1. Introduction
Classical antibiotic drugs have reduced their capacity in front of widespread infectious diseases and become needed for many medical interventions. Antibiotics paved the way for unheard medical and societal developments, and are today essential in all health systems [1], [2]. In recent years, multi drug resistance has widely increased among many species of pathogenic bacteria in worldwide and led to the most commonly antibiotics no longer effective in controlling of infectious diseases and thus created concern and challenge in the healthcare sector [3]. So, the development of new antibacterial systems in front of drug resistant pathogens is a serious threat for the successful treatment of microbial disease [4], [5], [6].
Multidrug-resistant organisms (MDR) were lately named as the ‘ESKAPE’ pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.), denoting their escape from the effects of antibacterial agents or the non-existence of new active antibiotics [7]. The reasons of antibiotic resistance are complex and include human behavior at many levels of society from which the consequences affect everybody in the world [8]. Many attempts have been made to explain the antibiotic resistance and the interventions needed to meet the challenge [9]. The rising resistance of bacteria and fungi to classical antibiotics have created massive clinical problems in immunocompromised, AIDS and cancer patients [10]. In addition, the identification and treatment of antibiotic-resistant microorganisms are difficult and costly [10], [11]. Furthermore complications associated with antibiotic-resistant bacterial infections, provide a high morbidity and mortality rate [12]. For example, annually in the United States, at least 2 million people are infected by antibiotic-resistant bacteria and every year, at least 23,000 people lose their lives due to these infections.
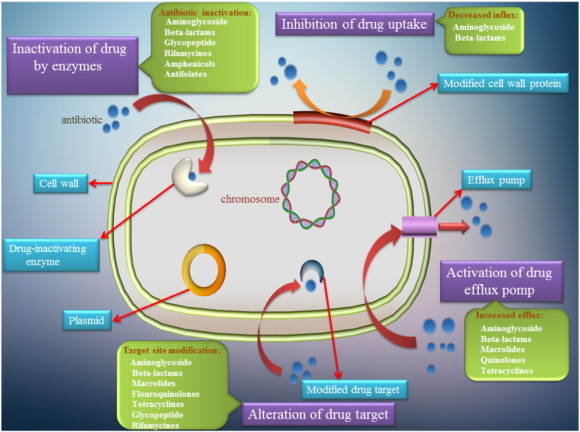
Resistance to antibiotics could be intrinsic or acquired and may be transferred horizontally or vertically [13]. There are several mechanisms of antibiotic resistance such as inhibition of drug uptake, enzymatic modification of antibiotics, alteration of target molecules, transformation, drug sequestration and active efflux out of the cytoplasm [4], [9], [14] as it has been summarized in (Fig. 1).
 Fig. 1. Mechanism involved in the development of cell antibiotic resistance.
Fig. 1. Mechanism involved in the development of cell antibiotic resistance.So the emergence of antimicrobial resistance is creating challenges that require a multidisciplinary approach including: (i) biomedical innovation, (ii) precise control of antibiotic consumption and antimicrobial-resistance rates, (iii) inhibition of health-care-associated infections and spread of multidrug-resistant (MDR) bacteria and environmental propagation, (iv) rapid microbiological diagnosis, and (v) elimination of clinical and veterinary misuse [15], [16].
According to the guidelines listed above, a critical mass of researches focused on the development of new antimicrobial agents or formulations of common antibacterial compounds to combat pathogenic bacteria [17]. So, a variety of new antimicrobial drugs for the treatment of resistant pathogens have been synthesized, being an important group that based on the use of nanoparticle-based materials [18], [19].
Antimicrobial nanoparticles (NPs) compared to conventional antibiotics have some obvious advantages, including low toxicity, overcome resistance and reduce cost [20]. Today, by the emergence of nanotechnology, many nanomaterials (NMs) with antibacterial properties have been produced to fill the gap antibiotic treatment failure [21]. Nanoparticles with antibacterial properties include: iron oxide (Fe3O4), zinc oxide (ZnO) [22], copper oxide (CuO) [23],titanium oxide (TiO2) [24], silver (Ag) [25], Magnesium oxide (MgO) [26], graphene oxide (GO) and reduced graphene (rGO) [27], Nitric oxide (NO) nanoparticles [28], Polyethylenimine (PEI), quaternary ammonium compounds and etc. [21].
2. Application of nanoparticles antibacterial agents
Nanoparticles (NPs) have several applications in medicine and biology including drug and gene delivery [29], [30], fluorescent biological labeling [31], bio detection of pathogens [32], tissue engineering [33], [34], tumor destruction via heating (hyperthermia) [35], isolation and purification of biological molecules and cells [36], cancer therapy [37] as well as antibacterial agent [20].
Researchers already have identified many nanomaterials with antibacterial properties to infections related to antibiotic-resistant bacteria. Antimicrobial NMs are divided into two big categories including organic and inorganic NPs [38].
NPs with antibacterial properties that have been identified so far include silver (Ag), iron oxide (Fe3O4), titanium oxide (TiO2), copper oxide (CuO), Au NPs, zinc oxide (ZnO), chitosan, fullerenes, carbon nanotubes (CNTs), NO-releasing NPs and nanoemulsion [39], [40], [41], [42], [43]. The high surface to volume ratio and unique physical and chemical properties of nanomaterials have increased antimicrobial activity [44], being the mechanism different for each type of nanoparticle [45]. Table 1 summarizes antibacterial mechanisms of various nanoparticles. In general it can be identified two main lethal routes, which are related to each other and in many occasion occur simultaneously: (i) infraction of membrane potential and integrity and (ii) generation of reactive oxygen species (ROS), also called as oxygen-free radicals, the NM acting as nanocatalyst [46], more over it has been summarized in (Fig. 2). In addition, some of NM are effective that due to their physical structure and metal ion release [20], [21].
Table 1. Antimicrobial nanomaterials, action mechanisms and applications.
| Nanomaterial | Antimicrobial mechanism | Clinical and industrial applications | References |
|---|---|---|---|
| CuO Nps | Production of ROS; disruption of membrane | Antimicrobial agent | [190], [191] |
| ZnO NPs | Intracellular repletion of NPs; H2O2 production; release of Zn2 + ions; cell membrane damage | Antibacterial creams; lotions and ointment; surface coating of medical devices; mouthwash | [39], [144], [192] |
| Au NPs | Interaction with cell membranes; powerful electrostatic attraction | Photothermal therapy with near infrared light; adjuvant treatment after critical infections antibacterial agent; antifungal agent | [41], [193] |
| Ag NPs | Release of Ag+ ions; infraction of cell membrane and electron transport; DNA damage | Coatings for medical devices; portable water filters; dressing for surgical wound and diabetic foot; antibacterial agent; antifungal agent | [194] |
| TiO2 NPs | Production of ROS; cell membrane and wall damage | Antibacterial agent; food sterilizing agent; air purifiers; water treatment systems | [195], [196], [197] |
| CNTs | Cell membrane damage by ROS; oxidation of cell membrane proteins and lipids | Biofouling-resistant membranes; water filter; surface-coating; antibacterial agent | [97], [198], [199] |
|
NO-releasing NPs |
Production of ROS and NO release | Infected wound and diabetic foot treatment | [200] |
| Chitosan | Chelation of trace metals; increased permeability and laceration of membrane; enzyme inactivation | Bacteria immobilizer; microbiocide in biomedical products; drinking water disinfectants | [46], [201], [202] |
| Fullerenes | Extinction of cell membrane integrity; enhancing activity of infiltrating neutrophil | Possible sterilization applications | [40], [203] |
| Nanoemulsion | Membrane infraction; infraction of the spore coat | Anti-biofilm agent; nasal application; vaccine delivery agents; antimicrobial inhaler | [204], [205]. |
 Fig. 2. Various antimicrobial mechanisms provided by nanomaterials.
Fig. 2. Various antimicrobial mechanisms provided by nanomaterials.Recent studies showed that graphene and graphene based nanocomposites could be used as antibacterial coatings for food products and water treatment due to their minimal or no cytotoxicity to human and animal cells [47].
3. Graphene oxide
Graphene, a two-dimensions (2D) layer of sp2-hybridized carbon atoms located into a honeycomb lattice, has led to intense interest in physics, chemistry, materials science and biotechnology [47], [48], [49]. Graphene, in 2004 was obtained for the first time through micromechanical exfoliation of graphite (Gt) [50]. Lately, graphene and graphene oxide (GO) has appeared as a promising nanoplatform with massive potential for biomedical applications and translational research, because graphene compared to other carbon allotropes, i.e., fullerenes, carbon nanotubes and graphite has unique chemical and physical properties [51], [52].
Graphene and graphene oxide, owing to the geometry and construction possess high Young's modulus, high diffraction strength, fast mobility of charge carriers, large specific surface area and biocompatibility, excellent electrical and thermal conductivity [47], [53], [54], [55].
So far, different techniques have been developed for the synthesis of graphene and graphene oxide, including epitaxial growth, mechanical exfoliation, unzipping carbon nanotubes, excoriation of graphene oxide, liquid phase exfoliation of graphite, electrochemical exfoliation of ionic-liquid, bottom-up organic synthesis, electrical arc discharge between two graphitic electrodes and sonochemistry [55], [56], [57].
Graphene and graphene oxide with an acutely high specific surface area can interact with different biomolecules for applications in sensing [58], biological imaging and molecular imaging [59], drug/gene delivery [60], and cancer therapy [61], tissue scaffolds [34] and as antibacterial agents [62].
4. Surface modification of nano-graphene for biomedical applications
Since 2008, graphene-based drug delivery systems have been proposed. Due to single-layered structure GO has very high surface area, therefore it has a high efficiency of drug loading. The success obtained from the use of carbon nanotubes as carrier of a variety of chemotherapy drugs including doxorubicin (DOX) [60], [63], camptothecin (CPT) [64], [65], SN38 (an analog of CPT) [66], ellagic acid [67], b-lapachone [68], and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) [69], loaded on GO with various surface functionalization for treatment of cancers. Recently, significant progress in the development of graphene-based biosensors has been made. For example GO has been used for the detection of drugs [47].
There are diverse surface adjustment strategies; such as covalent, non-covalent and incorporation with inorganic nanostructures approaches suitable for designed engineer functionalized graphene-based materials to be utilized in biomedicine [70], [71], [72], [73]. Graphene oxide possesses chemically reactive oxygen groups; such as hydroxyl, carboxylic acid and epoxy groups and, because of that, most of covalent functionalization procedures use reactions of these groups [57], [74].
Graphene oxide can also be non-covalently functionalized by biomolecules and polymers through hydrophobic interactions, electrostatic binding, or p-p stacking [75] and there are several hydrophilic macromolecules utilized for covalent functionalization of GO [74]. Polyethylene glycol (PEG) is a hydrophilic biocompatible polymer widely used to functionalize various nanomaterials to refine their biocompatibility and to decline their nonspecific binding to biological molecules and cells, and improve their in vivo pharmacokinetics for superior tumor targeting [59], [64], [66], [76]. For example incorporation of PEG to GOs enhances dispersion in biological media and applications such as drug delivery [77]. Other hydrophilic macromolecules and small molecules that can be used to covalent functionalization of graphene oxide are dextran, chitosan (CS), sulfonic acid, polyacrylic acid (PAA), poly-l-lysine (PLL) and others [74], [78], [79], [80].
In non-covalent functionalization methods, surfactant or amphiphilic polymers have been used in order to obtain stability enhancement of GO in aqueous solutions. In these methods, Tween, Polyethylenimine (PEI), Pluronic F127, Protein and DNA have been used as amphiphilic polymers for surface coating of GO [75], [81], [82], [83]. Furthermore, several other polymer such as (poly diallyldimethyl ammonium chloride (PDDA) and poly-dopamine (PDA) have been used for synthesis of nanocomposite and enhancement of their stability by different methods including in situ reduction, in situ grafting and one-pot hydrothermal [84]. The results showed that, hybridization of rGO-Ag NPs composites with polymeric material especially have high level inhibitory about (100%) compare with rGO-Ag NPs [85]. It seems that polymers with cationic charge such as polylysine, polyallylamine, poly (ethylenimine) (PEI), polyionenes, quaternized poly (vinylpyridine) (PVP) could be disrupted outer membrane (OM) and the cytoplasmic membrane (CM) of bacterial and finally led to bactericidal activity [86]. On the other hand some polymers such as polyvinylpyrrolidone (PVP) and PEG don't show significant antibacterial activity while led to enhancement of NPs stability in suspension [87].
Incorporation of nanoparticles on graphene oxide is another functionalization method. In this method several nanoparticles; such as Au, Ag, Pd, Pt, Ni, Cu, TiO2, ZnO, MnO2, Co3O4, Fe3O4 and Quantum dots (QDs) have been incorporated with graphene and graphene oxide, for different applications [88], [89], [90].
Nanoparticle-based drug delivery systems have been widely studied for the treatment of cancer, increasing therapeutic efficacy and minimizing toxic side effect [91].
The nanomaterials safety is very important for their biomedical applications. Recent studies shown that both, surface coatings and size, play basic roles in controlling NPs behaviors such as biodistribution, excretion and toxicity [73]. Therefore, like to other NMs, suitable surface modification can successfully reduce the in vivo toxicity of graphene and their derivatives. Many previous studies have demonstrated that the functionalized graphene and GO forms have no toxicity to cells, but results obtained so far are under discussion [92], [93].
5. Graphene oxide as a new generation of anti-bacterial nanomaterials
In recent years, graphene oxide based nanocomposites have increasing attention in many different areas such as the control of antimicrobial resistance in pathogens [94]. Combination of various functionalization strategies could efficiently increase the antibacterial properties of GOs [95]. Many studies show that the effectiveness of graphene oxide as an antibacterial is probably due to its high surface area, great thermal stability, particular physiochemical properties, great electronic conductivity and amazing mechanical power [96].
Recent studies show that the high antibacterial efficiency of graphene oxide is due to the damage of cell membranes via generation of reactive oxygen species and exceptionally sharp edges of graphene oxide [96], [97], [98].
The advantage of graphene oxide to kill or inhibit bacteria by comparison with other antibacterial materials can be summarized in these three particular characteristics: (i), the antibacterial mechanism of graphene oxide is affected by both, physical destruction and chemical oxidation, which decrease bacterial resistance. (ii), graphene oxide has mild cytotoxicity to mammalian cells in low dose, and (iii), in comparison to other carbon nanomaterials, easy processing, large scale production, and low cost of production, guaranteed them as a good antibacterial agent [66], [96], [99]. For example, Han et al. showed that graphene oxide had a strong effect on the proliferation of bacterial and fungal pathogens (killed almost 90% of the bacteria and suppressed 80% macroconidia germination [100].
In 2013, Murugan et al. demonstrated that the minimum bactericidal concentration (MIC) of UV irradiated graphene oxide nanosheets for Escherichia coli, Salmonella typhimurium, Bacillus subtilis and Enterococcus faecalis is lower than that of normal graphene oxide nanosheets and antibiotic kanamycin [95]. Several studies in recent years have shown that graphene oxide has high power antibacterial properties.
6. Hybridization of GOs with different NMs for enhancement of antibacterial capacity
Materials development at the nanoscale have been improved from single piece synthesis to multi-component groups where two or more pre-synthesized nanomaterials are mixed for a multi-functionality [101]. These groups are called as nanohybrids (NHs). The underlying focus of NH synthesis which results in conversion to inherent physico-chemical traits [102]. Hybrid nanoparticles (HNPs) mix several materials onto a single nano-system so supplying a powerful approach for bottom-up design of novel architectures [103], [104], [105]. Nanohybrid applications are classified as follows: i) electronic like imaging and sensing ii) environmental like contaminant sorption and iii) medical like cancer treatment and detection [106]. Also Nanomaterials like metallic NMs (Ag, CuO and etc.) could have toxicity, bactericidal and growth inhibitory effects on biological species that can link or act as linker with GO to create a new weapon against bacteria [107], [108]. Table 2 summarizes data related to the antibacterial activity of GO and GO hybrid nanomaterials, thus evidencing the importance of NHs in fields like pharmacokinetics, medicine, and other ones related to anti-microbial properties [109], [110], [111].
Table 2. Antibacterial activity of GO and GO-hybrid nanomaterials.
| Base nanoparticle | Linker | Linked material | Linked nanoparticle | Effected microorganism | Antibacterial activity and sterilizing rate [%] | Reference |
|---|---|---|---|---|---|---|
| GO | PDDA | – | Ag | E.coli and B. subtilis | 54 and 60 | [27] |
| – | – | Ag | P. aeruginosa | 100 | [206] | |
| – | – | Ag | E. coli | + | [89] | |
| – | – | Ag | E. coli and S. aureus | 100 and 87.6 | [207] | |
| – | – | Ag | Staphylococcus aureus and Bacillus subtilis | 100 | [208] | |
| – | – | Ag | E. coli and S. aureus | 94 and 74 | [94] | |
| – | – | Ag-TiO2 | E. coli | + | [209] | |
| – | Ethylenediamine triacetic acid (EDTA) | – | Bacillus subtilisand Cupriavidus metallidurans CH4 | 92.3 ± 10 and 99.1 ± 1.3 | [210] | |
| – | Nisin antimicrobials peptide | – | MRSA | 100 | [211] | |
| – | Gramicidin (GD) | – | Pseudomonas aeruginosa and Staphylococcus aureus | + | [212] | |
| – | – | ZnO | E. coli (ATCC 25922) | 99.5 | [213] | |
| – | BKB and BKB/PDA | – | E. coli and Gram-positive bacterium (list) | + | [214] | |
| – | – | TiO2 NRCs | E. coli | + | [215] | |
| – | – | Ag-ZnO BM | E. coli | 98 | [216] | |
| – | Poly(N-vinylcarbazole) (PVK) | – | E.coli and B. subtilis | + | [217] | |
| PEG | – | Au | Ca Ski cells | 38.5 | [218] | |
| Sodium dodecyl benzene sulfonate (SDBS) | – | Au-TiO2 | E. coli K12, Rhodopseudanonas palustris and Candida | 90.21, 100 and 92.13 | [162] | |
| Sodium dodecyl benzene sulfonate (SDBS) | – | TiO2 | E. coli K12, Rhodopseudanonas palustris and Candida | 68.93, 65.16 and 77.64 | [162] | |
| – | – | TiO2 | A549 cells | + | [142] | |
| – | – | Fe3O4 | S. aureus, E. faecalis, E. faecalis, E. coli, Shigella, and Salmonella | 88.5, 92.8, 92.3, 93.1, 60.5 and 82.5 | [219] | |
| – | – | ZnO | E. coli and HeLacell | 63 and 48 | [158] | |
| – | P25 | Ag3PO4 | E. coli, S. aureus, S. typhi and P. aeruginosa | 100, 12.5, 6.25 and 6.25 | [220] | |
| – | Polyvinyl-N-carbazole (PVK) | – | E. coli, Cupriavidus metallidurans, Bacillus subtilisand Rhodococcus opacus | 89.1, 92.3, 89.4 and 90.5 | [217] | |
| – | Chitosan | Fe3O4 | E. coli | 98.76 ± 0.16 | [221] | |
| – | Polyethylene (PE) | – | E. coli | 99 | [222] | |
| – | Fe3O4 | Ag | E. coli and S. aureus | 99.99 and 99.96 | [186] | |
| rGO | – | – | Ag | E. coli | 100 | [223] |
| PDDA | – | Ag | E. coli | + | [224] | |
| NA | – | Ag | E. coli and S. aureus | 92.4 And 96.9 | [131] | |
| PEI | – | Ag | E. coli and S. aureus | 93.7 and 96.1 | [85] | |
| – | – | Au | S. aureus, B. subtilis, E. coli and P. aeruginosa | 100.00, 99.76, 97.47 and 100.00 | [163] | |
| – | Brilliant blue (BB) | – | E. coli and S. aureus | + | [130] | |
| – | BB-TTP | – | E. coli and S. aureus | + | [130] | |
| – | – | Iron oxide (IO) Fe3O4 | Staphylococcus aureus (MRSA) | + | [185] | |
| Poly-l-lysine (PLL) | – | Cu | E. coli and S. aureus | 99.99 and 99.58 | [181] |
6.1. Go-Ag nanohybrid
AgNPs have been reported to exhibit antimicrobial virtues and have engrossed attention for medical and chemical applications, because of their high resistance to oxidation, wide-ranging of antimicrobial activity, as well as a high thermal conductivity [112], [113], [114], [115], [116].
Furthermore it has been evidenced that AgNPs have effective cytoprotective activity toward cells infected with social immunodeficiency virus and having antibacterial activity against Staphylococcus aureus, methicillin-resistant S. aureus (MRSA) bacteria, Escherichia coli, and methicillin-resistant Staphylococcus epidermidis [117], [118], [119].
Ag nanoparticles can unswervingly cause bacterial cell membrane damage, disturbed in DNA replication and finally leading to increased permeability and ultimately result in cell death [89], [120], [121].Three possible mechanisms are usually proposed: (i) gradual release of silver ions, which can affect DNA replication and ATP production, (ii) direct damage of cellular membranes by AgNPs, or (iii) generation of reactive oxygen species (ROS) from AgNPs and Ag [27].
In addition, many models have been considered to displaying comprehensive diversity of applications, such as bactericidal effects of Ag+ ions by the size of the particles, being confirmed that smaller size of AgNPs has greater antimicrobial effects, Therefore it is so important to control this NP [122], [123], [124], [125] but, additionally, it seems that the whole structure of the nanoparticles has a great influence on the antimicrobial activity and, thus, the construction of GO-based hybrid is presently a main subject to attracting worldwide attentions [126], [127].
The GO-Ag hybrid composites have been exploited as novel antibacterial systems [128], [129]. Modifying the GO nanosheets surface by Ag nanoparticles could enhance antimicrobial features and increase stability and dispersity of GONPs. Conjugated forms of GO-Ag nanocomposites lead to increasing antibacterial activity by synergistic effect and the negative surface charge that would reduce the interaction between the bacterial cells [27], [89].
For better understanding these features some experiment have been reported. For example, Zhu et al. have used poly (diallyldimethylammonium chloride) (PDDA) as a linker with different densities, sizes and forms for conjugating AgNPs to GO sheets (Fig. 3A). This approach permits AgNPs to be easily attached onto GO with several morphologies, that provides a common and simple method to formulate GO-metal NPs composites. The result showed that antibacterial activity of GO-PDDA-AgNPs could be significantly increased in comparison with Ag NPs (Fig. 3B) [27].
 Fig. 3. (A) procedure for the self-assembly of AgNPs and PDDA onto GO nano sheets (B) Bacterial growth curve in LB media (a) for E. coli, (b) for B. subtilis with different concentrations of nano composites. Adapted from published papers [27].
Fig. 3. (A) procedure for the self-assembly of AgNPs and PDDA onto GO nano sheets (B) Bacterial growth curve in LB media (a) for E. coli, (b) for B. subtilis with different concentrations of nano composites. Adapted from published papers [27].In another study Cai et al. used polyethyleneimine (PEI) as linker for conjugation of rGO to AgNP (Fig. 4A). This fabrication shows high solubility, stability, slight cytotoxicity, elongated term antibacterial effect, and permits that GO remains unchanged with negative zeta potential of 46.7 mV [85]. In this study, the mechanism of antibacterial activity of rGO-PEI-AgNP is based on ‘the blade like edge’ that can be made the Ag+ and finally damage the bacterial cell [130]. Obtained results demonstrate that GO-PEI-Ag NPs have long time antimicrobial activity than polyvinylpyrrolidone (PVP)-Ag NPs and show excellent stability. On the other hand PEI-r GO–Ag NP has a gentle cytotoxicity on treated CNE1 cells with PEI-rGO-Ag NP in comparison with PVP-Ag NP(Fig. 4B) [85].
 Fig. 4. (A) The synthesis of water-soluble PEI-rGO-Ag NPs hybrid (B) the antibacterial activity of Nanohybrid. This figure was obtained with permission from reference [85].
Fig. 4. (A) The synthesis of water-soluble PEI-rGO-Ag NPs hybrid (B) the antibacterial activity of Nanohybrid. This figure was obtained with permission from reference [85].In a similar study Cai et al. [131] link AgNPs to sodium 1-naphthalenesulfonatefunctionalized reduced graphene oxide (NA-rGO) as a substrate to produce an AgNP-NA-rGO hybrid. This nanostructure showed a tremendous stability, extensive antibacterial ability, and minor cytotoxicity. The results demonstrated that antimicrobial activity of AgNP-NA-rGO hybrid is higher than polyvinylpyrrolidone (PVP)-stabilized Ag NPs and NA-rGO.
6.2. GO-TiO2 nanohybrid
Titanium dioxide (TiO2) is as semiconductor commonly employed as photo catalyst for detoxification of industrial wastes using light energy. It was recognized, from the discovery of Fujishima in 1972 [132] as a compound with high stability, low-cost and safety toward both, humans and the environment [133]. Additionally it has been established that TiO2 could be used for the killing or growth inhibition of bacteria due to its strong oxidation activity and super hydrophilicity [134], [135]. TiO2 based photocatalyst and antibacterial systems can be used as a pattern for the photocatalytic damage of microorganism cells by different mechanisms [136]: (i) makes electron-hole pairs in the TiO2surface, (ii) photo generates holes which can react with adsorbed H2O or OH– to produce the extremely reactive hydroxyl radicals and the electrons can act in response with oxygen to form superoxide ions, (iii) organic compounds/cells adsorbed on the TiO2 surface are oxidized by various active oxygen species providing the death of the microorganisms [137].
As well as antibacterial activities and effectiveness of TiO2 based photocatalyst directly depends on TiO2 stability, moving the excitation wavelength to the visible light led to generate electron-hole pairs [136], [137]. In addition there are several strategies for increasing TiO2 photocatalytic ability, including modification of particle size and/or surface by adding other semiconductors or metal nanoparticles [137], [138], [139].
Modification of GO layers with TiO2 provides nanocomposite which show combined properties of carbon and TiO2 (Fig. 5) [140]. Consequently, the linking of TiO2 and graphene is able to concurrently present outstanding control ability, absorptivity, conductivity, and clearness [141]. Jin et al. used GO and TiO2/GO to demonstrate cytotoxicity and distribution effects for damaging of A549 cells. Results in this study showed that after enter GO and TiO2/GO into the cells they can injured the mitochondria and cause grow number of lysosomes that led to disruption and damage of cells [142].
 Fig. 5. graphene decorated with TiO2 nanoparticles. Adapted from published papers [140].
Fig. 5. graphene decorated with TiO2 nanoparticles. Adapted from published papers [140].6.3. GO-ZnO nanohybrid
Zinc Oxide (ZnO) is one of the famous NPs which have antibacterial activity against both
Gram-positive and Gram-negative bacteria and even bacterial spores. These NPs are very stable and has high bactericidal activity by damages the membrane wall of the bacteria [87]. ZnO NPs showed positive antimicrobial effects on Staphylococcus aureus compare with other metal oxide that reported by Jones et al. [143] In addition Huang et al. revealed that the antibacterial activity of ZnO that cause increasing permeability against S. agalactiae and S. aureus whereas led to disruption of cells inside [144].
Additional nanoparticles, based on modified GO, with antimicrobial properties [112], [145] can incorporate ZnO, which is a highly safety compound led to its special chemical and physical properties, stability, and high surface area to volume ratio [143], [146], [147].
Due to their low toxicity and bio safety, ZnO NPs frequently have been used in drug delivery studies and cosmetic products [148]. Antimicrobial activity of ZnO depends on size and shape of particle [149], aqueous suspensions, UV light [150], concentration and hybridation with other nanoparticles. The mechanism of ZnO antibacterial activity may occur by two pathways: (i) Generation of reactive oxygen species (ROS) [151], [152], [153]and (ii) accumulation of NPs in the cytoplasm or in the periplasmic space or, alternatively, assembly of NPs on the surface of bacteria that led to interruption and inhibition of membrane and cellular task. The procedure of synthesis of ZnO/GO nanocomposites, is illustrated in (Fig. 6A), Recently, GO-Zn hybrid NPs have been used in the different areas such as targeted drug carriers, optics, electronics, catalysts, sensors and antibacterial activity [154], [155], [156], [157], [158]. For investigation of antibacterial activity of ZnO/GO composites Wang and Cao have used ZnO/GO nanocomposites on E. coli and HeLa cell. The results of this study demonstrated that ZnO/GO have high bactericidal activity and low effect on the cell viability in different concentrations (Fig. 6B) [158].
 Fig. 6. (A) Procedure for self-assembly of ZnO NPs on to GO Nano sheets. (B) Photographs of the inhibition zone by the disk diffusion assay: (a) control; (b) GO; (c) 1 μg of ZnO/GO-2; (d) 2 μg of ZnO/GO-2; (e) 2 μg of ZnO/GO-1; (f) 4 μg of ZnO/GO-1. Adapted from published papers [154], [155], [156], [157], [158].
Fig. 6. (A) Procedure for self-assembly of ZnO NPs on to GO Nano sheets. (B) Photographs of the inhibition zone by the disk diffusion assay: (a) control; (b) GO; (c) 1 μg of ZnO/GO-2; (d) 2 μg of ZnO/GO-2; (e) 2 μg of ZnO/GO-1; (f) 4 μg of ZnO/GO-1. Adapted from published papers [154], [155], [156], [157], [158].6.4. GO-AU nanohybrid
The antibacterial effects of metal NPs depends on size, surface area and shape of NPs [159]. Among the NPs, gold NPs (AuNPs) have been used in biomedical applications for drug and gene delivery, as contrast enhancer, biosensor technology and photo thermal therapy [160]. It has been show that Au NPs has antibacterial activity against E. coli but not in S. aureus [101].
To enhance AuNPs activity, the surface of them can bio conjugated with different groups or NPs including GO. For instance, AuNPs-GO, AuNPs-rGO hybrids showed potent antimicrobial activity. Lin et al. reported the bactericidal activity, under 785 nm irradiation, of Au-GO nanocomposites, being exploited their photo thermal properties [161]. The TiO2-Au-rGO hybrid composite, under solar light irradiation for 2 h, has antibacterial activity against gram-positive, gram-negative and fungus especially on gram-positive bacteria as reported by He and coworkers [162]. In addition, Hussain et al. decorated rGO nano sheets with AuNPs to study their antibacterial activity against gram-positive and gram-negative bacteria and its biocompatibility in HeLa cells. This study evidenced the good biocompatibility and high bactericidal activity of Au-rGO NPa on some gram-positive and gram-negative bacteria [163].
6.5. GO-CuO nanohybrid
Copper (II)-oxide NPs (CuO-NPs) show a range of useful physical and chemical properties, such as high temperature superconductivity [164], their easy integration in electronic chips, heat transfer nano fluids [165], photovoltaicproperties, relative stability, low cost [166], antibacterial and antiviral activity [167], [168]. CuO NPs has anti-bacterial activity against negative and gram positive bacteria. This antibacterial activity originate from increasing permeability of outer membrane that led to increasing entrance of CuO NPs into the cells and eventually led to disrupting cells membrane and kill the bacteria [169], [170]. Bactericidal properties of CuO-NPs include mechanisms based on: genotoxicity, oxidative stress, non-homeostasis and coordination effects that kill the organisms [171].
Recently, GO or rGO have been decorated with CuO-NPs synthesized through in situ chemical methods to be used in applications as: Li-ion batteries [172], [173], [174], supercapacitors [175], polyaniline batteries [176], [177], non-enzymatic bio sensing [178], and enhanced catalytic activity [179], [180].
Furthermore rGO-CuO nano composite modified via poly-l-lysine (PLL) showed the high bactericidal activity against some bacterial strains. For more understand Ouyang et al. investigate antibacterial activity of PLL-rGO-CuNPs nanohybrid against E. coli and Staphylococcus aureus. The results show that PLL-rGO-CuNPs nanohybrid had a highest rate of killing bacteria (99.9%) and long-term bactericidal activity compare with other form of rGO. In addition mechanism of killing bacteria by PLL-rGO-CuNPs is disrupting the ion concentrations of intracellular fluid [181].
6.6. GO-Fe nanohybrid
Fe3O4 nanoparticles are antibacterial agents which bactericidal activity depends on size. Bactericidal properties of these NPs were previously reported by Shahzeidi et al. in front of P. aeruginosa, E. coli and Staphylococcus aureus, being concluded the role of Fe3O4 size in cytotoxicity [182]. Fe3O4 NPs have some other applications as detoxification of biological fluids and hyperthermia, tissue repair and increasing resolution of MRI [183]. Additional studies have evidenced the antibacterial activity of GO-Fe hybrids, being also used as controlled targeted drug carriers [184].
Pan et al. evaluate the effect of a nanocomposite system of (rGO)-iron oxide nanoparticles (rGO-IONP) to show their antibacterial effect on Staphylococcus aureus. The mechanism of rGO-IONP interaction with bacteria was based on the generated heat and large amounts of hydroxyl radicals, which own to inactivation and finally death of bacteria in both, in-vivo and in-vitro. There are several conditions that could affect the H2O2 + rGO-IONP + bacteria interactions and NIR (near-infrared) spectroscopy was used for testing the antimicrobial activity. Results found exhibited noticeably reduction in cell viability than photothermal effect of rGO-IONP (Fig. 7) [185].
 Fig. 7. (A) Schematic illustrations showing synthesis of rGO-INOP and mechanism by which it inactivates MRSA in subcutaneous abscesses that were experimentally created in a mouse model. (B) Qualitative results from live/dead staining images, (C) SEM images of MRSA following treatment of rGO-IONP. This figure was obtained with permission from reference [185].
Fig. 7. (A) Schematic illustrations showing synthesis of rGO-INOP and mechanism by which it inactivates MRSA in subcutaneous abscesses that were experimentally created in a mouse model. (B) Qualitative results from live/dead staining images, (C) SEM images of MRSA following treatment of rGO-IONP. This figure was obtained with permission from reference [185].In another study of GO doped on the surface with Fe3O4 and silver nanoparticles evidenced the MGO-Ag antimicrobial activity. Zhang and coworkers [186]have evidenced that MGO-Ag displayed high antibacterial efficiency in different conditions of temperature, time and pH, even if the rate of nanohybrid was low. In conclusion, after treating E. coli and S. aureus with MGO-Ag losses of viability were 99.99% and 99.96%, respectively. The involved mechanism includes physical pressure, oxidative stress and ROS. In this nano composite Ag could come into connection with the membrane of bacteria, decline lipopolysaccharide molecules and own to increment membrane permeability and Fe3O4 nanoparticles and nano-Ag destroyed DNA inside the bacterial cell [187], [188], [189].
7. Conclusion and future perspectives
After a half century of clinical antibiotic use, resistance and, in particular, MDR has emerged as a strong health problem with no signs of reduction. To solve this situation, the cooperation of numerous disciplines and innovation concerning new therapeutics and favorable antimicrobial strategies are required. Recently nanotechnology supplies a good platform to solve the problem of resistance, based on the use of antimicrobial nanomaterials, being identified many nanomaterials with antibacterial properties to combat infections by antibiotic-resistant bacteria. Up to now, various types of nano-materials with antibacterial properties have been identified, including silver (Ag), iron oxide (Fe3O4), titanium oxide (TiO2), copper oxide (CuO), Au NPs, zinc oxide (ZnO) and graphene oxide (GO). In recent years, graphene oxide (GO) based nanocomposites have increased considerable attention to control resistant pathogens. Recent studies shows GO has high efficiency in antibacterial activity by damage of cell membranes. To increase the antibacterial effect of graphene oxide, functionalization of graphene surface by different covalent and non-covalent strategies and the incorporation of inorganic nanostructures has been increased the graphene antibacterial efficiency. Macromolecules and small molecules have been used to functionalize graphene oxide such as, dextran, chitosan, sulfonic acid, polyacrylic acid (PAA), poly-l-lysine (PLL) and tween, polyethylenimine (PEI), pluronic F127, proteins or DNA. So, it can be concluded that modified graphene structures will provide new highly efficient antibacterial systems in front of multidrug-resistant pathogens.