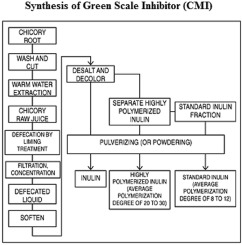
1. Introduction
The scales are mostly inorganic salts which deposit by precipitation at the supersaturation condition of water. They account for major maintenance problem in many industries where industrial water processing is required. They are associated with impurity of water.
In petroleum industry, water is produced with crude oil as unwanted by product. They are associated with oil and contain various inorganic (mineral) salts. Occurrence of scale depends on specific condition of temperature, pressure, pH, partial pressure of CO2 etc. At extreme conditions the salts remain soluble in the water and scaling problem is not encountered. However, when condition changes and water become supersaturated at optimum reservoir condition, the scales start to precipitate.
Water is one of the three major fluids present in the reservoir. Fluids produced from the reservoir contain typically oil, gas and water. Water produced from the well is not pure water and it contains dissolved impurities in the form of mineral salts. These mineral salts remain dissolved in under-saturated condition. However, when the condition changes and water becomes supersaturated due to change of temperature, pressure, pH, partial pressure of CO2 etc., it tends to precipitate undissolved salts (Mackay, 2003a; Hamdy et al., 2014). These mineral salts are known as scales. Scale deposits are impervious in nature and tend to accumulate on solid surfaces. Fig. 1 shows scale formed in oilfield equipment and its severity. The tendency of scale deposition increases as the roughness of the surface increases. Once the deposition starts it becomes easier for the next layer of deposition and thus gradually more and more scale layers deposit over the surface. In an oil well the surface such as internal surface of pipeline, choke, underground pumps and other equipment, surface facilities such as separators and heater treaters are major candidates for scale deposition. Scale formed becomes so problematic that gradually it can lead to total loss of production caused by chocking of net diameter of flow conduits (Dickson et al., 2011; Liu et al., 2012b). Fig. 2 shows causes and different locations of possible scaling in a typical water flood operation.
 Fig. 1. Scale deposition a) in the pipeline, b) on separator surface and c) on clean separator surface (Boak, 2012).
Fig. 1. Scale deposition a) in the pipeline, b) on separator surface and c) on clean separator surface (Boak, 2012). Fig. 2. Occurrence of scale at different locations during water flooding(Moghadasi et al., 2004a).
Fig. 2. Occurrence of scale at different locations during water flooding(Moghadasi et al., 2004a).Scale deposition once occurred is tough to deal with. Prevention of scale formation is the most suitable option. The chemicals used for prevention of scale formation are called scale inhibitors. These chemicals delay scale build up by disrupting agglomeration of small nuclei which add up to form solid precipitate. The scale inhibitor treatment is the most used chemical method in prevention of scale deposition. However, it can only be applicable in the case of prevention but it is not suitable for already existing scale deposition. Although Scale inhibitors used as primary control mechanism, it may not solve scale deposition issue and another type of chemical known as scale dissolver may be required. Inhibitors alone may not be sufficient for handling scaling problem in some of these scenarios:
-
i.
When scaling tendency has not been studied accurately for a reservoir.
-
ii.
Non optimal inhibitor placement into the reservoir due to reservoir heterogeneity or different pressure regimes encountered within the well or cross flow.
-
iii.
When squeeze treatment for the scale inhibitors may have exhausted and another squeeze treatment may not be economical.
-
iv.
When it is economical to use scale dissolver than scale inhibitor (Williams et al., 2016).
Scale inhibitors have primarily been used for fighting scaling. Majority of the scale inhibitors are harmful to the environment. Due to stricter regulation, the industry has now moved towards alternative of the conventional scale inhibitors. More recently many researchers have been working on to develop green scale inhibitors which may replace the conventional scale inhibitors.
This review aims at better understanding of the various types of scales, the mechanism of formation of scale, functioning of scale inhibitors and mathematical models to predict scaling. Finally, inhibition and remediation of scales using conventional scale inhibitors and development of various green inhibitors by different sources is also reported by researchers.
2. Different types of oil field scales
Based on their frequent occurrence and nature of their toughness the scales can be very tough or in some cases easy to handle. They can be categorized based on their sensitivity to pH. Some scales are pH dependent and some are pH independent. Normally pH dependent scales are easier to treat than pH independent scales (Olajire, 2015).
2.1. Major oil filed scales
There are various types of mineral salt precipitation which are known as scales. However, some scales occur more readily and cause great hindrance to the facilities and production. For example Calcium carbonate is the most common scale in the oil field. It, however, is relatively easy to deal with. This is a pH dependent scale and diluted HCl acid treatment is most widely used industry practice for its remedy. Sulfate of calcium, barium and strontium are also common scales. These scales are pH independent, hence acid treatment is not suitable for their remediation. It is better to inhibit their occurrence. Special chemical additives are used for this purpose called scale inhibitors.
2.1.1. Calcium carbonate
Calcium carbonate is one of the most frequently occurring scales in the oil field. It occurs in colloidal and amorphous state. It occurs in nature in three forms namely aragonite, calcite and vaterite (Helalizadeh et al., 2000). Calcite is the most stable polymorph of calcium carbonate. Its presence as solid deposits on metal surface is known as scale. Its deposition mechanism involves conversion of water soluble calcium bicarbonate to sparingly soluble calcium carbonate caused by reduction in CO2 partial pressure and increase in temperature (Moghadasi et al., 2004a). Although it is most abundant of mineral scale, it can be easily removed with acid cleaning. Solubility of all forms of Calcium carbonate is inversely proportional to temperature (Kumar and Choudhary, 1998). Solubility of all three form of calcium carbonate is shown in Fig. 3.
 Fig. 3. Solubility of different forms of calcium carbonate in water as a function of temperature (Plummer and Busenberg, 1982).
Fig. 3. Solubility of different forms of calcium carbonate in water as a function of temperature (Plummer and Busenberg, 1982).Equation (1) shows calcium carbonate formation:(1)
This equation is combined form of the following equilibrium equations as shown in equations (2), (3), (4), (5), (6):(2)(3)(4)(5)(6)
2.1.2. Calcium sulfate
Calcium Sulfate is another frequently occurring mineral scale. It is not readily soluble in water and causes permanent hardness of water. It occurs mainly in three forms:
i) dihydrate (CaSO4·2H2O, gypsum); ii) hemihydrate (CaSO4· H2O, plaster of Paris); and iii) anhydrite (CaSO4). Stability curves of CaSO4 are shown below in Fig. 4 for all three forms. It is evident that gypsum is least soluble below 40 °C, so it is most deposited calcium sulfate form whereas hemihydrate and anhydrite are commonly found on heat exchangers in distillation column (Dydo et al., 2003; Hamdy et al., 2014).
 Fig. 4. Solubility of different forms of calcium sulfate in water as a function of temperature (Linke, 1958; Marshall and Slusher, 1966; Silcock, 1979).
Fig. 4. Solubility of different forms of calcium sulfate in water as a function of temperature (Linke, 1958; Marshall and Slusher, 1966; Silcock, 1979).2.1.3. Barium sulfate
Barium sulfate is among the toughest kind of scale. It is very stable chemically and thermally and hard in nature. It is unique in a way that its solubility increases with the increase in temperature as opposed to calcium carbonate scale. It is a pH independent scale thus acidizing seldom gives satisfactory output. It is least soluble in water among common scales. Once formed it is very costly operation to remove and original productivity can never be achieved. Thus it is important to develop preventive measure than to apply remedial measures (Vetter, 1975). Barite scaling is a major problem for offshore fields. Normally formation water is rich in Ba2+ and sea water contains SO42− in excess. When both the incompatible waters come in contact, they tend to form insoluble BaSO4. The formation of BaSO4 is an exothermic reaction, as shown in equation (7).(7)
2.1.4. Strontium sulfate
Strontium sulfate is also very tough scale. It is relatively more soluble than barium sulfate in water but less soluble than calcium carbonate scale. It is also a pH independent scale. Its solubility decreases with the increase of temperature i.e. at higher temperature scaling become severe (Merdhah and Yassin, 2009).
2.2. Other oilfield scales
These scales are relatively less problematic than major oilfield scales and often occur alongside major scales. Examples of these scales are Magnesium hydroxide, Magnesium oxide, halite, iron/lead/zinc sulfide salts (Sutherland and Jordan, 2016). Common oilfield scales and the affecting factors are shown in Table 1. It can be observed that factors mostly responsible for scaling are temperature, pressure and total dissolved salts.
Table 1. Parameters affecting common oil field scales (Moghadasi et al., 2003a).
| Name of scale | Chemical formulae | Primary factors affecting deposition |
|---|---|---|
| Calcium carbonate | CaCO3 | partial pressure of CO2, temperature, total dissolved salts, pH |
| Calcium Sulfate: | temperature, total dissolved salts, pressure | |
| a) Gypsum | CaSO4·2H2O | |
| b) Hemihydrate | CaSO4· H2O | |
| c) Anhydrite | CaSO4 | |
| Barium Sulfate | BaSO4 | temperature, pressure |
| Strontium Sulfate | SrSO4 | temperature, pressure, total dissolved salts |
3. Factors responsible for oil field scale formation
Under favorable conditions homogeneous and heterogeneous nucleation result into formation of scales. Nucleation start if solution contains both cationic and anionic species and supersaturation condition occurs. Supersaturation is the function of temperature and pressure.
If solution temperature is increased then due to evaporation the unsaturated solution may become saturated and eventually supersaturated, resulting in exceeding the solubility limit and it may start the formation of scales. This kind of scaling can be termed as “autoscaling”. The carbonate and sulfate scales can be formed as a result of pressure change in the system. Halite can also form in the similar way from highly saline water.
The degree of supersaturation leads to the degree of scaling potential of the water. Several factors affect the scaling tendencies of the water (El-Hattab, 1985; Hamdy et al., 2014).
-
i.
Excess concentration of minerals
-
ii.
Condition of temperature, pressure and pH of the solution
-
iii.
Mixing of incompatible water such as injection water for pressure maintenance and formation water
-
iv.
Change in thermodynamic conditions i.e. pressure and temperature
-
v.
Agitation and velocity (hydrodynamics)
-
vi.
Particle size
-
vii.
Environment of deposition
-
viii.
Roughness of surface
Another condition required for the scaling is presence of cations and anions in equivalent ratio. If either of the species is deficient in the solution, scale cannot form. It should be noted that the formation water is cation rich (Ca2+, Ba2+ etc.) and seawater is anion rich (SO42−) (Itoro et al., 2015). When sea water in injected into the formation for water flooding or enhanced oil recoveryoperation, the incompatible water mixes and become supersaturated and thus can start sulfate scale deposition. The scale tendency of the fluid mixture is related to the ratio of sea water and formation water in the mixture. The factor responsible for this kind of scaling is “incompatible mixing”. Other examples of scaling due to incompatible mixing are sulfide scales where hydrogen sulfide is mixed with zinc, iron and lead ion rich water as shown in equation (8).(8)
Some scales are pH dependent i.e. their tendency to precipitate is a function of the system pH. Calcium carbonate scale is one of the examples of such kind of scales. CO2 is used in secondary recovery process. When CO2 partially dissolved into water, it changes to carbonic acid and the solution pH decreases and the calcium carbonate would be dissolved due to increase in acidity. Subsequent pressure drop due to production of fluid causes CO2 to break out from the solution and again pH changes and the increase in pH causes calcium carbonate scale to deposit. Thus “gas flooding” is also one of the conditions responsible for scaling (Frenier and Ziauddin, 2008). Equations (9), (10), (11), (12), (13)represent the reaction in gas flooding scaling.(9)(10)(11)(12)(13)
4. Mechanism of oil field scale deposition
Scaling is a complex phenomenon and involves crystallization mechanisms. The crystallization and subsequent precipitation of scales occurs once the activity of cations and anions in the solution exceeds their saturation limit and the solution becomes supersaturated. In addition, kinetics of the reaction is also a key factor responsible for the extent of scaling (Al-Roomi and Hussain, 2016).
The formation of scale occurs by two crystallization mechanisms, surface and bulk crystallization (Hasson et al., 2001; Lee and Lee, 2005; Antony et al., 2011). Scaling is a combination of both these mechanisms. Surface crystallization occurs due to heterogeneous nucleation mechanisms and bulk crystallization occurs due to homogeneous nucleation mechanisms (Olajire, 2015). In homogeneous nucleation there is no role of foreign material and the nucleation occurs in bulk solution in liquid phase. Thus in this case formed scale particle may flow through the system and do no deposit or in the other way they may get deposited as sediments to form cake layer. On the other hand heterogeneous nucleation occurs in the presence of foreign substances which act as trigger for the deposition of formed scales on the solid surface of equipment encountered. The foreign substance can be suspended solids, scale nuclei, welds/stress joint on the metals, corrosion site present on metal surface etc. (Olajire, 2015).
One of the factors responsible for scaling is corrosion. It is often ignored but the fact is that the corroded metal provides Fe2+ and Fe3+ cations in flowing fluid which results is formation of iron sulfides, iron oxides and iron carbonate scales (Frenier and Ziauddin, 2008).
The scale formation can be described in the following steps (Al-Roomi and Hussain, 2016).
Aggregation
After the solution attains supersaturation, cations and anions, such as Ca2+ and CO32−/SO42−, collides to form ion pairs. Next, they form micro-aggregates which act as small center of crystals, embryo, and micro-nuclei.
Nucleation
These micro-aggregates acts as nucleation center which acts in the formation of micro-crystals. Nucleation may develop on the substrate and possibly in the bulk fluid at relatively higher saturation ratio.
Crystal growth
Microcrystals formed in the solution agglomerates and/or absorbed to solid surface and grow into larger microcrystals and on further growth fuse to form depositional microcrystals.
Agglomeration
Formed micro crystals start growing continuously by further adsorption of additional scaling ions in the solution and the scales starts to form on the surface. On further growth these converts to deposit.
4.1. Development of mathematical model for prediction of scale formation
Several mathematical models have been proposed by different researchers for the prediction of reduced production due to scaling in porous media. Woods and Harker (2003) applied conservation of mass principle in their mathematical model. They simulated the barium and sulfate ions reaction when two chemically incompatible brines mixed in the formation. They solved the model using finite difference approach. In their model, concentration of barium and sulfate were calculated as a function of time and space considering the kinetics of barium sulfate formation (Shokrollahi et al., 2015). Similar mathematical model was developed by Bedrikovetsky et al. (2004). They also discussed on deposition characteristics of barite as a result of chemical incompatibility of two mixing waters. They additionally incorporated hydrodynamics apart from kinetic model for barite formation and deposition. However, both the models had some limitations and shortcomings. Their models were limited only for barite deposition and did not incorporate other types of scale minerals. In real case mixed scales often occurs rather than one particular scale. Effect of concentration of others ions on deposition was not presented. They also did not link their output to the permeability decline due to scaling in the porous media. Similar models were developed by a number of researchers considering on kinetic and hydrodynamic behaviour in the process of scaling and study the formation damage due to it (Bertero et al., 1988; Yeboah et al., 1993; Thomas et al., 1995).
Mackay (2003b) developed a model highlighting location of maximum scaling and composition of resulting brine. This model also incorporated reservoir geometry (1D, 2D and 3D), wellbore geometry and the rate of reaction. In this model the kinetics of reaction and impact of deposition of scale on permeability reduction were not considered.
Jamialahmadi and Muller-Steinhagen (2008) worked on some of the shortcoming in the previous mathematical models and developed model for scale deposition and removal of calcium sulfate dihydrate based on kinetics and hydrodynamics of scale deposition. In this model the effect of temperature, salt saturation and injection rate were incorporated (Shokrollahi et al., 2015). This model may predict individual calcium sulfate dihydrate scaling effectively but lack of accuracy in predicting permeability ratio when several scales co-precipitate simultaneously from the brine.
Kumar and Choudhary (2005) discussed the scaling problem in Indian oil filed and mechanism of scale deposition. Experimental study was conducted on the solubility aspect of various scales prevalent in Indian oil fields and on some of the commercial scale inhibitors (HEDP, polyacrylamide, sodium polyacrylateetc.) were used in the study. They developed three empirical models from the earlier work of Jacques and Bourland (1983) for strontium sulfate scaling under atmospheric pressure condition. The equation relates solubility as dependent variable and pH, salinity, pressure and temperature as independent variables. This model lacks the pressure variation parameter and this limits its uses only in atmospheric condition.
Kundu (2009) in his thesis proposed three mathematical models to predict calcium carbonate scaling under Mumbai High conditions. The parameters used were temperature, exposure period, pH, ionic strength and concentration of scaling ions (Ca2+ and HCO3−). He successfully developed two software ‘SCALEGUARD-I’ and ‘SCALEGUARD-II’ (in visual basic) to predict CaCO3 scaling tendency qualitatively and quantitatively in temperature range of 0–100 °C with variables pH (0–14), exposure period (4–48 h) and ionic concentration of various ions. This model predicts amount of scaling in mili gram per liter terms. Its limitation is the temperature which at maximum is up to 100 °C while in oil filed the temperature may be significantly higher. However, at greater than 90 °C temperature, CaCO3 solubility becomes practically constant (Kundu, 2009).
Most recently Shokrollahi et al. (2015) developed a hybrid model based on machine learning approach for all these shortcomings in the previous models. They built a model based on available data in literature to test the accuracy of the model. The machine learning approach they used is known as Least Squares Support Vector Machine (LSSVM) and Coupled Simulated Annealing (CSA), also called as CSA–LSSVM. Table 2 shows some important oil field scale studies by various researchers.
Table 2. A list of some important studies in scale formation (Shokrollahi et al., 2015).
| Inventor (s) | Porous media | Type of scale (s) | Temperature (°C) | Pressure (psig) | Flow rate (ml/min) |
|---|---|---|---|---|---|
| Mitchell et al., 1980 | Sand pack | Calcium carbonate/barium sulfate | 20–90 | 4–40 | – |
| Read and Ringen, 1982 | Glass bead/synthetic alumina cores | Calcium carbonate/barium, strontium, calcium sulfates | 20–70 | 1–22 | 2 |
| Lindlof and Stoffer, 1983 | Arab-D core (Saudi Arabia) | Strontium and calcium sulfates | – | – | – |
| Bezerra et al., 1990 | Sand stone cores (Outcrop/Brazil) | Barium and strontium sulfates | 80 | – | 0.5–1 |
| Wat et al., 1992 | Sand pack | Barium sulfate | – | – | 0.5 |
| Aliaga et al., 1992 | Sand pack | Calcium and barium sulfates | – | – | 0.2 |
| Todd and Yuan, 1992 | Clashach sandstone core | Barium and strontium sulfates | 20–70 | 1–2 | 7.5 |
| McElhiney et al., 2001 | Berea sand stone | Barium, strontium, calcium sulfates | 21 | 14.7 | – |
| Moghadasi et al., 2002 | Glass beads and Sand packs | Barium, strontium, calcium sulfates | 25–80 | 1–145 | 1–200 |
| Moghadasi et al., 2003b; Moghadasi et al., 2004b | Glass beads and Sand packs | Calcium carbonate/calcium sulfate | 50–80 | 17.7 | 25–100 |
| Abu-Khamsin and Ahmad, 2005 | Berea sand stone | Calcium sulfate | 45–95 | 100–3000 | 0.12–15 |
| Strachan et al., 2004 | BP Magnus core | Barium and strontium sulfate | 116 | 1500 | 1 |
| Bedrikovetsky et al., 2005; Bedrikovetsky et al., 2006 | Cores from outcrop RB (Brazil) | Barium sulfate | – | – | – |
| Merdhah, 2008 | Sandstone core (Malaysia) | Calcium, strontium and barium sulfates | 50–80 | 100–200 | – |
| Merdhah and Yassin, 2009 | Berea sand stone | Calcium, strontium and barium sulfates | 60–90 | 75–100 | – |
| Carageorgos et al., 2010 | Synthetic alumina cores | Barium, strontium, calcium sulfates | 20–70 | 1–22 | 2 |
4.2. The theory of thermodynamic scale prediction vs. kinetics of surface deposition
Thermodynamics and kinetics of the reaction are two tools that help to understand the possibility of scaling. Both of these signify different aspect of a reaction. Thermodynamics state whether a reaction be feasible or not based on the principles of Gibbs free energy (ΔG). It correlates between energy transformation between reactants and products. Suppose A is the reactant and B is the product. Fig. 5 shows the illustration of energy change in a course of a reaction.
 Fig. 5. Free energy vs. course of the reaction (Chen et al., 2016).
Fig. 5. Free energy vs. course of the reaction (Chen et al., 2016).When the Gibbs free energy of the product is less than the reactant then the energy is released during the reaction and it is spontaneous and exothermic reaction. Energy state of the product is less than the reactant and thus it is favorable reaction. The value of the Gibbs free energy in this case is negative. In other case if the energy is consumed during the reaction, it is endothermic and non-spontaneous. It is an unfavorable case and requires continuous extra supplement of energy and the product is less stable.
Thermodynamics only gives information regarding the equilibrium condition of the reactants and the products. However, it does not give information about the rate of reactions. The kinetics describes the rate of the reaction. It tells about how fast or slow a chemical reaction occurs. This is based on the concept of activation energy threshold which can be considered as the energy barrier the reactant must cross to form the product as shown in above Fig. 5 (Chen et al., 2016). Commonly there are two mechanisms by which scale inhibitor works, Kinetic and Thermodynamic inhibition.
4.2.1. Kinetic inhibition
Kinetic inhibition works by adsorption of inhibitors onto the crystal surface, preventing further growth of crystal. It is also known as threshold inhibitor and is normally favored over thermodynamic inhibitors because a little quantity of kinetic inhibitor is sufficient to block the site of crystal growth. This is further characterized in two main categories: nucleation inhibition and crystal growth inhibition (Sorbie and Laing, 2004).
4.2.1.1. Nucleation inhibition
Nucleation inhibition causes disruption in formed scale crystal and then they redissolved by scale inhibitor molecules. For homogeneous crystallization, this disruption affects thermodynamic stability of the growing nucleons. Generally this mechanism is shown by small polymeric species such as polyvinyl sulphonate (PVS), and sulphonated polymaleic acid co-polymer (S-PMA) (Yuan, 2001). The inhibition efficiency of such compounds tends to improve at a low range of pH and temperature (high saturation ratio) compared to those inhibitors that follow crystal growth mechanism. The common scale inhibitor that follows this mechanism is phosphino-polycarboxylic acid (PPCA). Studies on this mechanism suggest that nucleation inhibitors work by modification in the actual scale crystal structure in a way that the crystal tends thermodynamically towards redissolution rather than crystal growth phase. During the nucleation process, a crystal to become stable must exceed critical ratio of surface area to volume. Nucleation inhibitors change the shape of the proto-crystal in a way that the ratio of surface area to volume increases thus effectively creating additional energy barrier for the crystal to exist and grow (De Yoreo and Vekilov, 2003).
4.2.1.2. Crystal growth inhibition
Crystal growth mechanism or crystal growth retardation mechanism tends to impede the crystal growth of scales. In this case inhibitor molecules adsorb at the active growth site of an existing scale crystal and results in blockage and prevention of further growth. However, Scale inhibitors don't stop precipitation; they cause delay in it by increasing induction time (Ferguson, 2011).
Commonly scale inhibitors which show this type of mechanism are small phosphonate groups, such as diethylenetriamine penta-(methylphosphoric acid) (DTPMP). These inhibitors can work over wide range of pH's and temperature. But, it shows better inhibition in static bottle tests condition and at higher pH and temperature, and low supersaturation. Its effectiveness is also affected by the presence of divalent cations (Ca2+, Mg2+) (Laing et al., 2003; Sorbie and Laing, 2004).
4.2.2. Thermodynamic inhibition
This type of inhibition works by decreasing the solution supersaturation. This is achieved by decreasing ion activity product of ions by either acidizing the solution (reducing pH) or by adding chelating agents which binds the cations and avoid its reaction with anions present in the solution.
4.3. Kinetic model of scale deposition
Kinetics of a reaction states about the rate of the reaction. A number of models have been proposed in the literature for the kinetic model of scaling (Bedrikovetsky et al., 2003, Bedrikovetsky et al., 2004; Bin Merdhah et al., 2010). Al-Roomi and Hussain (2016) interpreted the rate of scale inhibition by simultaneously working on two different models having different assumption about adsorption of scale inhibitor of maleic anhydride based polymers (YMR-series). Model-I hypothesize adsorption of scale inhibitor to the micro aggregate and prevention of growth before the critical size. Model-II assumes scale inhibitor adsorption on two places. First to the micro aggregate as was in model-I and additionally also at microcrystal which imparts high electronegative charge to the crystal. This effectively slowed the agglomeration, and deposition of crystals.
These two models are shown in Fig. 6 for the prediction of scale formation step and inhibition by scale inhibitor.
 Fig. 6. Models of Scale inhibition mechanism (Model I and II) (Al-Roomi and Hussain, 2016).
Fig. 6. Models of Scale inhibition mechanism (Model I and II) (Al-Roomi and Hussain, 2016).The Inhibitor constants Ki and Ki′ represent dissociation constants and can be given by equation (14):(14)Here IC = micro aggregate, S = inorganic salt, MC = microcrystal, D = inorganic scale, I = scale-inhibitor, ICI = micro aggregate with adsorbed scale inhibitor and, MCI = micro-crystal with adsorbed scale inhibitor. The value of rate constant k1is taken for nucleation and k2 is taken for crystallization.
After validation with experimental data Model-II showed acceptable level of accuracy with average error less than 3% compared to the computed data value for entire experiment range. In Model-I huge error encountered. That signifies that assumption in Model-I is not satisfactory and Model-II is preferred to Model-I.
Reaction rate constants depend on the temperature. The relationship can be obtained by the Arrhenius expression shown in equation (15) (Al-Roomi and Hussain, 2016).(15)
Activation energy and pre-exponential factor can be calculated by observing the graph between and inverse of initial surface temperature and gas constant ().
4.4. Thermodynamic model for scale deposition prediction
Thermodynamic and kinetic theory of scale deposition have been studied extensively and several models have been proposed. Thermodynamic modelprovide framework for prediction of mineral behaviour. Saturation Index (SI) model is one of the model to predict equilibrium condition in brines.
Water is an excellent solvent for most of the polar compounds. Several models are available for the investigation of solubility and saturation of different ions in water. Some famous models are Langelier saturation index (LSI) (Langelier, 1936), Ryznar Stability Index (RSI) (Ryznar, 1944), Puckorius Scaling Index (PSI) (Puckorius, 1983; Puckorius and Brooke, 1991), Larson-Skold Index (Liu et al., 2013), the Stiff & Davis Saturation Index (Stiff and Davis, 1952), and the Oddo-Tomson Index (Shams et al., 2012). Most of these models are based on pH measurement of water versus calculated pH of water. Langelier saturation index (LSI), Stiff & Davis Saturation Index (S&DSI), Ryznar Stability Index (RSI) and, Puckorius Scaling Index (PSI) models shall be later discussed in this paper for determination of condition of scaling and corrosion. However, in the present time saturation index (SI) is the most prevalent and frequently used method for the determination of solubility of the minerals and scaling tendencies.
4.4.1. Modeling
Saturation ratio (SR) is defined as the ratio of ion activity product to solubility product and can be calculated by dividing the composition of the product in real state to that of saturation state.
Saturation index is the criteria to state about solution saturation state.
-
If it is undersaturated
-
If it is supersaturated
-
If it is saturated
It is defined as logarithmic of saturation ratio (SR).
Followings are the reaction for precipitation of Anhydrite, gypsum, barite and calcite as shown in equations (16), (17), (18), (19), (20).(16)(17)(18)(19)(20)
Followings are the equations of solubility product, SR and SI of Calcite as given in equations (21), (22), (23), (24), (25), (26).(21)(22)(23)(24)(25)(26)
For Gypsum, Anhydrite and Barite equation (27) follows:(27)where γ, m, a and K represents activity coefficient, the concentration, the activity and the solubility product respectively. SI is the function of concentration, activity coefficient and equilibrium constant of the reactions.
In their work Doubra et al. (2017) have investigated Saturation index (SI) model for the solubility calculation of Barite, Calcium carbonate, Anhydrite of CaSO4and Gypsum at different thermodynamic conditions of brine. In SI model one important parameter is activity coefficient. There are different models studied and verified for accuracy based on available data in the literature. Pitzer, e-NRTL and e-UNIQUAC electrolyte models were analyzed and reported by them. Pitzer's model was the most precise and the second accurate was e-UNIQUAC model.
4.5. Prediction of scale formation based on water parameters
Prediction methodology for scale formation has been given by many authors. The qualitative prediction of corrosion & carbonate scale formation can be found by the help of various water parameters such as pH, total dissolved solids(TDS), Temperature (°C) of the water, alkalinity and the calcium hardness (mg L−1 Ca2+ as CaCO3) (Puchorius and Brooke, 1988, 1990).
The Langelier Saturation Index (LSI) is an indicator of scale potential in cooling water system, (Langelier, 1936). Langelier Saturation Index (LSI), Stiff and Davis Saturation Index (S&DSI) (Stiff and Davis, 1952), Ryznar Stability Index (RSI) (Ryznar, 1944) & Puckorius Scaling Index (PSI) (Puckorius, 1983) gives numerical value that can be indicative of the possible corrosiveness and scale potential of the produced water. Equations (28), (29), (30) show relation between water parameters and three indices LSI, RSI and PSI respectively.(28)(29)(30)where is the actual of water at given temperature and is saturation .
The can be calculated from the relation, shown in equation (31):(31)where, A, B, C and, D can be calculated from the following equations (32), (33), (34), (35):(32)(33)(34)(35)
is calculated as per the following equation (36),(36)
[Alkalinity] can be calculated from equation (37) as per the following:(37)
Condition of corrosion and Scaling based on the three indices:
-
LSI: Negative value indicates the non-scaling conditions and positive value indicates CaCO3 precipitation condition.
-
RSI: If RSI Value is less than 6, it indicates scale formation and when the value is above 6 it is corrosion indication.
-
PSI: It gives similar indication as RSI.
Interpretation of LSI & RSI is shown in Table 3 which indicates values indicating condition of scaling and corrosion.