may be reflected as a raise in the Li-ion battery surface temperature and accordingly, the overall temperature of the battery pack will be increased. Moreover, the abnormal increase of the external temperature of the cell may happen as a result of one or more of the external battery faults i.e., faults of cell connection, cooling system, temperature, voltage, and current sensor. In general, both BTMS strategies significantly improve the overall maximum temperature and temperature uniformity of the Li-ion battery; however, each strategy has its advantages and disadvantages [25,26].
Several reviews have been done relevant to the Li-ion battery, for instance, the temperature effect on Li-ion battery was addressed in [27,28], Li-ion battery modeling was discussed in [29,30], and external heating or cooling system strategies were reviewed in [31,32]. In addition, other review articles focused on presenting a specific cooling system technique and its applications [[33], [34], [35], [36]]. On the other side, BTMS via internal cooling of Li-ion battery utilizing advanced cathode materials have been reviewed in [37], whereas [38], [39], [[39a]] show internal cooling techniques via enhancing the efficiency of the anode materials. Generally, most of the current reviews concentrated on enhancing the internal thermal performance of Li-ion batteries through improving cathode, anode, electrolyte, and separator characteristics. However, this review focuses on key technologies for enhancing the external thermal performance of Li-ion batteries including their merits, drawbacks, and applications. Moreover, the sparse literature on external thermal management systems of Li-ion battery pack presented BTMS strategy as stand-alone technique; however, recent researchers utilize two or more external cooling strategies. Accordingly, this review article focuses on both stand-alone and combined external cooling systems. Furthermore, based on analyzing the results of recent conducted experimental works in the literature, effective solutions for battery cycling aging slowdown are summarized in this review paper. To conclude, this article introduces an updated review of researches interested in external thermal management strategies to provide a deep understanding of the recent key technologies utilized for external BTMS. Accordingly, summarizing recently conducted studies for developing the external cooling system of Li-ion batteries utilizing air cooling, liquid cooling, and/or PCM cooling technologies, as well as their features and current challenges is the main objective of this review article. Furthermore, recommendations for appropriate enhancement for each external cooling technique have been provided.
This review is arranged as follows: In Section I, an introduction to the Li-ion battery has been given. Section II, provides an explanation of the effects of high and low temperatures on Li-ion batteries as well as explanation for a Li-ion battery aging. A review on external battery thermal management has been conducted i.e., the different coolant strategies have been described and the recent research conducted on them has been discussed in Section III. The conclusion and recommendations have been given in Sections IV & V, respectively. Finally, list of terms and abbreviations has been given.
2. Temperature impacts on Li-ion battery pack performance
The advanced storage applications, e.g., electric vehicles and hybrid power systems, need large-scale lithium battery packs in Li-ion batteries utilization is the thermal condition managing. The performance of a battery cell depends strongly on its temperature, accordingly, for battery safety, enhanced performance, service life, and cycle stability, the operating temperature of the battery cell must be maintained within a specific range of temperature, i.e. between 15 °C and 35 °C [18], [[40], [41], [42]]. Accordingly, the core of the challenge is designing an energy-efficient battery energy storage system that can mitigate cell overheating more effectively. The impact of battery operation outside the desired temperature range is discussed as follows:
2.1. a. High-temperature impact
Li-ion battery operation at high-temperature results in performance degradation, and poor temperature distribution, and may be led to thermal runaway [43,44]. Jiang and Zhang [45,46] further explained the necessary storage requirements for Li-ion batteries and that the storage environment ought to be dry and clean with an adequate ventilation system and within the desirable ambient temperature, whereas considering battery operation away from direct sunlight is highly recommended. Moreover, the space between the cell and any heat source ought to be more than 2 m long. In addition, the battery cell should not be operating under any mechanical stress. As a rule, during battery cycling (i.e., battery charging/discharging), heat is generated due to the electrochemical reactions and internal resistances. Non-effective heat removal leads to the accumulation of the head inside the battery pack. Commonly, surfaces of the external batteries of the pack have a higher convection coefficient than surfaces of the interior ones; consequently, better heat dissipation can be obtained at the external cells. Thus, there is temperature non-uniformity from cell to cell in the pack, i.e., between the cells at the battery pack center and those at its edges, leading to capacity variation from cell to cell which negatively impacts the performance of the battery pack as whole [18].
On the other hand, a severe problem might occur when the Li-ion battery operates at a high temperature i.e., if the battery temperature overtakes a critical point, thermal runaway occurs [6]. The battery thermal runaway is a chain of internal chemical reactions that can be nearly impossible to stop once it has begun. In thermal runaway, the temperature of a battery cell increases quickly within a fraction of seconds and the saved energy in that battery is unexpectedly released. Thermal runaway risk started at a temperature of 90 °C when the solid electrolyte interphase layer, which is a thin film coating the negative electrode to prohibit the carbon from interacting with the organic solvent in the electrolyte, started to decompose and self-heating is initiated. However, the situation turns into so critical at 100 °C, in these extreme conditions, thermal runaway gives rise to the battery burst and catching fires, whereas, in minor conditions, it causes irreparable damage or melt of the battery, for example, for the lithium iron phosphate batteries (LiFePO4batteries), the solid electrolyte interphase layer decomposes at 100 °C, the separator melts and shrinks at 143 °C, where at a temperature above 150 °C thermal runaway occurs [[47], [48], [49]]. Furthermore, what can worsen the situation in case of a fire outbreak and explosion is the generated oxygen from the electrolyte reaction. In this case and with the fast release of a lot of energy, the safety of individuals is being threatened as a consequence of generating a large amount of toxic gases [[50], [51], [52]]. In summary, the occurrence of the thermal runaway of a battery cell may be due to one of three causes i.e. excessive increase of the internal thermal resistance, short circuit with uncontrollable heat generation, and poor external cooling [48] & [53].
2.2. b. Low-temperature impact
Design of the Li-ion battery cells with a complex material system leads to performance degradation at low temperatures i.e. Li-ion batteries perform poorly at temperatures under 0 °C, whereas the batteries' power and energy are virtually missed when the temperature below −20 °C, [54,55]. This degradation can be attributed to various provenances which are related to the total internal resistance of the Li-ion cells [56,57]. The total internal resistance of the Li-ion cells is fundamentally consisting of a bulk resistance, a solid-state interface resistance, and a charge-transfer resistance. The former indicates the electric conductivity of the electrolyte, separator, and electrodes, while the latter two resistances represent the resistance of the interface layer created on the electrodes' surface, and the faradaic charge-transfer resistance, respectively [55]. The first source is the change of electrolyte properties. The electrolyte viscosity is inversely related to temperature i.e. the viscosity increases as a result of temperature decrease, leading to ionic conductivity reduction, hence, the total battery's internal resistance will increase as a consequence of the increment of the impedance of the directional migration of chemical ions [55], [[58], [59], [60]]. Electrolyte with modified property has been introduced in the literature to develop the operating temperature range [45] & [61]. Charge-transfer resistance is considered an important operator that gives a share in the Li-ion battery's performance degradation at low temperatures. Virtually, the charging process of the battery at low temperatures is more difficult than discharging process as the charge-transfer resistance of an empty battery is immensely larger than that of a charged battery [55] & [62], as well as, the performance degradation at lower temperatures is accompanied with slow diffusion of lithium ions within the electrodes [63].
2.3. c. Lithium-ion battery aging
In recent decades, batteries have become the focus of the attention of many scientists and researchers because of their uses in many applications [64]. However, battery aging is one of the major limitations of these technologies. New and recent applications concentrate on the phenomenon of battery aging while taking into account other requirements. Therefore, this phenomenon is widely utilized to evoke the major consequences and problems of use and time on the battery [65]. Battery aging causes severe harmful effects such as decreasing the battery lifespan and reducing the power efficiency and energy. Subsequently, it raises the failure risk and the cost of applications that utilize those batteries [66]. Thus, knowledge of the mechanisms of battery aging and degradation has become the most challenging and essential goal. Over the years, many studies have attempted to explore battery aging as understanding this process is very complex and difficult due to the interaction of many factors from the mode of use or from the environment to generate different effects of aging [[67], [68], [69]]. Indeed, the different types of batteries have different aging mechanisms, which mainly rely on the material type of anode and cathode. Additionally, the electrolyte impact and the aging of the electrolyte and separator mainly occur at the positive and negative electrodes and in interaction with them. Furthermore, there are important factors that affect battery aging over time such as temperature, energy consumed, mechanical stress, and charging/discharging rate [[70], [71], [72], [73], [74]] [[70], [71], [72], [73], [74]]. In general, the battery aging phenomenon occurs via multiple simultaneous physicochemical processes, including a loss of lithium stock, loss of active lithium ions, and resistance increase [[75], [76], [77], [78]]. Many researchers have studied the aging Mechanisms of the positive and negative electrodes that are presented in [65], [69], [[79], [80], [81]]. Also, several methodologies and models have been done to estimate Battery aging as described in references [78], [[82], [83], [84]]. Two primary criteria for battery aging exist: the first is the reduction in the battery's capacity compared to its nominal value, which is represented by the battery's state of charge. The second criterion is the increase in the battery's internal resistance, which is indicated by the battery's state of health [85]. Two terms are used to express the battery life: cycle-life and calendar-life. Cycle-life equals a complete cycles number that batteries can charge and discharge before their capacity degrade to eighty percent of the initial capacity, hence, cycle-life loss increases with battery usage. Calendar-life represents the time at which the batteries can be stored as inactive until their capacity degrade to eighty percent of the initial capacity. Therefore, the loss of calendar-life begins instantly after manufacturing Li-ion battery and increases with time [86,87]. In another word, the two major battery life loss/degradation features are capacity fade and power fade. Capacity fade means the ability of the battery to store energy is decreased over time and it is grown by usage. The rate of capacity loss depends significantly on its operating conditions such as charge/discharge rate, temperature, charge voltage during cycles, current, and load profiles. Power fade represents a reduction in the amount of power that a battery can supply as a result of the raise in the internal resistance of the battery [86], [88]. Nowadays, battery aging is a challenge for battery energy storage systems. For instance, in [89], the real-time battery degradations under multidirectional energy interactions were characterized by a developed mathematical model. A technical solution for battery protective control was proposed in [64] to ensure the techno-economic sustainability of flexible renewable energy design.
To summarize the effect of the operating temperature on Li-ion batteries, the followings can be presented: the temperature has a critical influence on the Li-ion battery performance. As stated by the scientists, chemical reactions inside Li-ion battery can be excited by a high operating temperature, which is a leading cause for thermal runaway. Contrarily, low temperature decelerates the lithium ions activity as the total internal resistance of the Li-ion cells is increased, resulting in failing of the battery charging/discharging process. The optimal temperature range for safe and reliable Li-ion battery operation is between 15 °C - 35 °C. Concerning battery aging, it is difficult to identify the different mechanisms of the aging phenomenon as they are complex to understand and interdependent with each other.
3. Battery thermal management systems
The main objectives of the BTMSs are maintaining Li-ion batteries at appropriate operating temperatures and guaranteeing their efficient performance, safety during operation, and long life [90,91]. Where the operating temperature of batteries has to be within allowable limits, which is done by dissipating the maximum generated heat [92,93]. Indeed, BTMSs should have some important characteristics like high reliability, low cost, low parasitic power use, and easy maintenance [94,95]. Battery cooling systems such as BTMSs are used to reduce the generated heat in the battery to a reasonable value and consequently control the operating temperature. Cooling systems of batteries can be classified relying on the medium into liquid, air, and phase change material (PCM) cooling systems [96], whereas they can be categorized into active cooling and passive cooling systems [97], or direct cooling and indirect cooling ones [98], according the consumed power.
This article focuses on the recent strategies of the external cooling systems of the batteries that operate in conditions of high temperature. The existing external cooling systems rely primarily on three cooling strategies, i.e., air, liquid, and PCM cooling strategies (the main external cooling systems). In each cooling strategy, the difference between the inlet and outlet temperature of the coolant should be reduced to achieve the greatest possible uniformity of the cell temperature, and also reduce the variance between the cell surface and coolant temperatures to manage the battery heat generation that appears during the operating conditions [99].
3.1. Cooling strategies for battery thermal management system
To mitigate and solve the difficulties and inefficiencies that appear because of overheating, different cooling systems have been employed to manage those conditions. These systems can be divided in the literature into three main categories: those with air, liquid, and PCM cooling strategies [[100], [101], [102], [103]]. In addition, each of the first two categories may include active or passive cooling techniques, while the third cooling category can be classified as passive cooling technology. In contrast to active cooling systems, passive cooling systems can be defined as those systems that are used to preserve the temperature of the cell and its thermal profile in the target extent, and maintain uniformity of the temperature along the battery pack without the need for blowers or flow distributors as well [100], [[104], [105], [106]].
3.1.1. Air cooling strategies
Air cooling systems are widely used in BTMS because of their various features like simple structure, high reliability, low cost, and easy maintenance [[107], [108], [109]]. Those cooling systems can be categorized into two major types; the first is cooling utilizing natural air and the second is cooling utilizing forced air. Due to the many advantages that the forced cooling system offers compared to that offered by the natural cooling system, this research focuses on the cooling system that utilizes the forced air. Forced air cooling can significantly decrease the whole battery module temperature that depending on many factors like the battery module layout, ambient temperature, cooling the air temperature, flow rate, flow area, and path length of the airflow. On the other side, the air cooling system has a notable disadvantage which is poor thermal management, i.e., insufficient cooling effect. This matter leads to undesired operating temperature and non-homogeneous temperature distribution, consequently spreading the thermal runaway [[110], [111], [112]]. The high values of ambient temperatures in the range of 45 °C–50 °C cause raising the internal temperature of the battery pack to a temperature higher than the operating temperature (about 55 °C) hence the thermal runaway [107]. The uniformity of temperature distribution, which depends on the flow rate, is a crucial factor and it has an impact on battery degradation and cycle-life [113]. The maximum temperature difference of the cell rises by increasing the flow rate, consequently increasing degradation, and decreasing cycle-life. This degradation occurs because the heat transfer from the battery pack to air is lower than the heat transfer between cells. As a result, fail of one cell within the battery pack causes the prevalence of thermal runaway over all the battery pack. On the other hand, air cooling strategies can be divided into two types as indicated in Fig (2a) and (2b). The passive air system which does not utilize external power sources for cooling the battery pack as presented in Fig. (2a) is one type, while the other type is the active air system which needs external energy to power fans, pumps, or other actuators used to cool Li-ion batteries as shown in Fig. (2b)) [114]. Furthermore, additional cooling or heating energy can be provided via active system such as evaporative air conditioner or heater, respectively. The power of the active systems is limited to 1 kW, whereas the passive systems have the ability to provide hundreds of watts of cooling or heating power.
 Fig. (2). Cooling systems, (a) passive air (b) active air (c) passive liquid (d) active liquid.
Fig. (2). Cooling systems, (a) passive air (b) active air (c) passive liquid (d) active liquid.A heat pipe is a method to enhance the passive air cooling systems [[114], [115], [116], [117]]. Figure (3) depicts the structure of a heat pipe. A partial vacuum was present in the heat tube's flat copper sheath. The sintered copper powder makes up the capillary structure whereas water is used as the operating liquid of a heat tube. Because of the low pressure and heat absorption by the water on the evaporator's side, the water turns to vapor at a temperature below 100 °C. On the condenser, the heat will be dissipated by the water to be changed back into a liquid. This cycle continues indefinitely [118]. Another option for improving passive air systems is to install a heating, ventilation, and air conditioning system. The thermo-electric module converts the difference in temperature to electric voltage and vice versa. Thermo-electrics feature compact and light constructions, and by changing the polarity, they may transform a heating element into an efficient cooling element [119].
 Fig. (3). Structure of heat pipe [121].
Fig. (3). Structure of heat pipe [121].Thermo-electrics are durable, dependable, and have minimal maintenance because there are no moving parts to wear out. Also, in the event of failure, replacement is easy. Furthermore, the operation is both quiet and vibration-free. Thermo-electrics have a drawback in that they have low cooling efficiency. The highest efficiency is about 0.8 which needs additional power over the dissipated heat. However, cooling using a heat pipe is more reliable as it does not include moving parts and no power usage. However, due to its fixed structural design, the heat pipe cannot heat the battery [107], [118] & [120]. A comparison of the features of active air systems versus heat pipe and thermo-electric cooling systems in terms of cooling performance, cost, reliability, weight, size, energy consumption, and safety can be given as follows: the heat pipe and thermo-electric cooling systems outperform active air cooling systems in terms of cooling performance, although active air cooling is more cost-effective and reliable, and weighs less than heat pipe cooling systems, while all the three techniques consume nearly the same amount of energy [107]. Furthermore, compared with the active air cooling systems, thermo-electric cooling systems are smaller in size, whereas heat pipe cooling systems can provide more safety.
3.1.2. Liquid cooling strategies
Direct and indirect liquid cooling procedures are two kinds of BTMS liquid cooling methodologies. Holding the battery pack in an insulating coolant liquid which has no chemical reaction with any of the materials on the outside of the cells, mineral and silicone oils as examples, is a direct liquid cooling technique, whereas indirect liquid cooling is performed utilizing liquid coolant, such as deionized water, propylene glycol, and ethylene glycol as examples, circulating in tubes/separate casing surrounding the battery pack or the tray on which the battery pack is mounted. As a result of the direct contact between the coolant and the battery cells, the direct liquid cooling strategy is very effective compared to the indirect liquid cooling strategy; furthermore, in the latter strategy, heat passes through the surface of the tubes/panels prior be removed by the coolant, resulting in an inevitable increase in thermal resistance [107]. Moreover, when a high forced flow rate is necessary, dielectric fluids with high viscosity, such as mineral oil, consume a lot of energy; this is not the case with indirect cooling, as low-viscous liquids like water can be employed.
On the other hand, liquid cooling strategies that are used for BTMSs can be divided into passive and active systems as illustrated by Fig. (2c) and Fig. (2d), [114] & [122]. As shown in Fig. (2c), the passive cooling strategy depends on the radiator as a heat sink. The electric pump circulates the coolant within a closed system. Heat is absorbed from the units via the circulating liquid and then released by the radiator to the ambient. The effectiveness of the cooling is determined according to the difference between the ambient air temperatureand cell temperature. However, in case this difference is exceedingly small, or the surrounding has a higher temperature than the temperature the cell, the passive cooling strategy becomes inefficient. To improve the cooling performance of the passive cooling system, adding fans beyond the radiator is considered an effective way.
As indicated in Fig. (2d), the active liquid system comprises two loops [107], [115] & [122]. The upper and lower parts are called the primary and secondary loops, respectively. The primary loop resembles that of the passive fluid system, which uses a pump to circulate the heat transfer fluid. The secondary loop represents the air conditioning loop. Instead of the cooler, the upper heat exchanger works as an evaporator for the cooling process and provides the link between the primary and the secondary loops. The four-way valve will be switched during the heating process, the heat exchanger of the primary loop acts as a condenser while the heat exchanger of the secondary loop acts as an evaporator. Regardless of which liquid cooling technology is applied water/glycols and mineral oils are the most common liquid coolants. That is due to their cooling features, e.g., low freezing point, and high heat capacity, however mineral oil coolant has higher heat transfer coefficient.
3.1.3. Phase change material cooling strategies
Phase change materials are the materials that can release or absorb an amount of thermal energy at phase change, i.e., thawing, and freezing processes, to allow a kind of heating or cooling. In the freezing process, PCMs release certain amounts of thermal energy as latent heat of fusion, or crystallization energy [123]. Conversely, whereas in the dissolving process, PCMs absorb from the surrounding environment a certain amount of thermal energy to be turned from the solid phase to the liquid phase, accordingly, the PCM can be used efficiently as a cooling strategy [124].
PCM-based cooling systems result powerfully for battery pack thermal management, as at the time of failure of a single battery cell in the pack, the PCM system provides a fast response by absorbing the generated heat and distributing it preventing thermal runaway from prevailing. Having high thermal conductivity and the ability to store heat are the most prominent features of the PCM cooling strategy, accordingly, in case of operation under high ambient temperature, which is between 45 °C and 50 °C, the temperature inside the battery pack is ensured to be beneath the maximum operating temperature, i.e., 55 °C. However, its suitability for cold operation conditions is one of the PCM cooling strategy weaknesses. Generally, the structure of the PCM cooling systems is characterized by simplicity, lightweight space efficient, and low parasitic energy consumption, however, up to date the PCM cooling systems suffer from flammability and electrical conductivity issues and resizing due to graphite PCM property as well [125]. As illustrated in Fig. (4), PCM cooling systems can be classified as passive cooling systems as they can be used as a blanket for each Li-ion cell without the need for external devices to enhance heat transfer [126]. According to [110], compared to the other external cooling systems, properties of the PCM cooling systems can be evaluated as follows: performance, safety, and size of the PCM cooling systems can be evaluated as the best, while their reliability, cost, and energy consumption can be rated with least grade. Finally, the weight of PCM cooling systems can be rated as middle-level among the main external cooling systems.
 Fig (4). Phase change material cooling system [126].
Fig (4). Phase change material cooling system [126].A comparative evaluation of the discussed external cooling techniques in this article is illustrated by Table (1). In this Table, the features of the cooling systems such as safety, cost, size, structure, weight, energy consumption, reliability, and performance efficiency have been described relative to each other to indicate the capabilities of each cooling system. Regarding choosing a cooling system to be implemented for a specific application, the designer should select the proper one based on the features of the cooling system that suit the needs and restrictions of the application. For example, the active air cooling system is considered the most suitable cooling system for EVs' Li-ion battery pack, as this system provides least weight, higher safety, and more reliability in addition to having a structure that fits EVs construction. However, due to PCM cooling system characteristics such as heavy weight, less energy consumption, and high performance efficiency, it's recommended for cooling the Li-ion battery pack that is used in renewable energy applications especially in the cold countries.
Table (1). Comparative evaluation of external cooling systems.
| Feature | Active Air | Heat Pipe | Passive Liquid | Active Liquid | PCM |
|---|---|---|---|---|---|
| Performance Efficiency | Low | High | Moderate | Very High | High |
| Structure | Complex | Moderate Complexity | Simple | Complex | Simple |
| Safety | High | High | Low | High | High |
| Weight | Light | Heavy | Medium | Heavy | Heavy |
| Size | Large | Small | Small | Small | Small |
| Reliability | High | Low | Medium | Low | Low |
| Energy Consumption | High | High | Low | Low | Low |
| Cost | Economical | Expensive | Middle Priced | Most Expensive | Expensive |
In order to sum up, the main strategies for BTMS are as follows: air, liquid, and PCM cooling systems represent the main cooling techniques for Li-ion battery. The air cooling strategy can be categorized into passive and active cooling systems. Simple-structure, easy-maintenance, and low-cost are the main features of the air cooling system. Heat pipe and thermoelectric cooling systems are technologies to increase air cooling efficiency. The liquid cooling systems can be classified into direct and indirect cooling systems depending on properties of the used coolant. Moreover, liquid cooling systems can be categorized to passive and active cooling systems. Finally, the PCM cooling strategy was introduced as a passive cooling system. PCM cooling systems have advantages of more safety and smallest size than other external cooling systems. Table (2) summarizes the features, and drawbacks of the main strategies of BTMSs.
Table (2). Comparison between cooling strategies and Recommendations.