1. Introduction
Nanotechnology is a recent field implicated into the scientific world, but is not a new word. It is based on the study of molecules, structures and substances with nano-scale diameters (at least one dimension between 1 and 100 nm), termed as nanoparticles (NPs). Due to their exceptionally high surface area to volume ratio, NPs possess several distinctive and unique physico-chemical properties, which are normally not encountered in their corresponding bulk materials. These unique properties are provided by the fraction of atoms present on the surface of NPs which increases the thermodynamic stability (phase transition) of NPs, whereas presence of less atoms in case of bulk material shows less defined phase transition upon temperature gradient [1]. It has been observed that as the surface area to volume ratio increases, the dominance behavior of atoms on the nanoparticle's surface also changes, which is a major contributing factor behind their unique properties [2]. This higher ratio also provides sufficient binding sites for substrates during chemical reactions, which confers wonderful catalytic reactivity [3].
Inorganic material like metals have attracted great interest of the scientific community to design their functional NPs for both basic and applied research due to their unique properties such as resistance to corrosion and oxidation, non-reactiveness, unusual high reduction potential, high melting point and high ionization energy. Metals constitute a large portion of periodic table and have a huge range of chemical activity, which controls their reactivity towards living cells. In this regard, use of inorganic molecule as metal NPs has been known since long back. The physico-chemical properties of metal and metal oxide NPs can be dictated by various attributes like size, shape, architecture, crystallinity and composition [4]. Due to these exceptional properties, metal NPs have been widely applied in fast moving fields like environmental remediation, water purification, antimicrobials and industries involved in manufacturing consumer goods such as cosmetic products, toothpaste, soaps, shampoos, detergents etc. [[5], [6], [7], [8]].
Due to the worldwide escalation & augmentation of multi-drug resistance in microorganisms, there is a need to develop a new and effective antimicrobial agent [9]. Antibacterial agents are compounds that locally kill or inhibit the growth of microorganism (specifically bacteria) without affecting the nearby tissues. Metals can be used as potential antibacterial agent because even at very less concentration metals can cause toxicity to bacteria and selectively interrupts the metabolic regulation network of microorganisms. Metals like Na, Mg, K, Ca, V, Cr, Mn, Fe, Co, Ni, Zn, etc. participate in the fulfillment of cellular biochemical functions and known as “essential metals”. Whereas other metals, which do not have any known biological function are called as “non-essential metals” viz. Ag, Au, Te, Hg, etc. [6]. These non-essential metals are exceptionally poisonous to most of the microorganisms and have microbicidal activity even at very low concentrations [10].
Among various metal and metal oxide NPs, Ag, Au, CuO and ZnO NPs are widely used as antibacterial agents. Action of various metal NPs as an antibacterial agents have been studied by various research groups but the center of attraction to be an antibacterial agent is credited to silver nanoparticles (Ag NPs) because of their vast and well known antimicrobial activity [[11], [12], [13], [14], [15]]. Silver has a very rich history of its use as an antimicrobial agent. Evidence for the use of silver nitrate as an antibacterial agent has been trace back to Roman pharmacopeia. Use of silver in clinical field has been stepped by Dr. J. Marion Sims, who has used silver for the treatment of vesico-vaginal fistulas in the year 1852 [16]. Considering the antimicrobial properties of silver, its varak has been used for garnishing sweets in Indian cuisine since ancient time. However, due to emergence of antibiotics as an economical and efficient alternative as well as lack of proper understanding of mechanism of toxic effects of silver, for a long period of time modification and development of new silver and other metal based antimicrobial formulations have been neglected.
The unprecedented increase of multi-drug resistant microorganism in recent years, has resuscitated the attentiveness of scientists to exploit silver and its NPs as antimicrobial agents. The chemical form of silver vary from cationic silver, metallic Ag 0 and silver oxide particulates; and can transform through dissolution, agglomeration and chemical speciation into alternate forms when it comes in contact with a particular environment such as aqueous biological media, body fluids etc. [12]. Hence, before development of new Ag NPs based drug formulations, in-depth understanding of interactions of Ag NPs with biological media is necessary perquisite [17]. In biological media, Ag NPs are reported to possess a wide range of activity depending upon its form (nano or ionic) and physico-chemical properties (surface coating, size, shape and crystallinity), which ultimately decides their antimicrobial action [18,19]. In recent years, extensive research has been done on the antibacterial activity of Ag NPs [5,[20], [21], [22], [23], [24], [25]]. However, very less attention have been paid to understand the mode of action responsible for the reduction in microbial growth and machinery of cells involved in the reduction process. Most of the studies indicated that different metals cause discrete and distinct type of injuries to microbial cells ranging from the cell wall and membrane damage, oxidative stress, protein dysfunction to DNA damage [6]. However, the precise molecular mechanism of their activity has remained unclear until date. Recently, it has been proposed that physico-chemical properties (surface properties, form of silver, size and shape) of Ag NPs can also influence their antimicrobial efficacy even at molecular level [[26], [27], [28]]. However, the available reports only deal with selected variations in the physico-chemical properties. Systemic studies on the interaction of Ag NPs with biological media and subsequently their effect on microbial cells at transcriptomic and proteomic level are not yet well established and is still an active area of research. Sporadic attempts have been made to understand the bacterial gene regulation upon exposure to NPs, but only one size of particles were used and few genes have been verified from the total transcriptome [[27], [28], [29], [30], [31]]. Further, in-depth studies are required to understand the effect of physico-chemical properties of Ag NPs on their toxic potential to ensure their safe use, exposure over extended period, fate in human body as well as in environment.
This review aims to discuss the up-to-date information about the bacterial resistance mechanism against various antimicrobial agents, which will help in identifying the mechanism(s) that can be a therapeutic target. Further, we discuss the interaction of NPs with biological media and bacterial cells, which decides the ultimate fate, potentiality, subsequent dissolution, internalization and accumulation of bactericidal silver species. This depends on the immediate surrounding and physico-chemical properties of Ag NPs. The review also highlights the differential gene regulation in bacteria in response to Ag NPs induced stress at transcriptomic and proteomic levels. Finally, this review states the future prospective for complete understanding on the mechanistic aspects of the antibacterial activity of Ag NPs, which will open a possibility to synthesize new Ag NPs based formulations with desired properties such as high solubility, stability, effectiveness, and controlled release activity at effective concentration against various pathogenic bacterial species.
2. Mechanism of antimicrobial resistance
All the way through the history, there has been a brutal fight between the humans and pathogenic microorganisms due to the severe infections caused by pathogens. To overcome the infection of microorganisms, use of antimicrobial agents came in existence since long back. In 1940s, first and foremost antibacterial agent revolutionized the whole world with an antibiotic (specific antimicrobial agent against bacteria) called penicillin. The widespread use of antimicrobial drugs (penicillin and sulfa drugs) began in the 1950s. Since then, a number of antibiotics have been developed and used for the treatment of various diseases. However, elation over the victory on pathogenic microorganisms was very short lived owing to the development of antimicrobial drug resistance in microorganisms. Antimicrobial drug resistance is the ability of microorganisms to defend against the effect of drug that was previously used to kill them and is an extremely serious problem in medical science. Massive quantities of antibiotics have been developed and are frequently used, essentially misused in high dose in hospitals to treat patients suffering from various diseases; in agriculture; aquaculture; in animal feeds as well as in fast moving consumer goods (FMCGs) such as soaps, detergents, toothpastes, deodorants etc. The inappropriate and overuse of antibiotics has resulted in rapid development of drug resistance in many pathogenic microorganisms. With the drastic increase in antibiotic consumption worldwide, the challenge posed by antibiotic resistance is likely to get worse and represents a major threat to the human health [32].
According to an estimate, over 50% of the antibiotic prescriptions given by doctors in developing countries are without clear evidence of infection or adequate identification of pathogens. Hence, it contain broad-spectrum antibiotic instead of narrow-spectrum drugs. The situation become worst when the patients do not take complete course of medication [33]. All these factors results in development of drug resistance in microbial strains. Microorganisms that acquired resistance to more than two drugs are called as multidrug resistant (MDR) microbes [34]. The knowledge of biological mechanisms involved with the use of antibiotics can be helpful to overcome the resistance problem. In general, cell wall/cell membrane synthesis, protein synthesis, nucleic acid synthesis and intermediary metabolism are the common targets exploited for the development of various antibiotics (Table 1) [[35], [36], [37], [38], [39]]. Bacteria can use single or multiple resistance mechanism against these drug targets (Fig. 1). The various drug resistance mechanisms (natural/intrinsic or acquired) present in bacteria have been discussed below [[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]]:
-
a)
Components of bacterial envelope (glycocalyx, cell wall or plasma membrane) can prevent the penetration and entry of drug inside, thus making the bacterial cell resistant to a particular drug. For example, presence of outer membrane in many gram-negative bacteria make them resistant to various antibiotics such as rifampin, penicillin etc.
-
b)
Several pathogenic bacteria have non-specific transporter proteins known as “efflux pump” in their plasma membrane, which can expel the drug from cell and make the bacteria resistant.
-
c)
Bacteria can become resistant by inducing modification in the structure of applied drug thus making it inactive. For example, the enzyme β-lactamase present in several Staphylococci species hydrolyzes the β-lactam ring of penicillins due to which antibiotic gets inactivated and cannot kill bacterial cell. Similarly, the acetyltransferase enzyme present in certain bacterial species make them resistant particularly to chloramphenicol as it cause acetylation of the amino group present in chloramphenicol which results in its deactivation.
-
d)
Bacteria can also become resistant to a drug by modifying their cellular structures (like peptide bridge in cell wall, ribosome etc.) and enzymes which are targets for the drug. The resulting changes in bacterial metabolism make them less susceptible to the antibiotic.
-
e)
Antibiotic resistance can also be due to the presence of pre-existing genes on the bacterial chromosome or on plasmid. These genes can also be acquired by a bacterial cell due to lateral or horizontal gene transfer.
Table 1. Biological mechanism involved with the common antibiotics.
| Antibiotic | Mechanism of action | Target |
|---|---|---|
| Penicillin | Inhibit the cross linking of polysaccharides in cell wall by preventing action of transpeptidation enzymes | Cell wall synthesis |
| Vancomycin | Inhibits the transpeptidation reaction by binding to d-Alanine present in the peptide-bridge | |
| Bacitracin | Inhibits the synthesis of cell wall | |
| Polymyxine B | Disrupts the permeability of plasma membrane | Cell membrane |
| Sulfonamides | Blocks the synthesis of folic acid by having a competition with p-aminobenzoic acid | Metabolic pathway for production of purines |
| Trimethoprim | Inhibit the synthesis of tetrahydrofolate | |
|
Ciprofloxacin, Norfloxacin and Naldixic acid |
Inhibits the activity of DNA gyrase A | DNA replication |
| Novobiocin | Inhibits the activity of DNA gyrase B | |
| Hydroxyurea | Inhibits the function of ribonucleotide reductase | |
| Mitomycin C | Inhibits the cross-linking of DNA | |
| Streptolydigin and Rifampin | Inactivates the DNA polymerase by binding to its β-subunit | Transcription |
| Actinomycin D | Binds directly to DNA | |
| Bleomycin | Induce DNA strand scission | |
| Kanamycin, Neomycin and Gentamycin | Binds to 16S rRNA | Translation |
| Thiostreptone and Erythromycin | Binds to 23S rRNA | |
| Fusidic acid | Binds to translation elongation factor G | |
| Kirromycin | Binds to translation elongation factor Tu |
 Fig. 1. Antimicrobial drug resistance mechanisms in bacteria (1) Prevention of antimicrobial drug penetration into the bacterial cell by bacterial cell envelope; (2) Removal of antimicrobial drug from bacterial cell by non-specific efflux pumps; (3) Acquisition of new genetic material from drug resistant bacterial strain and (4) Inactivation of antimicrobial drug by intracellular modification.
Fig. 1. Antimicrobial drug resistance mechanisms in bacteria (1) Prevention of antimicrobial drug penetration into the bacterial cell by bacterial cell envelope; (2) Removal of antimicrobial drug from bacterial cell by non-specific efflux pumps; (3) Acquisition of new genetic material from drug resistant bacterial strain and (4) Inactivation of antimicrobial drug by intracellular modification.Due to increased drug resistance, there has been development of several MDR bacterial species that can cause severe infections in humans and lead to the prolonged illness and increased mortality rate [46]. Hence, to circumvent this challenge, there is a constant need to search new and effective antimicrobial drugs or compounds. Last one decade has witnessed the use of metal NPs in various biological and medical applications such as diagnostics, imaging, drug delivery, therapy etc. [[47], [48], [49], [50]]. The knowledge of antimicrobial properties of metal leads to turn researchers to exploit the antimicrobial potential of metal and metal oxide NPs and development of “nano-weapons” against microbes [5,6,24,51]. The cytotoxic potential of these metal NPs (silver, gold, copper, zinc oxide etc.) has reported to be much better than their bulk materials [33]. In recent years, Ag NPs have developed as a new hope to treat MDR pathogenic bacteria. However, the precise molecular mechanism of their mode of action has remained unclear. In addition, the effect of various physico-chemical properties of Ag NPs on their interactions with biological media, bacterial cells and cytotoxic action mechanism has not been explored in-depth. In view of this statement, the next sections of this review specifically highlights the mechanistic aspects of antimicrobial activity of Ag NPs, starting from the interaction of Ag NPs with biological media (culture media in which Ag NPs get suspended) to the evaluation of bacterial transcriptomic and proteomic profile upon the exposure of Ag NPs (varying in their physico-chemical properties).
3. Interaction of silver nanoparticles with aqueous biological medium
Ag NPs are known to be the most extensively used NPs in consumer products because of their vast application as an antimicrobial agent [15,21,25]. There is a growing argument in the scientific community regarding the actual mechanistic aspects of antibacterial activity of Ag NPs. However, the behavior of Ag species (Ag0NPs/Ag+ ions) in the aqueous biological media in which their antibacterial activity has been tested remains unclear. Due to presence of O2 in the aqueous biological media used in antibacterial experiments, the oxidative dissolution of Ag+ ions from Ag NPs cannot be ignored. It has been reported that Ag NPs showed much higher bactericidal activity in oxygenic conditions as compared to anaerobic conditions [52,53]. This suggests that antibacterial action is actually due to Ag+ ions, and Ag NPs only act as a reservoir and release Ag+ ions due to their oxidative dissolution [54]. Multiple reports are available on the dissolution of ions from NPs under different conditions [53,[55], [56], [57]]. It is important to note that the Ag+ ions dissolution rate decrease with time. It has been reported that initially there is a fast release of ions which turns slow as the reaction step towards the completion [58]. Hence, the possibility of NPs size and shape conversion with time cannot be ignored [54]. Even concerns have been raised regarding the possibility of microbial resistance against Ag NPs, which could be either due to the multiple use of Ag NPs or higher concentration of Ag NPs, which form silver precipitates and reduce the active concentration of silver species in the undefined aqueous biological culture media [12,44]. Thus, our first focus is to explore the interaction of Ag NPs with undefined aqueous biological culture media to find out their possible fate in term of dissolution, agglomeration etc.
The rate of dissolution from nanoparticle's can be regulated by various parameters like surrounding medium, physico-chemical properties (size, shape, capping material) of NPs, physical state of immediate environment (pH, ionic strength and presence of organic material), etc., which ultimately makes a remarkable impact on the antibacterial activity of NPs [55,[58], [59], [60], [61]]. In addition, theses parameters also decide the state of NPs, i.e., whether NPs will remain in isolated single particle form, aggregated form or in dissolved form in the undefined aqueous biological media. Dissolution of ions is a medium (suspension medium for Ag NPs) dependent process, which eventually can affect the interaction of NPs to their surrounding environment. In order to understand the interactions of NPs with biological medium, initially there is a need to recognize the interface between NPs and water (as a suspension medium) because immersion of NPs in water will detail about the fate of immersed NPs in biological environment. NPs can also interact with various compounds and ions like sulfide, chloride, phosphate etc. present in the biological medium and can result in significant variation in the dissolution of ions from NPs and hence their potential activity [60].
To study the effect of physico-chemical properties of NPs towards the release kinetics of ions, Kittler et al. [58] used two differentially functionalized Ag NPs [i.e., citrate and polyvinyl pyrrolidone (PVP) capped] in the water suspension at different temperature range (5, 25 and 37 °C) and reported that dissolution rate of silver ion is directly proportional to the temperature due to increase in the kinetic energy. They observed that other than temperature, coating material present on the surface of NPs also put an impactful mark on the release of silver ions. PVP coated Ag NPs were found to have higher dissolution rate in comparison to citrate coated Ag NPs because being a charged ligand, surface coating of citrate act as a barrier by reducing the release of silver ions than the natural (uncharged) PVP molecules. In broad-spectrum, the release of silver ions from Ag NPs in biological medium can be obscured due to the interactions of silver with various components (like free salts, anions, inorganic sulfides, free thiol groups etc.) present in the media. Table 2 summarizes the interaction of various components of biological media with Ag NPs, which essentially leads to affect the release of silver ions and thereby the antibacterial action of Ag NPs towards its specific activity [56,60]. Liu and Hurt [65] added all the essential components of biological media to the immersion media (water) and reported that dissolution of ions is a collective process of release and its interaction with media components, which leads to the precipitation of silver due to formation of complexes with various components of medium. They also observed adverse effect on dissolution of ions with the increase in pH and addition of humic acid to the immersion media. Later on, Levard et al. [60] showed the role of surface sulfidation on the dissolution rate and stated that as the concentration of sulfur to silver increases, the release of ions decreases from its nano-form. In addition to Ag2S, having the solubility rate of 140 ng mL−1, many other kind of insoluble silver precipitates (AgCl and Ag3PO4 with a solubility rate of 1.9 μg mL−1 and 6.5 μg mL−1, respectively) also formed in the biological media, which reduces the antibacterial activity of Ag NPs by scavenging the free silver ions from the solution [63,66]. Fig. 2 pictorially depicts the effect of various parameters on the dissolution of silver ions from Ag NPs in aqueous biological medium. Furthermore, Loza et al. [19] also modified the simple immersion medium (water) by addition of components that are commonly available in biological medium such as anions, reducing agent (glucose) and oxidizing agent (dissolved oxygen) to study their effect on Ag NPs. By substituting the biological media components, they could find out the interaction of silver with these biomolecules and reported that chloride and phosphate ions (typical anion) eventually leads to the precipitation of least soluble silver salts (silver chloride and silver phosphate). Glucose as a reducing agent was found to have a decelerating effect on the release of silver ions. Whereas, oxidizing agent H2O2used instead of dissolved oxygen was found to enhance the dissolution process. This process was further studied in detail and silver dispersion in modified media was kept until equilibration, followed by ultracentrifugation in order to collect different fractions (precipitates and NPs). All the fractions were screened by various techniques. Based on the obtained results, they proposed that scanning electron microscope (SEM), energy-dispersive X-ray spectroscopy (EDS) and X-ray diffraction (XRD) are not adequate to estimate the dispersed ions and unchanged behavior of Ag NPs upon immersion into the substituted medium. Atomic absorption spectroscopy (AAS) and inductively coupled plasma mass spectrometry (ICP-MS) have been recommended for the analysis of dissolved silver whereas, dialysis, nanofiltration and ultracentrifugation should be used for separating silver ions form Ag NPs [19,65].
Table 2. Interaction of various components of biological media with Ag NPs and their effects on dissolution kinetics.
| Biological media component | Effect on Ag NPs | Rate of dissolution | Reference |
|---|---|---|---|
| Ca+2 and Mg+2 | It allows the aggregation of NPs | Decrease | Jin et al. [59] |
| Free thiol containing groups (cysteine and glutathione) | These groups can bind on to the surface of Ag NPs and prevent the oxidation by inhibiting oxygen exclusion | Decrease | Liu et al. [56] |
| Ascorbic acid | It behaves in a surprising way that at low concentration it increases the dissolution rate but at a concentration above 1 mM, completely inhibits the dissolution. This kind of activity is not understood completely but can be correlated to the generation of Reactive Oxygen Species (ROS) | Increase/decrease | Liu et al. [56] |
| Chloride and phosphate ions | Precipitation of silver in the form of least soluble forms such as silver chloride and silver phosphate | Decrease |
Li et al. [55,62] Levard et al. [60] Loza et al. [19] |
| Sodium chloride | It allows the aggregation of NPs | Decrease |
Li et al. [55,62] Levard et al. [60] |
| Sulfide ion | Formation of insoluble layer of Ag2S occur on Ag NPs, thus rendering it to release Ag+and decrease the activity | Decrease |
Reinsh et al. [106] Levard et al. [63] |
| Glucose | Decelerate the rate of ion release from NPs because it is acting as a reducing agent | Decrease | Loza et al. [19] |
| Hydrogen peroxide | Accelerate the rate of dissolution by forming peroxide intermediates | Increase | Loza et al. [19] |
| Chlorine | Increases the release of ions in comparison to peroxide because it would be needed in very low concentration (2 mol of Ag NPs can be oxidized by only 1 mol of chlorine) | Increase | Garg et al. [64] |
 Fig. 2. Effect of various physico-chemical parameters (temperature, pH, size, shape and surface capping) on the stability of Ag NPs and dissolution kinetics of Ag+ in aqueous biological medium.
Fig. 2. Effect of various physico-chemical parameters (temperature, pH, size, shape and surface capping) on the stability of Ag NPs and dissolution kinetics of Ag+ in aqueous biological medium.Once Ag NPs are exposed to biological media, a very basic doubt arises that what is the probability for the formation of Ag+ ion and clump of nanoparticle's? Thermodynamic studies proved that silver ions are the only equilibrium product under most of the above mentioned conditions. The oxidation of Ag NPs to its ionic form is simply a redox reaction, which produces peroxide intermediates and this released peroxide content can be measured through a fluorescence assay known as Amplex Red Peroxide Fluorescence Assay that quantify the possible H2O2 intermediates during the redox reaction and ultimately tells about the released silver ion content [56,65]. Direct oxidation of silver followed by the dissolution of phase in acidic condition, are the two mechanistic steps that finally leads to the formation of silver ions [67]. The rate of release of ions from NPs can be increased by substituting the oxidizing agent i.e. peroxide by chlorine because 2 mol of Ag NPs can be oxidized by only 1 mol of chlorine. However, at low concentration of chlorine, dioxygen plays an immense role towards the formation of silver ions [64].
Based on the above mentioned facts, it can be concluded that the actual behavior of Ag NPs in a particular biological media depends on the physico-chemical properties of Ag NPs, their dissolution kinetics and active concentration of released Ag+ ions. It also depends on their immediate surrounding environment (pH, temperature, etc.) that can make a noteworthy change in the status of NP's i.e. dissolution rate of ions, agglomeration etc. Hence, one should not use chemically undefined media having sulfur, chloride and phosphate ions for the Ag NPs based antibacterial activity assay as these ions can react with silver and reduce its active concentration, thereby, give a false result in terms of concentration. We also recommend that before finalizing a particular concentration for the antibacterial assay, one should first measure the interactive behavior of Ag NPs in the selected chemically defined media following techniques like EDS, XRD, AAS, ICP-MS etc.
4. Antibacterial mechanism of silver nanoparticles
Due to increasing antibiotic resistance, a variety of Ag NPs based formulations have been developed and commercialized in past few years. However, because of inadequate regulations and policies, there are multiple loopholes and knowledge gap in the use of Ag NPs at commercial scale. In recent years, serious concerns have been raised regarding the possibility of microbial resistance development against Ag NPs. Silver ions are microbicidal at very low concentration and do not cause any adverse effects on humans at such low concentrations [44,68]. However, the risk associated with the use of higher Ag NPs concentration in commercial products has not been clarified yet. Extensive use of Ag NPs based product can increase the release of silver in the environment. Continuous and long term silver exposure can result in the development of resistance in microbes [69]. It is very important to note that Ag NPs can act as reservoir and results in continuous release of silver ions [44,54,70], which can result in development of bacterial resistance to Ag NPs [71]. As per the recommendation of various International organizations, the human exposure limit for silver compounds in air is around 0.01 mg/m3 and in drinking water is around 0.10 mg L−1. This suggest that in general humans are unlikely to be exposed to such silver concentrations. However, people living in highly polluted areas with silver residues from factory wastes can be at increased risk to develop the argyria disease in which subdermal Ag deposits results in an irreversible purple-gray to blue-black coloring of the skin [17,72]. One should be very careful when using commercially available Ag NPs products containing unspecified levels of ionizable silver. Further studies need to tackle the precise toxicity mechanisms and behavior of Ag NPs. It is a challenge to distinguish precisely what portion of the toxicity is from the ionic form, and what portion is from nano form of silver. Hence, there is an urgent need for a judicial approach towards the use of Ag NPs to preserve their efficacy and to avoid development of microbial resistance [12]. In this perspective, systematic and in-depth mechanistic studies can help in developing an efficient Ag NPs based antibacterial system. These studies should involve two fundamental steps, which can make impact on the effectiveness of NPs antibacterial activity (Fig. 3):
-
•
First is proper understanding of the physical and chemical behavior of NPs, which will decide its way of action on bacterial cells. It will provide the knowledge regarding the effect of physico-chemical modifications (size, shape, surface coating etc.) of Ag NPs towards the variations in their antibacterial activity.
-
•
The second step is to have in-depth knowledge about the bacterial cell machinery, which can be affected due to exposure of Ag NPs. These include increased generation or dissipation of the regulation pathways. This knowledge can be achieved by critically studying their transcriptomic and proteomic profiles.
-
•
 Fig. 3. Study levels to understand the mechanistic aspects behind the bactericidal activity of Ag NPs.
Fig. 3. Study levels to understand the mechanistic aspects behind the bactericidal activity of Ag NPs.This section discusses the recent developments in the above-mentioned steps:
4.1. Effect of physico-chemical properties of silver nanoparticles on their bactericidal action
It is a well-known fact that silver ions and silver-based formulations (bulk and nano form) are extremely harmful for the microorganisms. The nano form of silver possess extensively high surface area to volume ratio as compare to its bulk form, which makes them more toxic for various microbial species. Rai et al. [24], have beautifully reviewed this phenomenon of NPs. Based on the antimicrobial properties of Ag NPs, a variety of new nano-formulations have been developed and commercialized in recent years. Now-a-day, washing machines are available in the market that contain “ion-generating” devices designed to release silver into the wash tub which releases silver ions (Ag+) from Ag NPs. As silver has been reported to be present in ionic, nano and aggregated form in the solution, its antimicrobial activity can be significantly manipulated by variation in the active form of silver [60,63].
In addition to the silver form, the antibacterial action also changes with variations in the physico-chemical properties of Ag NPs. Antibacterial action of Ag NPs starts with the interactions of NPs suspension with the microorganisms in the biological media, which involves release of silver ions that eventually interact with microbial cells. As discussed in earlier section, release of silver ions from its nano form can be controlled by manipulating various physico-chemical properties (size, shape, surface coating, etc.). In this context, Ma et al. [73] checked the size dependent dissolution of silver ions from organic material coated Ag NPs. They used gum arabic (GA) stabilized Ag NPs with two different size of 6 and 25 nm as well as PVP coated Ag NPs with a size range of 5, 8, 25 and 38 nm. The obtained results suggested that the increase in the size of NPs reduces the surface area to volume ratio that ultimately affect the dissolution rate of ions and antibacterial efficacy of the NPs. Small sized NPs were found to dissolve more rapidly, and thus possess higher antibacterial activity. Size is one of the most crucial parameter to decide the rate of dissolution because it gives the proper activity of NPs based on their accurate unit of mass or mole.
Likewise, surface coating also makes a terrific impact on the ion release kinetics from Ag NPs. Damm and Münstedt [74], studied the effect of polymer on the dissolution rate of Ag NPs by forming polymer/silver nanocomposites. They used different range of polyamides [polyamide (PA) 6 grade, PA 6.6 grade, PA 12 grade, PA 12 modified with poly-THF grade and cycloaliphatic PA 1] to form nanocomposites and noted the release of silver ions in following manner: PA12 < cycloaliphatic PA < PA12-poly-THF < <PA6. 6 < PA6. This pattern of dissolution was due to the increased water content of polymer. However, before using polymers as surface coating agents, some major points need to be looked into viz. (1) cross linking of polymer to NPs, because it can decrease the ion release rate; (2) coating with hydrophilic or hydrophobic polymers, as hydrophilic polymer coated NPs have high tendency to discharge ions; and (3) crystallinity of polymers, as the rate of dissolution increases with the decreased crystallinity of polymers [[74], [75], [76]].
It has been observed that the shape of Ag NPs also cause a critical impact on their antimicrobial activity [77,78]. Plate and rod shaped Ag NPs showed higher antibacterial activity as compared to spherical shaped Ag NPs and thus required in lesser concentrations. It was observed that the bactericidal activity of plate and rod shaped Ag NPs was favored by the presence of high atom density facets {111} whereas due to predominance of {100} facets on spherical Ag NPs they showed relatively lesser bactericidal activity [77]. The antibacterial activity of Ag NPs was found to be dependent on the surface area of a particular shape [78]. It is imperative to relate the shape-dependent bactericidal activity of Ag NPs with varied surface areas.
Based on the aforementioned discussion, we concluded that small sized Ag NPs represent much higher antibacterial activity due to their higher surface area to volume ratio as compared to the large sized Ag NPs and bulk silver. Selection of capping material should also be given equal importance, as it can significantly affect the dissolution kinetics and release of active silver ions from the surface of Ag NPs. In addition to size and capping material, the silver atom density facets on the surface of NP's also depends on their shape. The review of literature suggest that one should prefer rod or plate shaped Ag NPs in comparison to other shapes (spherical, triangle, etc.), as the presence of higher atomic density facets in rod or plate shaped Ag NPs found to enhance their antibacterial efficacy.
4.2. Effect of silver nanoparticles on bacterial cell machinery
As discussed in earlier sections, the bactericidal activity of Ag NPs is found to be dependent on their physico-chemical properties which ultimately cause the difference in their rate of dissolution of ions, oxidation potential etc. Virulence of silver is known to be either due to the aggregation on the surface of bacterial cells or by diminishing its molecular machinery directly by entering into the cells. However, the major challenge to understand the bactericidal aspects of silver is to distinguish the degree of toxicity caused by the released silver ions in comparison to the nano form, because ionic form of silver has a huge difference in their toxic potential over Ag NPs [26,28,79,80].
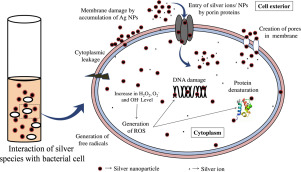
The action of Ag NPs has been speculated similar to the silver ions [70,77]. It has been anticipated that the release of silver ions from the core of Ag NPs in the ultimate basis of bactericidal activity of Ag NPs [44,81]. However, contrasting report indicating limited antibacterial efficacy of Ag+ ions in comparison to higher antibacterial activity of Ag NPs by cell membrane dissolution are also available [79]. The probable action of Ag NPs can be attributed by hindering the energy (ATP) generation due to uncoupling of oxidative phosphorylation from respiratory electron transport, intrusion in membrane permeability, inhibiting respiratory chain enzymes, decreasing the functionality of other intracellular components by generating free reactive oxygen species (ROS), etc. (Fig. 4) [14,62,[82], [83], [84]]. Due to disruption, the increased permeability of membrane leads to uncontrolled transport and finally death of bacterial cell. Most of the destructive pathways in bacterial cells utilize generation of ROS; hence, it is known to be most potent component behind the bacterial cell death. Upon Ag NPs exposure, bacteria experience an elevated level of oxidative stress due to the formation of peroxide, superoxide and free hydroxyl ions, which eventually leads to the cellular inactivation [[85], [86], [87]]. However, the exact molecular mechanism behind ROS evolution is yet to be established which would let us know that it is whether due to enhanced ROS generation or due to disruption of ROS regulatory pathways present in the bacterial cell. Moreover, the involvement of non-ROS mechanism cannot be ignored as bactericidal effects of Ag NPs have also been observed in dark and anaerobic conditions in which ROS cannot be evolved [88].