1. Introduction
Communication stands crucial in progress of a fraternity and it’s observed that bacteria too communicate with each, which is collectively called as Quorum sensing. This is a density dependent mechanism where communication is mediated by signaling molecules termed as autoinducers; it assists bacterial community to coordinate and work as a single unit in a population [1], [2], [3]. Numerous bacterial functions such as secondary metabolite production, sporulation, biofilm formation and symbiosis are regulated by quorum sensing mechanism [4], [5]. All bacterial quorum sensing systems fulfills three basic precepts i. Concentration dependent response to autoinducers; where autoinducers are secreted outside the cell and later inflexed depending on autoinducer concentration [6], [7], ii. Bacteria consists specialized receptors in its cell membrane or in cytoplasm that sense and respond to concentration of autoinducer, iii. Detection of autoinducers by receptors recommence quorum sensing loop thereby bacterial virulence [8], [9]. Quorum sensing was first observed in a gram negative marine bacteria Vibrio fischeri [10] as studies showed that phenotype bioluminescence was regulated by quorum sensing machinery [11], [12]. Since then many bacteria have been successfully scrutinized for their quorum sensing ability. Understanding of quorum sensing open up possibilities of regulating bacterial virulence as far as the immense role of quorum sensing upon it is concerned [13]. Recently many studies have been carried out to down regulate bacterial quorum sensing and this strategy is collectively called quorum quenching [14], [15]. Inhibiting communication of a bacterial population disables them to initiate most of its virulence activity which help host for an effective immunological clearance. In this review we discus about the types and mechanisms of quorum sensing and different approaches of quorum quenching through which bacterial communication can be inhibited.
2. Autoinducers: alphabets of bacterial dialect
Specialized signaling molecule; autoinducers play a key role in quorum sensing and consequently considered as alphabets of bacterial language. Autoinducers are studied under three different classes based on their structure and specific function; they are AHLs (Acyl Homoserine Lactones), AIP (Autoinducing Peptides) and Autoinducer-2 (AI-2) [16]. AHLs are small diffusible molecules with a core lactone ring and acyl side chain which is responsible for facilitating signaling in gram negative bacteria [17]. Quorum sensing in gram positive bacteria are found to be mediated by AIPs which are short peptide chains synthesized in cell. AIP lack free transportation across the cell membrane hence requires specialized membrane transport proteins [18], [19]. AI-2 are furonone derived signaling molecules found functioning in both gram negative and gram positive bacteria [20] also exhibit features of both AHLs and AIPs [21]. Signaling molecules are produced inside the bacterial cells which will be processed internally or externally hinge upon the organism. Despite of their functional and structural differences, autoinducers possess certain common characteristics such as high degree of receptor specificity and transport across cell membrane which may be active or passive.
3. AHL mediated bacterial communication
AHL mediated quorum sensing is intensely studied in gram negative bacteriathat constitutes maximum pathogenic strains in it. Numerous virulence factorsin gram negative bacteria such as bacterial adhesion, biofilm formation, exozyme secretion, pigment production are regulated by N- Acyl homoserine lactone(AHL) dependent quorum sensing [22], [23]. Typical quorum sensing system in gram negative bacteria contains two integrant; an autoinducer synthase which is responsible to synthesize AHLs [24], [25] and an autoinducer receptor cum transcriptional activator [26]. AHL mediated signaling is highly intra-specious specific due to peculiar receptor binding sites that recognize only precise AHLs [27], [28], [29] thus signals produced by one specious will not disturb the communication mechanism of other [30], [31]. Modification in AHL structure is accomplished by varying number of Carbon and modification upon R- group [32] which gives advantage to bacteria in an endosymbiontenvironment. Some important signaling systems mediated by AHLs are discussed here.
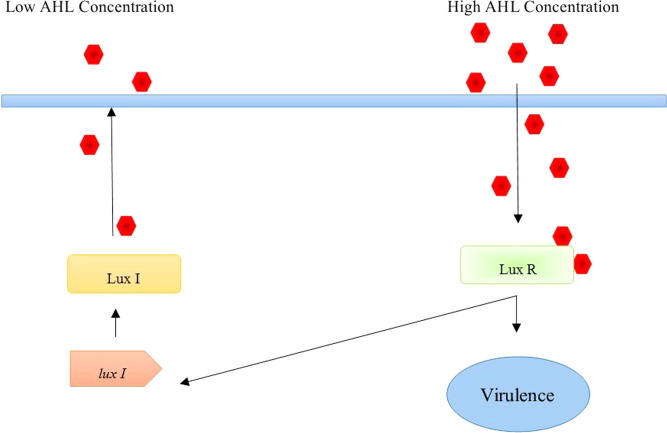
3.1. LuxIR: typical gram negative bacterial quorum sensing system
LuxIR quorum sensing circuit stands archetypal of gram negative bacterial communication (Fig. 1) as over hundred gram negative bacterial strainscommunicate by the engagement of LuxIR homologues genes [33]. SmaIR in Serratia marcescens [34], [35], CviIR in Chromobacterium violaceum [36], [37], hanIR in Halomonas anticariensis [38]and TraIR of Agrobacterium tumefaciens[39] all work based on the principle of LuxIR but with slight variations in AHLs. This is the first studied bacterial linguistics model and was discovered in marine bioluminescent bacteria Vibrio harveyi which is capable to lead a free and symbiotic lifestyle [40].
 Fig. 1. LuxIR signaling Circuit. Red hexagons indicate the autoinducer produced by LuxI.
Fig. 1. LuxIR signaling Circuit. Red hexagons indicate the autoinducer produced by LuxI.This system is based in the reactions mediated by LuxI and LuxR. LuxI is an autoinducer synthase that catalyze the interaction between S-adenosylmethionine and acyl carrier protein which leads into the formation of N-(3-Oxohexanoyl)-L-homoserine lactone which subsequently functions as autoinducer [25], [41], [42]. AHL will be dispersed out of cell until a specific threshold level is attained. In high external concentrations, AHL will be taken back into the cell which sequentially interact with LuxR. If not bound with AHL, in free state LuxR will be degraded inside bacterial cell, whereas after forming LuxR-AHL complex it will be capped from degrading [43]. LuxR-AHL complex binds upon Lux promoter region that initiate bioluminescence and other quorum sensing regulated functions [44], [45].
3.2. LasIR-RhlIR: overlapping quorum sensing system
LasIR-RhlIR is serially arranged overlapping quorum sensing circuits observed in Pseudomonas aeruginosa where LasIR and RhlIR are arranged one after other in a series (Fig. 2) [46], [47]. Pseudomonas aeruginosa is a widely observed human opportunistic pathogen which is mainly concerned with nosocomial infections upon patients suffering from Cancer, AIDS and cystic fibrosis [48], [49]. It produces virulent factors such as elastase, protease, exotoxin A that collectively cause serious tissue damage in mammals [50]. Such mechanisms are found to be controlled by Quorum sensing circuits [51].
 Fig. 2. LasIR signaling system, Red hexagons indicate the signaling molecules involved in LasIR circuit and grey pentagons denotes RhlIR signaling system.
Fig. 2. LasIR signaling system, Red hexagons indicate the signaling molecules involved in LasIR circuit and grey pentagons denotes RhlIR signaling system.Signaling is initiated by the production of AHL (3OC12-homoserine lactone) by LasI which act as a homologous of LuxI [52]. AHL will be diffused out of the cell and in high concentration it will be in taken and binds with LasR. Numerous activities such as production of elastase, protease and exotoxins are triggered by LasR-AHL complex [53]. Other than triggering this virulence factor production LasR-AHL also initiate the second system RhlIR. [54]. As a result RhlI produce a secondary AHL (C4-homoserine lactone) that binds with RhlR resulting in the production of subsequent products such as sidophores and pyocyanin [55], [56]. All Quorum sensing controlled mechanism in Pseudomonas aeruginosa is regulated by any one circuit but immense overlap between this systems are noted [57], [58] which enables to carry on virulence by any one system [59].
3.3. ExpIR: virulence down regulating quorum sensing system
ExpIR mediated quorum sensing is observed in opportunistic plant pathogen Erwinia carotovora (Fig. 3) that frequently causes soft rot in plants [60]. Study of Exp IR system have lot of economic importance on it as soft rot disease affect lot of economic crop plants such as potato, carrot, pineapple, cucumber, onion. It is also observed that Exp IR also regulate synthesis of antibiotics such as carbapenem ß-lactum which give dominance to the bacteria to survive in a high bacterial diverse rhizosphere [61].
 Fig. 3. ExpIR mediated signaling in Erwinia carotovora which is homologues to LuxIR signaling circuit.
Fig. 3. ExpIR mediated signaling in Erwinia carotovora which is homologues to LuxIR signaling circuit.Exp circuit is initiated by the production of OHHL by Exp I. Unlike other QS systems the virulence factors of bacteria Erwina carotovora is such as pectate lyase, pectin lyases, cellulases and proteases are positively influenced by the OHHL alone [62] OHHL also triggers the action of Exp R by forming conjugated complex but Exp R have no significant role in bacterial virulence in fact it is found decreasing exozyme production by bacteria [63]. This stand in contrast to many other bacterial virulence mechanism whereas virulence is triggered by regulatory protein not directly by AHL. The difference possessed by Exp IR system found to help bacteria against host defense mechanism [64], Exp R bind with low density of AHL would produce only less exozyme hence low level of effect; this indeed provokes host defense mechanism which challenges the existence of bacteria. Where as in Erwina carotovora ExpR neutralizes the low AHL density which inhibits the production of exozyme. Therefore exozymes will be produced only in high level of OHHL
4. Peptide mediated bacterial communication
The signaling in gram positive bacteria are controlled by Oligopeptide which is commonly referred as autoinducer peptides [AIPs] [65]. AIPs are produced inside the bacterial cell as pro-AIP which will be processed and modified inside or outside the cell hinge upon the organism [66]. Unlike AHL signaling molecule AIP's are impermeable to cell membrane hence requires specialized transport proteins for the inward and outward carriage of AIP's [67]. This transport of AIP's are generally accomplished by cell membrane bound sensor kinases [68]. Competence mechanism such as sporulation in Bacillus subtilis virulence initiation by Staphylococcus aureus, Listeria monocytogenes, Clostridium perfringens, Enterococcus faecalis are regulated by Quorum sensing systems [69], [70], [71], [72], [73]. Even though gram positive Quorum sensing circuits have a general resemblance some small variation in mechanism is observed depending on specious and living environment which is discussed here
4.1. Two component system in gram positive bacteria
Most of the gram positive bacterial linguistics are depending upon membrane bound two component system that identify signaling molecule autoinducer peptides (Fig. 4) [74], [75]. A classic example for two component quorum sensing system is found in Staphylococcus aureus [76]. This is a nosocomial pathogen which is normally concerned with skin infection if neglected leads to bacteremia and sepsis [77].
 Fig. 4. Agr circuit depended two component quorum sensing system is found in Staphylococcus aureus.
Fig. 4. Agr circuit depended two component quorum sensing system is found in Staphylococcus aureus.Virulence and communication of this bacteria is regulated by Arg locus which is a combination of two transcripts RNA II and RNA III [78]. Pro-AIP will be produced by ArgD one among the four component of Quorum sensing system. Pro-AIP will be processed and modified by Arg B after which it will be transported out by the same. [79]. During the course of modification pro-AIP that of 47 amino acid residue will be derived to 9 residue peptide. In high bacterial density, AIP will accumulate extra cellular environment and attain a certain threshold level. In this stage Arg C; a trans membrane protein will get activated which in turn bind with AIP [80]. Agr C is a histidine kinase which phosphorylate by the combination of AIP. Hence available phosphate group interacts with Agr A which is the response regulator [81]. Agr C and Agr A together constitute two component system. Activation of Agr C - Agr A activates the transportation of RNA II which continues the quorum sensing circuit and RNA III that is responsible for virulence [82].
4.2. Extracellular protease processed AIP quorum sensing circuit
In some gram positive bacteria processing of pro AIP is done in the external environment of bacterial cell by extracellular protease enzymes after which AIP will be transported back to cell for regulating transcription (Fig. 5) [83]. Many virulent factors like sporulation and enzyme production in Bacillus cereus, plasmid transfer in Enterococcus faecalis are regulated by such mechanism [84], [85]. Quorum sensing circuit in Bacillus cereus is a significant example for extracellular protease processed AIPs. A 48 amino acid long pap R intercellular pro-AIP is been produced by pap R gene. An amino terminal signaling peptidepresent in pro-AIP will initiate a secretory pathway due to which PRO-AIP is been taken out of bacterial cells and will be processed by an extracellular protease into an active AIP [86], [87]. Once the concentration of processed AIP reach a threshold level it will be transported inside bacterial cell by oligopeptide permease trans membrane protein [88]. It is observed that only processed pap R could interact with oligopeptide permease system whereas during the process pro -AIP will be degraded into peptides of 5, 7, 8 and 11. This is because of the specific activity of Intercellular transcription regulator plc R upon pentapeptide and heptapeptide [89]. Interaction of AIP on transcription factor plc R brings conformational changes and initiate plc R oligomerizationwhich subsequently induces production of virulence factors [90].
 Fig. 5. Extracellular protease processed AIP Quorum sensing circuit in Bacillus cereus.
Fig. 5. Extracellular protease processed AIP Quorum sensing circuit in Bacillus cereus.4.3. Competitive quorum-sensing system
In this type of Quorum sensing; network of different phenotype would antagonize each other based upon the desired lifestyle of bacteria [46]. This is moreover a combination of other two above mentioned systems. Bacillus subtilisstands as a perfect example of such signaling circuits (Fig. 6). In this bacteria signaling system of competence and sporulation influence each other based on necessity. Competence in bacteria is controlled by Com X peptide which is a ten amino acid [91] sized and processed and secreted by Com Q [92]. In high density: Com X is identified by an histidine kinase Com P that initiate formation of Com X - Com P complex which eventually trigger autophosphorylation that enable Com A to consume a phosphate group [93]. Com A is a DNA bindingresponse regulator that initiate numerous competence mechanism [94].
 Fig. 6. Competitive quorum-sensing system in Bacillus subtilis.
Fig. 6. Competitive quorum-sensing system in Bacillus subtilis.In other hand this bacteria also produce another oligopeptide by gene phr C and called as CSF (competence and sporulation factor). From cytoplasm CSF is effluxed out by transmembrane proteins [95]. In optimum threshold level CSF is taken back into the bacterial cell by by oligopeptide permease [96]. Internalized CSF have two positive fates depending upon its internal concentrations. In low concentrations CSF bind with cytoplasmic protein Rap C and promote bacterial competence [97]. Rap C protein in free state disturbs Com A hence bacterial competence. Therefore CFS-Rap C complex leads to smooth regulation of bacterial competence. In the high internal concentration level CSF form a complex with Rap B protein hence induce sporulation [98]. In free State Rap B block sporulation by phosphorylation of SPOOF gene which is responsible for sporulation [99]. Complex formation between CSF-Rap B also ensures the availability of Rap C to inhibit competence.
5. Bacterial silencing: taking antibacterial strategy to a new dimension
The mechanism through which bacteria are made “silent” by blocking quorum sensing system is called as quorum quenching. In this era of antibiotic depletion world is searching for new remedies against bacterial infections. Quorum sensing targeted antibacterial therapy has evolved new revolution in this field. Suppression of Quorum quenching have immense value in clearing bacterial infections such as chronic lung infections in CF patients, severe wound infection [100], [101]. Quorum sensing targeting drugs basically does not killing the bacteria but it is only attenuating bacterial virulence [102] and offers additional time to host defense mechanism that effect in better immunological clearance of pathogen. Quorum sensing circuit targeted treatment against Staphylococcus aureus [103], Pseudomonas aeruginosa [104], Vibrio cholera [105] were successful and comprehensive. Not only in pharmacological field but agriculture, aqua culture, industries are also found benefited by quorum quenching. There are many strategies which can be relied for blocking bacterial communication which is generally classified as enzymatic and non-enzymatic quorum quenching methods. There are many techniques to find appropriate quorum quencher such as cross streak assay, disc diffusion method, overlay assay, metagenomic analysis, microarray based screening. The choice of technique vary with the requirement. In this part we discuss some important quorum quenching methods and its applications.
6. Enzymatic quorum quenching
Enzymatic quorum quenching is concerned with altering conformation and structure of signaling molecule which eventually block bacterial communication. These quorum quenching enzymes are mostly derived from microorganisms which is found to give benefit to the producer in a competitive environment [106], [107]. This hypothesis of bacterial benefit by producing quorum quenching enzymes are based on the discovery co-existence between quorum sensing and quorum quenching bacteria [108], [109]. Four different types of chemical reactions are observed behind enzymatic quorum sensing they are decarboxylation, deaminisation acylase and lactonase activity [110]. So far enzymes those found degrading signal molecules are studied under three categories which is constituted by lactonase enzymes, acylase enzymes and oxydoreductase enzymes.
6.1. Lactonase mediated quorum quenching
AHL lactonanses hydrolyze lactonase ring of the signaling molecules due to which opened ring structure will be formed (Fig. 7). It is also observed that lactonase enzyme doesn’t disturb anything other than lactone ring. [111], [112]. It was believed that enzyme hydrolyze amide linkage between lactone and acyl side chain but recent studies on structure and function proved that ester link is affected. Based on phylogeny lactonase belongs to metallo-beta-lactonase superfamily and phosphotriesterase family among which maximum candidates belong to metallo-beta-lactamase superfamily [113], [114]. AHL lactonase are extensively produced by bacteria those have no phylogenetic relation suggests that enzyme production is not dependent upon taxonomic classification. First analyzed bacterial AHL-lactonase is AiiA 24B1, A product of aiiA gene possessed by Bacillus sp. 24B1 [115]. Since then many bacteria were found producing AHL lactonases which is homologous of AiiA [116]. A thermostable lactonase enzyme namely GKL was obtained from Geobacillus kaustophilus which belonged to phosphotriesterase family and showed relatively low paraoxonase activitysuggesting its non-involvement of phosphate ester as substrate [117]. Geobacillus stearothermophilus was observed to produce low catalytic enzyme but with high thermo stability [118]. Thermostable lactonase enzyme were also produced by Geobacillus caldoxylosilyticus YS-8 and Geobacilius kaustophilusHTA426 [119], [120].
 Fig. 7. Structural modification by the hydrolase action of lactonase which disables bacterial signaling.
Fig. 7. Structural modification by the hydrolase action of lactonase which disables bacterial signaling.It was also evident that some bacterial strains produce lactonase enzyme that show major deviation from aiiA gene product. For instance, AiiM lactonase enzyme was obtained from Agrobacterium tumefaciens [121] AhiD of Arthrobacter, Ochrobactrum produced Aid H Aiim of Microbacterium testaceum, Qsd A of Rhodococcus [122], [123], [124], [125] all were examples of lactonases that showed deviation from aiiA. It was believed that AiiA hydrolyze amide linkage between lactone and acyl side chain but recent studies on structure and function of AiiA proved that ester link is the one got attacked by enzyme. Crystal structure of AiiA suggested that there are two Zn2+ ions present in active center and these metal ions are very much essential for the catalytic activityand folding of enzyme [126]. Analysis on lactonase from Bacillus thuringiensisindicated that Zn1 binds to His 104, HiS 106 and HiS 109 however Z2 binds up on Asp 108, His 109 and His 235 [127] substitution of di-zinc by di-cobalt, di-manganese and di-cadmium suppressed the lactonase activity which proves the role of Zn ion in enzyme activity [128].
6.2. Acylase mediated quorum quenching
Acylase are the group of quorum sensing enzyme that hydrolyze amide bond between homoserine lactone and acyl side chain (Fig. 8) [129]. Major number of identified AHL acylases belong to Ntn Hydrolase superfamily and are classified into two clusters referred as AAC and Qui P cluster [130]. These two cluster differ in their substrate specificity whereas AAC specifically degrade AHL's longer than C8-HSL however Qui P cluster have a varying range of catalytic activity [131] initial reports of acylase enzyme was by gram negative bacterium Variovorax paradoxus that efficiently degraded AHL in growth media [129]. Till date many bacterial strains have been studied for its production of acylase enzyme. Gram positive Streptomyces Sp. was found producing acylase enzyme. This stand first such example for gram positive bacteria [132] AiiD from Ralstonia sp. XJ12B effectively degraded short and long AHLs. Actinoplanesutahensis and Brevundimonas diminuta produced acylase those with high similarities to Ralstonia acylase [133] acylase in substrate selection. Bacterial strain Pseudomonas syringae strain B728a were immensely studied for the ability to produce two acylase namely Hac A and Hac B [134]. Many other bacteria such as Shewanella sp. [135] Tenacibaculum maritimum [136], Comamonas testosterone [137] were also able to produce acylase. From the structural analysis it was observed that acylase enzyme is composed of two or more sub units. Amino acid sequence usually consists of four domains those are signal peptide, Alpha-subunit, Linear spacer and Beta-subunit [138]. Pro acylase enzyme is not functional and it will be converted to activate enzyme by proteolysis.
 Fig. 8. Structural modification by the hydrolase action of acylase because of which quorum sensing circuit is compromised.
Fig. 8. Structural modification by the hydrolase action of acylase because of which quorum sensing circuit is compromised.6.3. Oxidoreductase mediated quorum quenching
Oxidreducatase mediated Quorum quenching is targeting signal receptor specificity towards AHL signals. This enzymes modify the chemical structure of AHLs that disable them to interact with receptor and hence blocking signaling pathway (Fig. 9) such reports were first observed in Rhodococcus erythropolis[139]. AHL oxydoreductase obtained from Bacillus megaterium found oxidising ω-1,2,3 carbons of acyl chain [140]. There are not much reports obtained about oxydoreductases but NADH-dependent BpiBoa enzyme [141] obtained from Burkholderia GG4 give great hope to carry on more intense study in the possibility of structural remodeling of AHL signal molecules
 Fig. 9. Structural modification of AHL due to reduction by oxydoreductase enzyme which in turn mediate quorum quenching.
Fig. 9. Structural modification of AHL due to reduction by oxydoreductase enzyme which in turn mediate quorum quenching.