1. Introduction
There is a dire need for effective tissue engineering solutions for renal failure, as the global incidence of this disease is increasing disproportionately to advancements in therapies [1]. At present, the only definitive treatment for renal failure is donor transplantation, and the only viable management therapy is dialysis. Renal transplantation inherently relies on the availability of donor kidneys, of which there is a significant global shortage. For example, in the United States, patients wait for an average of 3–5 years to receive a transplant, with many patients passing away before that time point [2]. Furthermore, even though renal transplantation recovers the essential functions of the kidney, the necessity for immunosuppression puts patients at life-long risk of infection and is correlated with increased cancer risk [3], [4], [5], [6], [7], [8], [9]. Until patients are eligible to receive a transplant, they are treated with dialysis, which is not a true replacement for kidney function. Furthermore, dialysis poses a significant risk for patients, with an average 1 in 100 chance of death for every eight weeks on dialysis [10]. It is estimated that, by 2030, approximately 70% of renal failure cases will be in countries that lack the infrastructure to support treatment [1]. Thus, it is clear that the development of an alternative treatment modality is necessary.
Kidney tissue engineering approaches hold promise for developing permanent solutions that address many of the inadequacies in current care. If a true renal tissue replacement can be successfully engineered, tissue-engineered therapies will eliminate the dependency on viable donor tissue and thereby decrease the time requiring dialysis. Furthermore, the use of autologous primary or stem-cell-derived renal cells for tissue engineering may eliminate the need for immunosuppression. To that end, many advancements have been made, especially in the past decade; however, numerous technological and scientific challenges remain to be addressed in kidney tissue engineering.
One central challenge to the advancement of current tissue engineering approaches—and the focus of this review—is the establishment of vascularization for implanted tissue constructs. In any tissue engineering system, a key component of the success of the system is the supply of proper nutrition, metabolite exchange, and oxygenation of the resulting tissue. It is well known that cells need to be within 100–200 µm of a nutrient and oxygen supply in order to survive [11]. In vivo, this nutrition and metabolite exchange is made possible through the microvasculature distributed throughout tissue structures. Despite recent advances in this area, current engineering techniques are limited in designing sophisticated and precise vascular networks to maintain this capacity, hindering the development of larger tissue constructs as donor tissue replacements. This review focuses on the vascular challenge, highlighting recent advancements toward recapitulating and integrating renal vasculature for tissue engineering, as well as the shortcomings of these approaches.
2. Defining the vascular challenge specific to kidney tissue engineering
The need to establish adequate vascularization is a universal challenge in many tissue engineering applications but is particularly pronounced in renal tissue engineering, because adequate vasculature is central to the functionality of renal tissue, in addition to its role in nutrient and metabolite exchange. Blood flow through the kidney and, more specifically, its functional unit, the nephron, is essential for the functions of glomerular filtration, blood pressure regulation, and metabolite and chemical balance. Without proper flow through this vascular system, that occurs in many pathologies of renal failure, kidneys cannot properly remove waste from blood, remove acid produced by cells, regulate water and other fluid levels, make hormones that help produce red blood cells, and balance blood minerals. This loss of organ function has direct effects on overall body function and homeostasis. For example, kidney failure results in disequilibrium, as acid and waste build up in the body; anemia, as red blood cell production is hindered; irregular blood pressure and edema, with an imbalance of fluid levels; and osteoporosis, with the imbalance of minerals. The additional functionality that the vasculature of the kidney needs to provide increases the complexity of the vascular network in this organ and escalates the vascular challenge of kidney tissue engineering. An overview of the complexity and integration of the vascular network of the kidney discussed in this section highlights the depth of the challenge of engineering such a network.
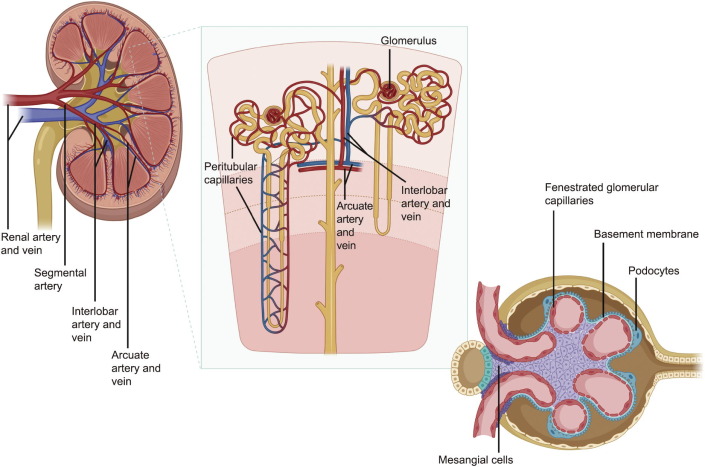
Blood flows through each kidney from the renal hilum toward the renal cortex and through the nephron, as follows: The renal artery at the renal hilum branches into segmental arteries, interlobar arteries, arcuate arteries, and afferent arterioles into the glomerular capillary network, where it is filtered into an initial urine product, then into the efferent arteriole out of the glomerulus, feeding into the peritubular capillaries. These capillaries then connect to the interlobular vein and arcuate vein, into the renal vein moving blood out of the kidney (Fig. 1) [12]. Several aspects of this vasculature are particularly important to renal function and are critical to consider when designing engineered tissues. These include ① the peritubular capillaries that sustain the nephron while playing a pivotal role in urine concentration, ② the three layers of filtration within the glomerulus that are paramount to kidney function, ③ the integration of renal vasculature with different body systems, and ④ the high amount of blood flow and pressure that the renal vascular network must withstand. Each of these aspects creates a vascular challenge unique to renal tissue engineering and is briefly detailed below.
 Fig. 1. Kidney vascular organization. Blood flow moves into the kidney via the renal artery at the renal hilum, which branches into segmental arteries, interlobar arteries, arcuate arteries, and afferent arterioles into the glomerular capillary network. The blood entering the glomerulus through the afferent arteriole is filtered through a three-layer sieve involving the fenestrated glomerular capillary, the basement membrane, and the slits between the podocyte foot processess to create a rudimentary urine product. The unfiltered component of the blood moves out of the glomerulus through the efferent arteriole, feeding into the peritubular capillaries. These capillaries then connect to the interlobular vein and arcuate vein, into the renal vein. Created with BioRender software.
Fig. 1. Kidney vascular organization. Blood flow moves into the kidney via the renal artery at the renal hilum, which branches into segmental arteries, interlobar arteries, arcuate arteries, and afferent arterioles into the glomerular capillary network. The blood entering the glomerulus through the afferent arteriole is filtered through a three-layer sieve involving the fenestrated glomerular capillary, the basement membrane, and the slits between the podocyte foot processess to create a rudimentary urine product. The unfiltered component of the blood moves out of the glomerulus through the efferent arteriole, feeding into the peritubular capillaries. These capillaries then connect to the interlobular vein and arcuate vein, into the renal vein. Created with BioRender software.(1) The peritubular capillaries support the majority of the oxygen and metabolite exchange to maintain the cells of the nephron, while also being critically important for the exchange of fluids and electrolytes from the initial urine product as it passes through the loop of Henle. Without this robust microvasculature, the viability of the nephron would suffer and, functionally, the body would not be able to excrete waste properly to maintain homeostasis.
(2) Three layers of filtration exist at the highly integrated junction between the glomerular capillaries and the Bowman’s capsule that collects the initial urine product. This junction consists of the fenestrated capillaries of the glomerulus, the charged basement membrane, and the slits between the podocyte foot processes. Each layer of this system acts as a sieve that retains charged and larger proteins and cells in the blood while allowing waste products and water through. It has also been found that flow through this three-layer system is critical for the paracrine signaling involved in the proper development and maintenance of the nephron tissue structures [13].
(3) As the renal vasculature interfaces with the Bowman’s capsule, the vasculature must also interface appropriately with a variety of cell populations for proper kidney function. Most notably, the proper placement of afferent arterioles at the glomerular hilum next to the juxtaglomerular cells that release renin is critical to regulating blood pressure. The vasculature also interfaces with the cell populations responsible for the release of erythropoietin and for the proper metabolism of vitamin D.
(4) All of the vascular components of the kidney need to withstand a large amount of flow and pressure, as the two kidneys together receive 20%–25% of cardiac output. In general, flow through developing vasculature is essential for the proper development and maintenance of vessels.
3. Engineering applications
Many different tissue engineering approaches have been introduced to address the challenge of engineering a vascular network. This review separates the approaches into categories based on their intended purpose—namely, engineering complexity and precise cell placement or the integration of vasculature into renal tissue structures. Although some of these approaches have not yet been directly applied to kidney tissue engineering and largely compartmentalize the challenges of tissue vascularization, these discrete approaches utilized together may provide a solution suitable for kidney tissue integration in the near future.
3.1. Engineering complexity and precise cell placement
Many different tissue engineering approaches have been introduced to fabricate a vascular network, including gas foaming, solvent casting, particulate leaching, fiber bonding, phase separation, electrospinning, and self-assembly [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]. While some success has been achieved with these methods, the fabrication of a long-lasting patent microvascular network has remained elusive, because these techniques currently lack the control and precision necessary to recapitulate the microarchitecture. Thus far, synthetic vascular grafts have largely only been successful for diameters greater than 8 mm. Smaller vascular substitutes (< 6 mm) often fail to maintain long-term patency and viability due to the absence of a healthy endothelial cell layer, diameter mismatch, synthetic material surface properties, and compliance mismatch with native tissue leading to intimal hyperplasia, thrombosis, or infections of the graft [24]. It is believed that, through better control of scaffold microarchitecture and cell placement, some of these challenges may be overcome.
For these reasons, there has been a recent focus on developing bioprinting techniques that offer a higher level of fabrication control using biocompatible cell-laden bioinks. A schematic image of the most prevalent bioprinting methods is shown in Fig. 2. This higher level of control in building scaffold architecture and cell placement is necessary to recapitulate the complex architecture of the renal vascular network. Many in-depth reviews of current methods in vascular bioprinting have recently been reported [24], [25], [26]. This section provides a brief overview of leading bioprinting strategies aimed at microvascular fabrication for tissue engineering, including extrusion-based bioprinting, droplet-based bioprinting, and laser-based bioprinting, all of which differ mainly in the level of control they provide.
 Fig. 2. Overview of main bioprinting techniques. (a) Extrusion-based bioprinting uses a pneumatic, piston, or screw mechanism to apply pressure to bioinks in a syringe in order to print shapes onto a flat surface. A coaxial or multiaxial nozzle can be used to extrude hollow shapes and shapes with more complex architecture. (b) Droplet-based bioprinting uses thermal, electrostatic, piezoelectric, or micro-valve technologies (schematically represented by a silverdisc) to release droplets of cell-laden hydrogel. The images to the right of parts (a) and (b) show the advantage of an even cell and scaffold distribution that droplet-based techniques provide over extrusion methods. (c) Laser-based droplet bioprinting methods use a titanium or gold film and a laser to create air bubbles in the underlying layer of bioink that push droplets of bioink onto a surface. The methods shown in parts (a–c) can be used in a supportive bath to allow for better stability and can be used to extrude sacrificial materials that are later used in indirect bioprinting methods. (d) Laser-based photopolymerizationuses a porous stage within a bioink bath to build a structure layer by layer using laser-based curing. Created with BioRender software.
Fig. 2. Overview of main bioprinting techniques. (a) Extrusion-based bioprinting uses a pneumatic, piston, or screw mechanism to apply pressure to bioinks in a syringe in order to print shapes onto a flat surface. A coaxial or multiaxial nozzle can be used to extrude hollow shapes and shapes with more complex architecture. (b) Droplet-based bioprinting uses thermal, electrostatic, piezoelectric, or micro-valve technologies (schematically represented by a silverdisc) to release droplets of cell-laden hydrogel. The images to the right of parts (a) and (b) show the advantage of an even cell and scaffold distribution that droplet-based techniques provide over extrusion methods. (c) Laser-based droplet bioprinting methods use a titanium or gold film and a laser to create air bubbles in the underlying layer of bioink that push droplets of bioink onto a surface. The methods shown in parts (a–c) can be used in a supportive bath to allow for better stability and can be used to extrude sacrificial materials that are later used in indirect bioprinting methods. (d) Laser-based photopolymerizationuses a porous stage within a bioink bath to build a structure layer by layer using laser-based curing. Created with BioRender software.3.1.1. Extrusion-based bioprinting
Extrusion-based bioprinting methods essentially deposit bioinks with or without cells into pre-programmed patterns by extruding them through syringe barrels onto a surface. Solidification of the dispensed bioinks occurs via physical or chemical crosslinking, depending on the material properties. For high accuracy of bioink placement and integrity of the resulting structure, bioinks typically need to be shear-thinning hydrogels that can be easily extruded from the printer’s dispensing nozzles and must be rapidly solidified upon extrusion. The bioinks also need to be biocompatible and support cell survival and growth. These requirements limit the choice of materials that can be used as bioinks. Different sizes and types of extrusion nozzles can also provide an additional layer of intricacy to bioprinted construct designs.
Direct extrusion-based bioprinting approaches place bioinks in patterns that will themselves comprise the vascular tissue. These patterns can be intricately designed using a mosaic of different bioinks and cells to allow for the precise placement of specific cell types and materials. Early studies of vessel bioprinting focused on methods to extrude bioinks or cell-laden bioinks to form tubular shapes. These studies directly printed NIH 3T3 cells into tubular structures using a thiolated hyaluronic acid and gelatin gel [27], [28]. The tubular structures demonstrated the feasibility of using bioprinting to create vascular and other tubular structures. Other groups took different approaches to build tubular structures, with several groups attempting to vertically bioprint large-diameter stand-alone tubes [29]. Despite the success in printing these shapes, these structures were not viable for extended periods, primarily due to structural instability. More recently developed approaches to bioprinting with enhanced structural stabilization have focused on providing a temporary support for scaffolds until complete crosslinking occurs or improving the speed and strength of crosslinking during printing. For example, Hinton et al. [30]attempted to enhance structural integrity by developing a technique of printing structures within a secondary hydrogel bath to stabilize the placement of bioinks. This technique prevents the spreading of bioinks once extruded, prior to crosslinking.
Utilizing improved crosslinking methods, many groups have used coaxial nozzles that extrude a core material coated in a second material to print hollow filaments onto surfaces in a single step on the scale of microvasculature. For example, Yu et al. [31] developed methods of coaxial extrusion followed by instantaneous crosslinking to support tubular fibers. These researchers demonstrated the potential of using this method of bioprinting hollow microfilaments for cell-laden bioprinting, as printed human umbilical veinsmooth muscle cells using this strategy resulted in an 84% cell viability after seven days of perfusion culture [31]. Dolati et al. [32] improved upon these methods to reinforce the integrity of the tubular filaments. Coaxial methods were further improved by developing methods that fuse adjacent hollow filaments to make tubular networks more akin to natural vascular networks [33]. Recent studies have expanded the concept of the coaxial nozzle to print bi- and tri-layered hollow channels within gel scaffolds using newly developed multiaxial extrusion nozzles to create further biomimetic networks [34]. Computer-aided design (CAD) models of organ microarchitecture have also been used for extrusion-based bioprinting to imitate biomimetic vascular constructs [35].
Another method to stabilize extruded vascular structures is to rely upon the stability of support scaffolds using indirect bioprinting methods. Indirect extrusion-based bioprinting approaches involve printing sacrificial material such as gelatin, alginate, agarose, or Pluronic in the desired pattern of vascular channels into a surrounding matrix material with or without cells. Often, the sacrificial material is then removed or washed out, and the hollow vascular tree is subsequently seeded with endothelial and/or vascular smooth muscle cells. The work by Lee et al. [36], [37] used this approach to fabricate a microvascular network that was anastomosed to two larger vessels. The researchers printed channels of gelatin into a collagen matrix adjacent to a bed of fibrin embedded with human umbilical vein endothelial cells (HUVECs) and lung fibroblasts. The gelatin was removed via warming, and the hollow tubes were seeded with endothelial cells. The entire structure was cultured for several weeks, resulting in a microvasculature that was anastomosed to the larger vessels.
Advancing from the capability of printing large vessels, microvessels, and now branching microvessels, extrusion-based bioprinting technology brings us closer to the ability to synthetically design and create microvasculature for tissue engineering applications. Although the stability and long-term viability of these constructs are improving, one main limitation of this technology is achieving the required level of control. In theory, bioinks should be evenly dispersed through the extruded bioink construct; however, in actuality, the uniformity of the distributed cells and hydrogels suffers. This lack of precise control becomes problematic for kidney applications due to the high degree of complexity and the necessity for precise interface integration between vasculature and tissue structures, as detailed above. Thus, it is important to consider methods of achieving better control over the placement of cells, that include droplet-based bioprinting and laser-based bioprinting.
3.1.2. Droplet-based bioprinting
The central approach to droplet-based bioprinting—both direct and indirect—is similar to extrusion-based bioprinting. The difference is that, instead of a steady stream of material, small droplets containing a specific number of cells are placed in a pattern. The advantage of this approach is that the resulting constructs are more uniform in their cellular distribution and material composition. The droplets are made by thermal, electrostatic, piezoelectric, or micro-valve technologies that push out a specific size and number of droplets at a time [35]. A very early study in direct droplet-based bioprinting used inkjet bioprinting to show proof of concept that this technique could be used to fabricate tubular hydrogel constructs [38]. The research group also showed that human hematopoietic stem cells encapsulated within these constructs could be differentiated into multiple cell types during culture. Subsequent studies performed by Cui et al. [39] showed that droplet-based bioprinting could be used in conjunction with human microvascular endothelial cells to fabricate microvascular constructs with a confluent vessel lining exhibiting robust cell proliferation and cell-to-cell adhesion. Another study demonstrated that these techniques could be better controlled by printing the alginate droplets into a CaCl2 pool to allow for rapid calcium diffusion into the alginate solution in order to stabilize it upon contact, much like the crosslinking solidification in the extrusion-based bioprinting examples above [40], [41]. Using a similar approach of direct droplet-based bioprinting into a CaCl2 pool, other studies applied this technique to bioprint more complex branching constructs in order to more accurately recapitulate native vascular structures [42], [43]. These groups achieved a high level of precision and detail in their constructs, as well as high cell viability after incubation.
Indirect droplet-based bioprinting is performed in much the same way as indirect extrusion-based bioprinting. For example, the same group that demonstrated microvascular bed formation in the extrusion-based examples above conducted a similar study using a droplet-based approach. In their study, a mixture of gelatin and HUVECs were made into droplets and deposited in two tubes adjacent to a bed of fibrin embedded with HUVECs and lung fibroblasts [37]. The entire structure was then warmed to melt the gelatin and incubated further to allow HUVEC attachment to the tube lining. Consequently, a confluent endothelial lining was developed [37].
3.1.3. Laser-based bioprinting
Laser-based bioprinting methods use either cell-transfer technologies or photopolymerization technologies to build tissue constructs. Cell-transfer technologies work by transferring the energy from a laser beam into heat energy, which then causes the physical ejection of a bioink droplet onto a surface. In brief, a laser beam is applied to a layer of gold or titanium sitting on top of a layer of bioink. The gold or titanium layer absorbs the energy from the laser beam at the precise area of contact and creates heat to vaporize a small amount of the bioink to form a high-pressure bubble that quickly pushes out a droplet of bioink below the bubble onto a surface for crosslinking upon contact [35]. While this droplet formation mechanism is more precise and controlled than the droplet-based bioprinting methods discussed above, the eventual patterning of droplets to create a three-dimensional (3D) construct uses the same concept.
Photopolymerization technologies use a pool or layer of photosensitive bioinks that solidify when irradiated [24]. Laser beams are applied in specific patterns to cure isolated regions of the bioink, and uncured ink is subsequently removed to leave only the cured 3D construct. To fabricate thicker tissue constructs, a porous table can be used, so that the high-energy laser beams do not dissipate but are rather used to cure the bioink layer by layer and build it up. Although this technology has significant potential to provide more controlled and precise methods of bioprinting vascular constructs, studies of their use in vascular biofabrication have been unsuccessful in developing structures with structural integrity and cell viability to the level seen with the other bioprinting methods discussed above.
A comparison of the different properties of these bioprinting methods is detailed in Table 1. Although these bioprinting methods have been used successfully to create simple vascular networks, their applications for renal tissue engineering continue to be challenged by the structural resolution and integrity needed to recapitulate the crucial vascular structures that make up the glomerular capillaries and peritubular capillaries (5–10 µm in diameter) of the kidney [44]. To clarify, the three-layer sieve of the glomerulus is made of fenestrated capillaries (fenestrations 70–100 nm in diameter), a uniquely charged basement membrane (240–370 nm thick), and podocyte foot processes with fenestrations (25–60 nm wide), all of which are beyond the current range of printing resolution [45], [46], [47], [48], [49], [50], [51]. Thus far, 3D bioprinting methods have been developed to recapitulate tubule structures with some degree of complexity using novel bioinks that exhibit improved structural integrity and biological relevance to the renal environment [17], [52], [53]. Some studies have also printed perfusable structures that mimic proximal tubular organization and geometry [53]. However, no study has been able to recapitulate the renal vasculature to a degree that allows for true filtration function, as the complexity of the glomerulus remains beyond the limit of current capabilities. Furthermore, the insufficient integrity of the printed vascular networks makes it difficult to successfully culture these networks under flow—a process that is vital to the proper maturation of vasculature. Importantly, it remains difficult to artificially integrate the vascular structure into kidney structures using bioprinting methods alone—a point that is critical to achieving proper tissue survival and filtration function.
Table 1. A comparison of extrusion-, droplet-, and laser-based bioprinting methods.
| Properties | Extrusion-based bioprinting | Droplet-based bioprinting | Laser-based bioprinting |
|---|---|---|---|
| Printing speed | 10–50 µm·s−1 | 1–10 000 droplets·s−1 | 200–1600 mm·s−1 |
| Resolution | 100 µm–1 mm | 50–300 µm | 50 µm |
| Accuracy | Medium to low | Medium | High |
| Structural integrity | High | Low | Low |
| Scalability | Yes | Yes | Limited |
| Cost | Medium | Low | High |
3.2. Integration of vasculature into renal tissue structures
As stated above, current methods of synthetically creating a vascular network are unable to recapitulate the native vascular network of the kidney at the resolution needed. Moreover, these methods do not support the integration of vascular networks with the necessary populations of renal tissue cells. Due to these limitations, there has been a recent surge of research focusing on using the micro and macro architecture of native kidney tissue, known as the extracellular matrix (ECM), as a foundation for renal tissue engineering. The ECM is the acellular scaffolding of the tissue that provides the physical tissue structure and local cell niches using biochemical cues, such as growth factors and vascular endothelial growth factor (VEGF), to allow for proper cell maintenance, development, and vascular ingrowth. Advancements in this approach to vascularize kidney tissue are discussed in this section, and a schematic of the general techniques discussed below is shown in Fig. 3.
 Fig. 3. Methods of vascularization utilizing native tissue structures. (a) Decellularization and reseeding approaches first decellularize kidneys using a series of washes with detergents, followed by a reseeding of these scaffolds with different cell types. This method relies on the remnant cell niches to guide cells to appropriate tissue locations. (b) Vascular casting methods use the vascular networks within decellularized kidneys for the perfusion of a sacrificial material that is maintained while the rest of the tissue is digested off. This sacrificial material is then coated or placed in a cell-laden or un-laden matrix material; next, it is removed to leave behind a hollow cast of the native renal vascular network. (c) Organoid vascularization methods utilize native VEGF–VEGF receptor signaling of the organoid to call in vasculature from surrounding areas either in vivo or in vitro. Created with BioRender software.
Fig. 3. Methods of vascularization utilizing native tissue structures. (a) Decellularization and reseeding approaches first decellularize kidneys using a series of washes with detergents, followed by a reseeding of these scaffolds with different cell types. This method relies on the remnant cell niches to guide cells to appropriate tissue locations. (b) Vascular casting methods use the vascular networks within decellularized kidneys for the perfusion of a sacrificial material that is maintained while the rest of the tissue is digested off. This sacrificial material is then coated or placed in a cell-laden or un-laden matrix material; next, it is removed to leave behind a hollow cast of the native renal vascular network. (c) Organoid vascularization methods utilize native VEGF–VEGF receptor signaling of the organoid to call in vasculature from surrounding areas either in vivo or in vitro. Created with BioRender software.3.2.1. Whole-organ decellularization and recellularization
The central paradigm for using whole-organ scaffolding as a foundation for kidney tissue vascularization is to remove cellular debris from an intact organ or tissue in a process known as decellularization, which is followed by a reseeding of cells onto the scaffold in a process referred to as recellularization. Removing cellular debris from the organ will remove much of the immunogenic reactions, because the immune-reactive donor DNA and human leukocyteantigen will be removed. Moreover, the ECM material is entirely biocompatible [54], [55], [56], [57]. The framework for the vascular network that these decellularized kidneys hold is necessarily biomimetic and has the resolution necessary for kidney tissues. The innate cell signaling provided by the cell niches also theoretically allows for proper localization of different cell populations for adequate vascular integration with different regions of the nephron. Below, we detail the progressive methods that have been developed to achieve successful decellularization and recellularization of the kidney.
Critical to the development of successful decellularization methods was the establishment of a set of criteria for adequate cell removal by Crapo et al. [58], who recommended using 4′,6-diamidino-2-phenylindole (DAPI) and hematoxylin and eosin (H&E) staining to visually evaluate the removal of cells in conjunction with gel electrophoresis in order to quantify the DNA content in the decellularized tissue. Their study suggests that 50 ng of double-stranded DNA per milligram tissue by dry weight would indicate adequate decellularization [58]. Furthermore, any remaining DNA in the scaffold from a small number of residual cells should be less than 200 base pairs in length [58]. These parameters reduce the immunogenicity of the scaffolds and make the scaffolds suitable for further applications.
For reseeding the scaffolds, it has been found that retaining the integrity of the collagen, laminin, and growth factors in the ECM is critically important. Preservation of the ECM composition translates to the maintenance of cell niches to support migration, maintenance, and proper differentiation. Previously, it was thought that the growth factors and chemotactic factors of the ECM play the most significant role in the fate of seeded cells. However, more recent studies on the relationship between the biophysical properties of the ECM—such as its stiffness and innate piezoelectric properties—and cell fate have highlighted the importance of maintaining the structural integrity of the ECM [59], [60], [61], [62], [63]. For these reasons, protocols developed to decellularize cells focus on removing a sufficient amount of cell debris from the scaffold while maintaining critical ECM proteins and structural properties.
It is challenging to balance these aims of decellularization, removal of cellular debris, and preservation of the ECM structure, because methods that quickly and successfully remove cell debris from the organ often damage the ECM, while methods that focus heavily on maintaining the ECM structures often do not remove adequate amounts of cellular debris. For example, kidney decellularization methods that primarily use Triton X-100 to decellularize scaffolds successfully maintain growth factors and the structural integrity of the ECM but fail to remove cells adequately. Decellularization methods that primarily use sodium dodecyl sulfate (SDS) have been shown to sufficiently remove cellular debris while maintaining the proteins and structures of the ECM [54], [55], [57], [64], [65], [66]. Currently, many effective decellularization methods use a mixture of Triton X-100 and SDS to achieve an optimal level of cell clearance and ECM maintenance. Through scanning electron microscope(SEM) imaging, Orlando et al. [57] demonstrated that this method of decellularization maintains the glomerular structures of the nephron that are critical to the filtration function of the kidney. It is important to note that these clearance methods have been reported to decrease the stiffness of the kidney ECM and impact the cell fate of reseeded stem cells.
Once the kidney is successfully decellularized, the challenge of reseeding the vascular network properly begins. Here, we focus our discussion on studies that have recently demonstrated successful reseeding of the vascular component of decellularized renal scaffolds. The most common method of reseeding the renal vascular ECM is via antegrade perfusion through the renal artery. Most research groups achieve perfusion using a perfusion pump. Due to the complex but delicate nature of the vascular tree, especially at the glomerulus level, determining an optimal perfusion pressure and flow rate is critical to the success of re-endothelialization [67]. Incomplete re-endothelialization introduces a significant risk of thrombosis in vivo, jeopardizing the integrity of the vascular network and, therefore, the long-term viability of the engineered tissue. One of the most successful attempts at whole-organ renal tissue engineering focused on using a perfusion-based approach to repopulate the renal vasculature and renal tissue. Another study decellularized rat kidneys by using an antegrade arterial perfusion of heparinized phosphate-buffered saline (PBS) at 30 mmHg (1 mmHg = 133 Pa) arterial pressure for 15 min to clear the kidney of residual blood, followed by the administration of 1% SDS in deionized water for 12 h at a constant pressure of 30 mmHg, 15 min of deionized water only, then 30 min of 1% Triton X-100 in deionized water [65]. Next, HUVECs suspended in media were seeded into the scaffold through the artery at a constant flow of 1 mL·min−1 [65]. The cells were allowed to attach overnight, after which the perfusion culture was resumed. After this vascular perfusion, rat neonatal kidney cells were seeded into the scaffold via the ureter while a pressure gradient was applied across the scaffold in an organ chamber to allow for better penetration into the kidney. The whole kidney was then placed in a custom-built bioreactor with constant perfusion through the renal artery at 1.5 mL·min−1 [65]. The ureter and renal vein were allowed to drain passively. Imaging of the resulting tissue showed the varied success of cells in localizing to the correct cell niches. Here, it is important to note that the endothelial cells had localized correctly to successfully re-endothelialize and fully line the blood vessels of the construct. Subsequent orthotopic implantation of these engineered constructs showed that, upon anastomosis with the renal artery and vein, the structure was successfully perfused without thrombi formation or hemorrhaging in the short term. Other groups have used different cell types to reseed the renal vasculature, with differing levels of success. Mouse embryonic stem cells (ESCs) were also seeded into the renal artery of decellularized rat kidneys and cultured without any additional growth factors. These cells matured into smooth sheets with the correct morphology in vascular structures [68], [69]. Another group also seeded murine ESCs into the renal artery of rat kidney scaffolds to evaluate the repopulation of the glomerular and peritubular capillaries. They found that these cells expressed Tie-2 and cluster of differentiation 31 (CD31) endothelial cell markers as early as 24 h after seeding, with CD31 expression increasing after 72 h [70]. Induced pluripotent stem cell(iPSC)-derived endothelial cells were also used as a cell source for renal vascular reseeding. In these studies, the iPSCderived cells successfully lined the vascular structures and, importantly, lined the glomerular structures as well [66], [71].
However, with all of these vascular reseeding methods, the occurrence of thrombosis in the long term remains a significant challenge. Several groups have applied anticoagulants at the time of perfusion, with little success [57], [72], [73]. Recently, one research group immobilized heparin onto the ECM of decellularized kidney scaffolds and observed an improved result compared with non-heparinized controls and anticoagulant injection upon perfusion [74]. This result could not be maintained in the long term; nevertheless, their techniques may be developed further for better results in the future. Furthermore, the localization signals provided by cell niches alone appear to be inadequate to prompt renal cells to populate the scaffolds properly, perhaps due to the cells’ inability to move freely through these scaffolds. This inadequacy hinders the ability to properly integrate renal cell populations with vascular networks, although the vascular network itself that results from this method has a higher resolution than that produced using the bioprinting methods listed above.
3.2.2. Vascular casting
Recently, a novel alternative approach to using native vascular structures for tissue engineering purposes was developed. This approach, which was founded in vascular casting methods, sought to address the challenges of current approaches: namely, the lack of resolution of bioprinting methods, the unsuccessful long-term patency, and the capacity for better integration with nephron structures. In this study, Huling et al. [75] aimed to create a biomimetic vascular scaffold that could be developed in isolation from the entirety of the kidney ECM. The benefit of this approach is that a vascular tree could be fabricated successfully to the glomerulus level and that this vascular tree could be allowed to mature more completely before being applied to different hydrogels or scaffolds to establish tissue. Building renal tissue around a predeveloped vascular bed potentially allows the development of larger tissue constructs in vitro and promises better integration when the construct is anastomosed in vivo.
The vascular tree was fabricated as follows: The renal artery of adult rats was perfused with 1 mL of acetone, followed immediately by perfusion with 1 mL of 10% (w/v) of polycaprolactone (PCL) dissolved in acetone [75]. The acetone was allowed to evaporate at room temperature for 24 h, and then at 4 °C for three days [75]. This evaporation created a solid vascular cast of the native vascular tree. Subsequently, the tissue surrounding the vascular cast was removed by placing the kidney in a 20% (w/v) sodium hydroxide solution for 48 h and then washing it with deionized water for 24 h [75]. From the now-isolated corrosion cast of the vascular tree, segments were separated at the level of the lobar arteries and dip-coated with 9.8 mg·mL−1 type 1 rat tail collagen, then placed in a crosslinking solution of 10 mmol·L–1 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride and 10 mmol·L–1 N-hydroxysuccinimide for 30 min after drying [75]. The PCL casts were then dissolved from the structure using warm acetone, leaving behind a hollow collagen-based vascular scaffold that was relatively easy to manipulate. Green fluorescent protein-transfected MS1 endothelial cells were seeded onto the scaffolds by simply coating the surface of the scaffolds suspended in media. The resulting biomimetic tree exhibited a 3D branching architecture with visible hollow channels that were interconnected and continuous. SEM images of the tips of the vascular scaffolds showed that the glomerular structures were seemingly intact. This endothelial cell-coated vascular scaffold was then put into a collagen hydrogel mixed with renal cells. Further in vivo studies implanted this entire construct onto rat kidneys after a cortical defect had been surgically made.
Though promising, this research is still in its early stages. The potential of this novel approach was demonstrated by the results of the in vivo studies, which found a significant increase in angiogenesis in implanted kidneys and, importantly, showed the presence of red blood cells and nucleated cells in the vascular construct—indicating that some anastomosis had occurred between the scaffold and host vasculature. The challenges that remain to be addressed in future studies of the vascular casting approach are the optimization of reseeding and scaffold maturation methods. It was found that, by day 14 after implantation, most MS1 cells had moved out of the scaffold into the surrounding tissue, indicating the instability of cell adherence to the scaffold [75]. As endothelial cells move away from the scaffold, they present a significant thrombosis risk, as the underlying basement membrane can be exposed to begin the clotting cascade. It was suggested that seeding the endothelial cells internally through the vascular scaffold may yield better results than external seeding, although the perfusion may damage the delicate vascular structures and introduce thrombosis, which also occurs in whole-organ recellularization methods. Another improvement could be allowing the vascular scaffold to mature further in order to form stronger connections before placing the scaffold in a tissue hydrogel; however, the patency of the vascular network may be challenging here.
3.2.3. Organoid vascularization
Another approach to better integrate engineered vascular networks and renal tissue constructs is to grow these two components together as they develop during embryogenesis, based on an improved understanding of developing vascularization and kidney tissue organogenesis. During development, the vascular network of the kidney is formed through a combination of vasculogenesis and angiogenesis. Vasculogenesis is the formation of primitive vasculature from a starting angioblast cell source originating from mesodermal cells of the developing tissue itself, while angiogenesis is the remodeling and ingrowth of vessels from the pre-existing vasculature surrounding the developing tissue. Several studies of kidney development suggest that the angiogenic source of vasculature has a more decisive influence than the vasculogenic source. However, the importance of the second population cannot be overlooked, as these cell populations may produce stronger chemotactic signals for vascular ingrowth [76], [77], [78], [79], [80], [81]. Using a mixture of these two mechanisms, a vascular network can be created that is integrated into the early nephron structures. This occurs as the early nephron structures are developed to create a region known as the vascular cleft with early podocyte-like cells that participate in VEGF–VEGF receptor signaling to bring in developing endothelial cell populations [82], [83], [84]. Once integrated and matured, the endothelial cells will become the fenestrated capillaries that are part of the three-layered filtration unit between the glomerulus and Bowman’s capsule.
Developing nephron structures that can participate in VEGF–VEGF receptor signaling can be grown in vitro in the form of renal organoids. Organoids are aggregates of the cell types necessary to create a seed tissue. Different combinations of cells can make tissues with differing functions. For example, some renal organoids have been developed only to contain isolated structures, such as tubules, while others have been designed to contain multiple renal cell types. In this context, we focus on organoids that mimic fetal kidney tissue and consist of progenitor cell populations that can build tubular and glomerular structures.
As discussed above, angiogenesis plays a major role in the vascularization of the developing kidney during embryogenesis. Therefore, it is believed that, if a vascular network with angiogenic potential is successfully established in vitro, co-culture with these organoids may result in a vascularized tissue that could be sustained with perfusion [85], [86], [87]. It has also been shown that some protocols for making these fetal kidney-like organoids can develop a population of the organoid’s cells into endothelial cells that may engender vasculogenesis as it occurs in embryogenesis [88]. Thus far, these de novo endothelial cells have been shown to mature into interconnected vascular beds when organoids are stimulated in a high-flow environment [88]. However, the vasculature that develops is not robust enough on its own to vascularize the whole tissue construct, with an expansion of the vascular network invading only a few of the glomerular structures [88]. Until successful in vitro angiogenic vasculature is developed, we can learn more about how renal progenitor organoids may connect to external vasculature and develop by transplanting them in vivo.