Introduction
For centuries, human beings have used biomedical devices to correct, augment, replace, or restore lost or malfunctioning biological structures [1], [2], [3], [4]. As a person grows older and the number of ailments increases, there is a growing demand for implants to replace hard tissues [1,5]. Biomaterials are materials that are used to repair or replaced damaged, compromised, or degenerated body parts [6] without causing any negative side effects or damage to the body parts [7]. They have had a huge impact on the enhancement of the quality of life for many patients around the globe [1] through functional restoration engineering of different body tissues [6].
Modern implantable biomaterials have improved mechanical, biological, and chemical properties and can be tailored for a specific medical application and a specific patient. They can be made of metallic alloys, composites, polymers, or ceramics, but the majority are made of metallic alloys, which are used to replace damaged hard tissues [8,9]. Modern metallic biomaterials used for making medical implants include cobalt alloys, titanium and its alloys, magnesium, tantalum or niobium alloys, medical-grade stainless steel, and gold alloys [4]. In most medical applications, titanium alloys are becoming the material of choice [6] and comprise 70–80% of the materials commonly used in making orthopaedic implants [10].
Due to their resistance to high-temperature effects and stress-resistance properties, titanium alloys were originally developed in the 1940s for use in aeronautical applications [11]. Because of their unique properties, they have also found applications in marine engineering, chemical processing, medical industries, pharmaceutical manufacturing, and many other industries. They are amongst the most useful for biomedical applications due to their excellent mechanical, chemical, and physical properties, which allow for successful implantation [12]. They are very vital in the manufacturing of medical implants [5] due to their favourable features such as higher biocompatibility, better bio-corrosion and wear resistance in the body fluids [11,13], a high strength-density ratio [14,15], high mechanical strength and low Young's modulus compared to other biomaterials [1].
Different varieties of medical implants have been developed from Ti and its alloys. Some of them include cardiovascular devices (such as pacemakers and mechanical heart valves), orthopaedic implants (such as hip implants), dental implants, stents in blood vessels, external prostheses, and operational devices (plates, pins, and hip joints) [3,16,17]. Dental devices such as dental implants, orthodontic brackets and wires, endodontic files, and prosthetic appliances have been manufactured using Ti alloys [18].
Although some fundamental information about biomaterials is known, the understanding of the behaviour of most titanium biomaterials is still limited. Therefore, the review aims to discuss different types of titanium alloys currently used in biomedical applications and their properties in relation to their performance. Despite the extensive knowledge about the grinding of titanium alloys, the potential for sustainable grinding has not yet been fully explored for most titanium alloys. Hence, the main motivation of the review is to present an updated overview of the sustainable grinding of titanium alloys used in medical device manufacturing.
This review starts with an introduction and description of the requirements and considerations of titanium alloys for medical applications. Secondly, the general characteristics of the currently used titanium alloys are described. The third part describes the sustainable grinding of titanium alloys, where different cooling and lubrication methods are highlighted. The advantages, limitations, and research gaps of these different sustainable techniques for grinding titanium biomaterials are highlighted. Finally, a conclusion on the main aspects presented previously and future recommendations are discussed.
Considerations for selecting Ti-based alloys for medical applications
A biomaterial's properties for body implant applications must be assessed to determine its suitability to avoid rejection in the human body. However, the location of the implant and the medical history of the patient may change the properties of an ideal biomaterial [1]. Some of the principal requirements stated below are discussed in detail in the subsections:
-
(i)
Biocompatibility
-
(ii)
Mechanical properties
- (iii)
-
(iv)
Wear resistance
-
(v)
Osteointegration
- (vi)
Biocompatibility
The capacity of a biomedical device to perform its function adequately over time without generating any unfavourable local or systemic effects at the site of implantation or on nearby tissues or organs is referred to as biocompatibility [3,19,20]. It is determined by the implant material's properties and mechanical design [21]. Overall biocompatibilities, including tissue-, cyto-, and haemo-compatibilities, are often evaluated using cell markers, histological sections, and metabolite measurements. The implant materials should be chemically inert, biocompatible with the human body, and should not produce toxic and unwanted immunogenicity. They should also not produce allergic reactions once implanted [22]. The biocompatibility of any implant material depends on how the human body reacts to it when it is implanted, and this reaction determines the success of the implantation process [19]. The problems associated with biocompatibility are thrombosis and the development of fibrous tissues around biomaterials implanted in the body [23].
Because no biomaterial is fully inert, some bodily reactions to implants must be measured and factored into the implant's material selection, design, and use [24]. The human body's reaction to the implant and the implant's deterioration in the body after implantation are two major factors that influence the implant's biocompatibility [23,25]. There are four types of implants based on the body's response to the implant: bio-tolerant, bioinert, bioactive, and bio-reabsorbable materials [3,25,26]. Once a bio-tolerant material has been implanted in the body, there is the development of a thin fibrous tissue interface between it and the body tissue, i.e. there is indirect contact between bone and the implant during bone formation (distant osteogenesis) [23]. Polymethyl methacrylate (PMMA) and Co-Cr-Mo alloys are examples of bio-tolerant materials. Titanium and its alloys, partly stabilized zirconia, 316 L stainless steel, and aluminium oxide are bioinert materials that often integrate well into the bone. They have limited interaction with their surroundings. For example, during bone formation, bone and implant are in direct contact (contact osteogenesis) [23].
Bioactive materials interact with the body tissues and bones, causing direct chemical and/or biological connections between them and the bone tissues (bonding osteogenesis) [23]. Synthetic hydroxyapatite, glass ceramics, and bio-glass fall into this category [25]. Materials that dissolve slowly once implanted in the human body and are slowly replaced by developing human tissue and bones are referred to as bio-resorbable materials. Tricalcium phosphate, calcium carbonate, calcium oxide, gypsum, and polylactic–polyglycolic acid copolymers are common bioresorbable materials that have been used in the manufacture of implants during the last three decades [26].
Titanium is biocompatible because of its inertness and resistance to corrosion by all bodily fluids and tissues [1,11]. Titanium implants are immune to the body's rejection and have a high rate of physical attachment to the host tissues and bones [1]. Ti alloys are non-toxic, hence they are useful in making surgical implants and prosthetic devices [1]. Titanium and its alloys have greater biocompatibility because, in any oxidative environment, a thin and adhesive passive native TiO2 layer forms spontaneously on their surfaces [5]. The presence of these spontaneous thin films, it was argued, plays a crucial role in Ti and its alloys' outstanding biocompatibility and corrosion resistance [23]. The titanium also has low electrical conductivity, which makes it biocompatible [27].
Mechanical properties
Biomaterials should have mechanical biocompatibility to ensure long-term implantation. Mechanical bio-compatibility generally refers to mechanical properties appropriate for the proper functioning of the implant once implanted in the body [1]. The mechanical properties of the biomaterials are important when selecting an appropriate implant for a specific application, and it is expected that those properties are almost similar to the mechanical properties of natural bone [23]. The mechanical properties determine how a biomaterial will respond to different related force and load conditions [2,28]. Regarding mechanical behaviours, the primary classes of clinical biomaterials include metals, ceramics, polymers, and composites of these biomaterials [2]. Standardized mechanical tests using standard specimens subjected to specific load conditions are used to determine the mechanical properties of the biomaterials, taking into consideration international standards such as ASTM (ASTM E8–04, ASTM E9, ASTM E92–17, ASTM 143, ASTM F1264, ASTM 1044, ASTM F2193, ASTM E2546–15) [2,29].
The mechanical properties considered when selecting a biomaterial for medical applications include tensile strength, hardness, Young's modulus, fatigue strength, and elongation [1,23,25]. These characteristics are influenced by the human body's components [30]. They depend on the microstructure formed during phase transformations, chemical compositions and element types, and processing and transformation techniques [2,30]. The degradation of biomaterials has been shown in many studies to have a substantial impact on the mechanical characteristics of biomaterials as well as biological functions due to interactions between body cells and biomaterials [2].
The hardness of the biomaterial is one of the vital engineering requirements considered in choosing implant materials. Machining processes such as grinding can improve the implant's hardness due to strain hardening on the surface. Hardness has a relationship with tribological behaviour, strength, toughness, and modulus of elasticity of the implant material [31]. It is a factor in determining the suitability of biomaterials for clinical use. In tribological behaviour, as hardness increases, the wear mechanism in implant materials decreases. It is a consideration that the hardness value of implant materials like Ti-6Al-4 V should be almost similar to the hardness value of bone (614–736 MPa) when selecting it for biomedical application [14].
The stiffness of the biomaterial is a material property measured by Young's modulus [26]. A human bone's Young's modulus varies from 4 to 30 GPa, depending on the type of bone and the measurement direction [25]. Because artificial implant materials are stiffer than human bone, the necessary stress is not transferred to the surrounding bone. This promotes the resorption of the bone around the implant, causing it to loosen. The "stress shielding effect" causes the death of natural bone cells due to the biomechanical incompatibility of the implants [25]. It is thus recommended that an implant material should have a low Young's modulus comparable to or closer to the natural human bone to avoid the loosening of implants. In comparison to other implant materials, Ti alloys, particularly β-titanium alloys, have a low Young's modulus of elasticity (50–120 GPa) [32]. This low modulus of elasticity of Ti alloy is advantageous biochemically because it generates a smaller stress shielding effect [1]. A smaller stress shield promotes faster and healthier bone regeneration and eliminates the need for revision surgery [33].
The implant should also have high mechanical strength (i.e., tensile and fatigue strength) in order to withstand the applied load and avoid fracture [34]. The implant's long-term success when subjected to repetitive cycle stress is determined by the material's fatigue strength. Fatigue strength depends on the composition of the alloy, thermomechanical processes, surface processes, finishing processes, and heat treatments. To have a longer service period in the body and avoid revision surgery, it is recommended that an implant material have high strength, such as tensile strength (1000 MPa).
Titanium-based alloys have sufficient fatigue strength to prevent implant fatigue and fractures [25] due to cyclic stresses. The density of titanium-based alloys is around 4700 kg.m−3[5]. This is a good feature as this will reduce the weight of the implant, and this makes Ti alloy present a behaviour mechanic similar to human bones [1].
Corrosion resistance
The corrosion behaviour of biomaterials, especially metallic ones, is of significant significance because ions released from the implant by bodily fluids can cause biocompatibility complications such as thrombosis [32]. Corrosion is the decomposition of a material into its constituent atoms as a result of chemical reactions between the material and its surroundings [28]. It is a critical consideration for a biomaterial intended for use as an implant in the human body since implant biocompatibility is highly dependant on it [6,35]. The higher the rate of corrosion, the more toxic ions are produced in the body, and consequently, the more adverse effects lead to implant incompatibility with human bones and tissues [28].
Corrosion affects the service and life of the implant made of metal and its alloy implanted in the body [7]. The corrosion behaviour of materials used for implantation has a strong influence on foreign body reactions at the implantation site [36]. Many factors influence the corrosion resistance of any implant, including the material's grain size, surface roughness, chemical composition (including the degree of interstitial elements), texture, mechanical stresses, and manufacturing procedures [1,24,35,37]. The implant's excellent corrosion resistance inhibits the discharge of metallic ions into the body due to the corrosion mechanism [35]. The service life of an implant in the body is determined by its corrosion resistance. Corrosion of the implant appears to impair the material's final strength and fatigue life, leading to biomechanical failure of the implants if they corrode [37,38]. Biomedical devices can undergo different and distinct types of corrosion after implantation, such as uniform, pitting, fretting, crevice cracking, galvanic, stress cracking, and fatigue corrosion [28]. Corroded implants can release harmful metal ions, which have been linked to allergic reactions, carcinogenicity, local tissue toxicity, hypersensitivity, inflammation, and genotoxicity in humans [1,32].
Titanium and its alloys have higher corrosion resistance to human bodily fluids over time when compared to other metallic biomaterials [24]. They form a stable, protective oxide layer quickly due to their extremely high affinity for oxygen [39]. Surface modification of titanium alloys used in implant fabrication is a promising technique to overcome implant problems and improve the performance of implants [28]. Surface modification procedures include applying a thin uniform coating to the implant, forming a stable passivationoxide layer, ion beam processing, and surface texturing [28]. Surface modification of the titanium implant leads to the formation of a thin protective layer which improves the corrosion resistance.
Wear resistance
The wear behaviour of the biomaterials is of great concern because the discharge of wear debris from the implant may give rise to an adverse cellular response that leads to inflammation, pain, bone resorption, and the release of harmful enzymes [1]. Wear of the implants occurs due to articulation of the artificial joints and has an impact on the longevity of metallic implants in the body [34]. The wear resistance of an implant is amongst the major factors considered when selecting a biomaterial because the biocompatibility of implants depends greatly on it [35]. The wear mechanism limits the discharge of metallic ions into the body due to its high wear resistance [35]. Metallic ions can stimulate allergic reactions in the body while others are toxic, which can cause health issues and diseases [32]. Implant loosening and tissue responses are reduced when the implants have high wear resistance.
Titanium and its alloys have low resistance to wear, and this has limited their usage in the making of implants used in situations involving wear. Titanium has been shown to suffer considerable wear when brushed against itself or other materials [23]. This limitation has been mediated by adequate surface treatment of the titanium alloy using methods such as plasma nitriding and coatings such as physical vapour deposition (PVD) [7]. Coating of the implants with thin films such as titanium nitride coating (TiN) increases their wear resistance, corrosion resistance, and surface hardness and lengthens the service life of the implant [7].
Osseointegration
Osseointegration is the integration of an implant with bone cells and tissues after implantation [11,17]. It is the anchoring of the implant to the bone through the production of bone tissue at the bone-implant interface without troublesome fibrous tissue development. The implant's successful osseointegration ensures that it is safe and effective throughout its useful life [23,40]. The loosening of an implant can occur if the implant surface does not integrate adequately with the bone organs and other bodily tissues [23,40].
The surface integrity of any implant is critical to the implant's integration with the bodily tissues. Right surface roughness, surface chemistry, and surface topography ensure the proper development of good osseointegration [25]. Studies have demonstrated that chemical modifications of implant materials, such as surface coating of the implants, promote osseointegration [9,41,42]. The coated surfaces induce initial adsorption of selected proteins, such as vitronectin and fibronectin, promoting osteoblast adhesion and osseointegration [43,44]. The osteoblast-like cells may be stimulated to recognise the implant surface by the nano-topological titanium oxide layer, which can resemble the natural topography of native bone [42].
Surface roughness at the micro-scale of a biomaterial can improve its biological performance as it facilitates attachment of cells, proliferation, and differentiation of osteogenic cells [5,43]. It also determines the ground surface characteristics such as fatigue strength, service life, and the medical component's chemical stability [21,45,46]. The osseointegration parameters differ depending on the extent of surface roughness, such as macro-roughness, micro-roughness, and nano-roughness. While most research finds mean surface roughness (Ra) of 1–1.5 μm appropriate for bone formation, there is no agreement on the adequate roughness needed to obtain optimum osseointegration [47]. The production of toxic ions by implant materials can also affect osseointegration. Poor osseointegration may be detrimental to the regeneration of the local cells [48].
Titanium and its alloys have a high osseointegration rate, which implies they can efficiently fuse with the bone and, as a result, increase the implanted device's lifetime [5]. However, studies have shown that titanium alloys form a direct bond with the bone but not a chemical bond with bone tissue [23]. This limitation has been mediated by proper surface treatment of the implant using methods such as electrochemical deposition, physical vapour deposition, and dipping techniques [23]. Surface treatment of titanium alloy implants can help them perform several times better than their natural capacity.
Processability
The material characteristics of titanium biomaterials are largely determined by their microstructure. Thermo-mechanical processing and heat treatment operations influence the microstructures of titanium and its alloys [2,30]. Alpha and beta-titanium alloys have single microstructures [24]. Through manipulation of their microstructures, alpha-beta titanium alloys can be improved or their biomaterial properties customised for medical applications. Titanium can have equiaxed, transformed, Widmanstatten, and metastable beta microstructures depending on the heat treatment. As-worked structures have higher anisotropic tensile strengths but are less ductile. Recrystallized equiaxed structures possess lower strengths but are more ductile [24]. In summary, in selecting titanium alloys for the manufacturing of implants, the objective is to develop implants that can last a long time once implanted and ensure the patient lives a quality life. Hence, the considerations above formed the basis for the selection of titanium alloys.
General characteristics of titanium and its alloys
Structures, stabilizers, and types of titanium and its alloys
Titanium is a d-transition element and it can exist as commercially pure (CP) titanium and as an alloy [13]. CP titanium is 99.9% pure and is graded 1, 2, 3, or 4 based on the amounts of interstitial elements such as carbon, iron, oxygen, nitrogen, and hydrogen [49]. Each grade of CP titanium has different corrosion resistance, ductility, and strength [13]. CP titanium (unalloyed) exists in two crystallographic forms and they are a hexagonal closed-packed crystalline structure (HCP) and a body-centred cubic crystalline structure (BCC) [11,13]. It has an HCP structure at room temperature as well as up to 888 °C [11,50]. At a transformation temperature of 888 °C, CP titanium transforms from an HCP structure to a BCC structure that is stable until melting [5,6].
Titanium alloys with various properties result from the manipulation of the crystal structure through alloying of CP titanium with stabilizers and thermo-mechanical processes [11]. To optimize mechanical qualities, including tensile strength and wear resistance, alloying elements are added to CP titanium [44]. Based on their effects on the transformation temperature of titanium metal, alloying elements are classified as beta (β) stabilizers, alpha (α) stabilizers, and neutral stabilizers [51]. Oxygen, aluminium, germanium, carbon, nitrogen, and gallium, amongst other alpha stabilizers, dissolve efficiently in the α-phase, raising the transition temperature of titanium [50]. Aluminium is the principal alpha stabilizer in titanium alloys [39], and it strengthens the titanium by increasing its tensile strength, creep strength, and modulus of elasticity through solid solution strengthening mechanisms. The aluminium content used in alloying titanium is typically below 7% to avoid embrittlement [39].
Molybdenum, chromium, vanadium, and niobium are beta stabilizers that lower the titanium transition temperature by stabilizing the β-phase [13,50]. Molybdenum improves hardenability, making it an effective stabiliser in the design of β-type alloys [52]. It however reduce long-term, elevated-temperature strength. The addition of niobium to titanium is largely used to strengthen the alloy's high-temperature oxidation resistance. Neutral stabilizers do not affect transformation temperature and include zirconium, silicon, and tin [53]. Tin is highly soluble in both phases and is commonly used with aluminium as a solid solution strengthener to provide greater strength without embrittlement. With titanium, zirconium forms a continuous solid solution. It operates as a neutral element and is isomorphic with both the α and β phases [54]. It is utilized to boost strength in low and mid-temperature environments. The zirconium content used in alloying titanium should be below 6% to avoid a reduction in ductility and creep strength [39]. Other alloying elements, for example, nickel, iron, silicon, copper, and boron, are commonly added to titanium alloys to improve castability, mechanical strength, and chemical stability.
Based on their atomic crystal structures, titanium alloys are divided into four groups: alpha (α), near-alpha, alpha-beta (α + β), and beta (β) alloys [55,56]. The α-type Ti alloys have a principal component phase called the alpha phase, which has the crystal structure HCP [57]. The major alloying ingredients in the alpha alloy are iron and oxygen. They are more resistant to creep than beta alloys. They are used in cryogenic and high-temperature applications. They offer adequate strength, hardness, and weldability, but they are difficult to forge. A commercially produced alpha alloy is the single-phase -alloy Ti-Al-2.5Sn. Near-alpha alloys have a higher percentage of α-stabilizers than β-stabilizers. Ti-3Al-2.5 V, Ti-8Al-1Mo-1 V, and Ti-6Al-2Sn-4Zr-2Mo are some of the alloys in this group. These alloys are used to make components that operate at temperatures between 400 and 520 °C [11]. Alpha-beta alloy has α- and β-phases and the beta-phase may vary from ten to fifty per cent of the whole phase at room temperature. Ti-6Al-4 V, Ti-6Al-2Sn-4Zr-6Mo and Ti-6Al-2Sn are the most common alloys. These alloys' characteristics are influenced by heat treatment, which modifies the amounts and types of β-phase elements. Components made from α-β alloys can operate at temperatures ranging from 315 to 400 °C [39].
The beta alloys have β-stabilizers in their phases and include Ti–15V–3Cr–3Al–3Sn, Timetal 21S, Ti–3Al–8V–6Cr–4Mo 4Zr, and Ti–10V–2Fe–3Al alloys [14]. The beta phase, which has a BCC crystal structure, is the most common component phase in -type Ti alloys [57]. They can be forged easily across a larger range of forging temperatures [39]. They are easy to work with, have good stress corrosion resistance and can also be heat-treated to high strength. Because of their cold rolling characteristics, they are frequently utilized to produce sheets. Some β-alloys, such Ti–10V–2Fe–3Al, have outstanding fatigue properties, whilst others, like Ti–15V–3Cr–3Al–3Sn, have poor fatigue qualities when compared to their strengths.
Some grades/types of titanium biomaterials and some of their mechanical properties
There are different grades and types of titanium and alloys that exist. The designation of CP titanium is commonly known by ASTM grades [55]. Table 3.1shows different types of titanium and its alloys utilized in medical applications, while Table 3.2 shows some of the mechanical properties of different grades of titanium and its alloys.
Table 3.1. Examples of Titanium biomaterials used for biomedical applications [14,58].
| S/No | Materials | S/No | Materials |
|---|---|---|---|
| 1 | CP Ti | 20 | Ti-15Mo-3Nb |
| 2 | Ti-6Al-4 V (TAV) | 21 | Ti–35.3Nb–5.1Ta–7.1Zr |
| 3 | Ti-6Al-4 V ELI | 22 | Ti–15Mo–5Zr–3Al |
| 4 | Ti-Zr | 23 | Ti–29Nb–13Ta–4.6Zr |
| 5 | Ti-Ni | 24 | Ti–20Cr–0.2Si |
| 6 | Ti-13Nb-13Zr (TNZ) | 25 | Ti–13Cu–4.5Ni |
| 7 | Ti–6Al–7Nb (TAN) | 26 | Ti–25Pd–5Cr |
| 8 | Ti–15Zr– 4Nb–4Ta (Ti-15–4–4) | 27 | Ti-50Ta |
| 9 | Ti–5Al–2.5Fe | 28 | Ti-25Nb-11Sn |
| 10 | Ti–5Al–3Mo–4Zr | 29 | Ti-12Mo-5Zr |
| 11 | Ti–12Mo–6Zr–2Fe | 30 | Ti-39.3Nb-13.3Zr-10.7Ta |
| 12 | Ti–15Sn–4Nb–2Ta–0.2Pd | 31 | Ti-35Nb-4Sn |
| 13 | Ti–15Mo | 32 | Ti-29Nb-13Ta-2Sn |
| 14 | Ti–15Zr–4Nb–2Ta–0.2Pd | 33 | Ti-36Nb-2.2Ta-3.7Zr-0.3O |
| 15 | Ti–16Nb–10Hf | 34 | Ti-30Zr-3Cr-3Mo |
| 16 | Ti-25Nb-2Mo-4Sn | 35 | Ti-12Mo-3Nb |
| 17 | Ti-31.0Fe-9.0Sn | 36 | Ti-12Mo-5Ta |
| 18 | Ti-30Zr-3Cr-3Mo | 37 | Ti-29Nb-13Ta-4Mo |
| 19 | Ti-29Nb-13Ta-6Sn | 38 | Other medical Ti alloy(s) |
Table 3.2. Mechanical Properties of Some Medical grade Titanium biomaterials [13,24, 25,55,[58], [59], [60], [61], [62]].
| S/N0. | Grade | Name | Type of Crystal Structure | Tensile Strength (MPa) | Fatigue Strength (MPa) | Hardness | Young's Modulus (GPa) | Elongation (%) |
|---|---|---|---|---|---|---|---|---|
| Commercially Pure Titanium (CP Titanium) | ||||||||
| 1 | Grade 1 | Titanium CP4 | α-type | 241 | 70 HRB | 102.7 | 24 | |
| 2 | Grade 2 | Titanium CP3 | α-type | 345 | 80 HRB | 102.7 | 20 | |
| 3 | Grade 3 | Titanium CP2 | α-type | 448 | 90 HRB | 103.4 | 18 | |
| 4 | Grade 4 | Titanium CP1 | α-type | 552 | 100 HRB | 104.1 | 15 | |
| Titanium Alloys | ||||||||
| 5 | Grade 5 | Ti-6Al-4V | α+β-type | 931 | 620–725 | 36 HRC | 110–114 | 6–10 |
| 6 | Grade 6 | Ti-5Al-2.5Sn | α-type | 828–972 | 290 | 36 HRC | 110 | 10–16 |
| 7 | Grade 7 | Ti-0.15Pd | α-type | 485 | 150 HV | 103 | 28 | |
| 8 | Grade 8 | Ti-7.35Al-1Mo-1V | Near α-type | 938 | 37 HRC | 121 | 17 | |
| 9 | Grade 9 | Ti-3Al-2.5V | α+β-type | 690 | 24 HRC | 102 | 21 | |
| 10 | Grade 10 | Ti-11.5Mo-6Zr-4.5Sn | β-type | 730 | 380 | 36 HRC | 80 | 13 |
| 11 | Grade 11 | Ti-0.15Pd | α-type | 345 | 120 BHN | 103 | 37 | |
| 12 | Grade 12 | Ti-0.3Mo-0.8Ni | Near α-type | 483–607 | 180 HBN | 103 | 18–22 | |
| 13 | Grade 13 | Ti-0.5Ni-0.05Ru | α-type | 275 | 24 | |||
| 14 | Grade 14 | Ti-0.5Ni-0.05Ru | α-type | 410 | 20 | |||
| 15 | Grade 15 | Ti-0.5Ni-0.05Ru | α-type | 484 | 106 | 19 | ||
| 16 | Grade 16 | Ti-0.06Pd | α-type | 345–483 | 185 HRB | ≥ 103 | 20–28 | |
| 17 | Grade 17 | Ti-0.06Pd | α-type | 241–345 | 120 HRB | ≥ 103 | 24–37 | |
| 18 | Grade 18 | Ti-3Al-2.5V-0.05Pd | Near α-type | 621–740 | 15 HRC | 91–107 | 15–17 | |
| 19 | Grade 19 | Titanium Beta-C (Ti-8V-6Cr-4Mo-4Zr-3Al) | β-type | 793 | 275 | 39 HRC | 103 | 12 |
| 20 | Grade 20 | Ti-8V-6Cr-4Zr-4Mo-3Al-0.06Pd | 795 | 105 | 8 | |||
| 21 | Grade 21 | Ti-15Mo-3Nb-3Al-0.2Si | β-type | 793 | 100 | 15 | ||
| 22 | Grade 23 | Ti-6Al-4 V ELI | α+β-type | 862 | 598–816 | 32 HRC | 101–110 | 10–15 |
| 23 | Grade 24 | Ti-6Al-4V-0.06Pd | α+β-type | 895 | 10 | |||
| 24 | Grade 25 | Ti-6Al-4V-0.5Ni-0.06Pd | α+β-type | 895 | 10 | |||
| 25 | Grade 26 | Ti-0.1Ru | α-type | 345 | 20 | |||
| 26 | Grade 27 | Ti-0.1Ru | α-type | 300 | 24 | |||
| 27 | Grade 28 | Ti-3Al-2.5V-0.5Ru | Near α-type | 625 | 25 HRC | 107 | 15 | |
| 28 | Grade 29 | Ti-6AL-4V-0.1Ru ELI | α+β-type | 898 | 110 | 16 | ||
| 29 | Grade 36 | 55Ti-45Nb | β-type | 546 | 63 | 22 | ||
| 30 | Ti-6Al-7Nb | α+β-type | 900–1050 | 580–710 | 114 | 8.1–15 | ||
| 31 | Ti-5Al-2.5Fe | α+β-type | 1020 | 740–780 | 112 | 15 | ||
| 32 | Ti–5Al–1.5B | α+β-type | 925–1080 | 720–750 | 110 | 15–17 | ||
| 33 | Ti–15Zr–4Nb–4Ta –0.2Pd | α+β-type | 881 | 100 | 27 | |||
| 34 | Ti-15Mo-5Zr-3Al | β-type | 920 | 580 | 80 | 18–25 | ||
| 35 | Ti-12Mo-6Zr-2Fe | β-type | 1085 | 580–620 | 74–85 | 20 | ||
| 36 | Ti-15Mo | β-type | 874 | 78 | 21 | |||
| 37 | Ti-15Mo-2.8Nb-0.2Si | β-type | 990 | 83 | 16–18 | |||
| 38 | Ti-13Nb-13Zr | β-type | 1005 | 490–550 | 81 | 13 | ||
| 39 | Ti-35Nb-7Zr-5Ta | β-type | 597 | 260–300 | 55 | 19 | ||
| 40 | Ti-35Nb-7Zr-5Ta-0.4O | β-type | 1012 | 66 | 18 | |||
| 41 | Ti–35.3Nb-5.1Ta–7.1Zr | β-type | 596.7 | 55 | 19 | |||
| 42 | Ti-16Nb-10Hf | β-type | 852 | 81 | 11 | |||
| 43 | Ti-29Nb-13Ta-4.6Zr | β-type | 912 | 80 | 13 | |||
| 44 | Ti-24Nb-4Zr-7.9Sn | β-type | 830 | 46 | 15 | |||
| 45 | Ti-35Nb-7Zr-5Ta-0.4O | β-type | 1012 | 66 | 20 | |||
| 46 | Ti–15Sn–4Nb–2Ta–0.2Pd | α+β-type | 860 | 89 | 21 | |||
| 47 | Ti–25Pd–5Cr | β-type | 880 | 261 HV | 5 | |||
| 48 | Ti-55Ni | Intermetallic | 875 | 190 HV | 8 | |||
| 49 | Ti-6Al-2Nb-1Ta-0.8Mo | α-type | 860 | 490 | 110 | 12 | ||
Notes:.
The values stated may vary depending on the heat treatment subjected to the alloy.
For fatigue strength, the values stated depend on the number of cycles used.
Titanium biomaterials in use, their applications, and limitations in biomedical applications
Titanium biomaterials are widely employed in medical applications due to their superior properties over other metallic biomaterials. Different types of titanium and their alloys used for medical applications are discussed in the following sections.
Commercially pure (CP) titanium
CP titanium available on the market can be categorised as grades 1, 2, 3, and 4 according to the ASTM B265 standard [63,64]. The grading determines the corrosion resistance, ductility, and strength of the CP titanium [13]. The lowest quantities of iron (Fe) and oxygen (O) in CP Titanium Grade 1 make it the most formable. Oxygen and iron content, as well as mechanical strength, increase progressively in grades 2, 3, and 4 [55]. In all four classes of CP titanium, corrosion resistance is almost similar. For applications requiring high corrosion resistance and good ductility, grade 2 is preferred compared to others [63]. CP titanium is used as an implant because it has more corrosion resistance and higher mechanical strength than Co-Cr and 316 L stainless steel [65]. Its tensile strength ranges from 240 to 550 MPa, its Young's modulus is 100 GPa, and its hardness is 70–100 HRB [24]. Due to its limited mechanical qualities, it is primarily employed in the production of dental implants.
Ti-6Al-4 V alloy
It is the most common titanium alloy and belongs to the category of α+β titanium alloys [38]. There are two commercially available grades of Ti-6Al-4V: grade 5 and grade 23 (ELI). Grade 5 was amongst the first generation of biomaterials to be used for making orthopaedic implants. In the Ti-6Al-4 V alloy, Al (5.5–6.1%) serves as an α-phase stabilizer and V (3.9–4.2%) serves as a β-phase stabilizer [5]. This alloy can be processed through mill-annealing, solution treatment, and ageing, and when used in its annealed state, its microstructure has a considerable impact on its mechanical properties [54].
Ti-6Al-4V-ELI (extra low interstitials) is an alloyed grade of titanium, also known as Ti grade 23 [66]. This alloy has fewer iron inclusions and more carefully regulated interstitial components of carbon and oxygen than standard Ti-6Al-4 V. It has a lower degree of interstitial impurities than standard Ti-6Al-4 V, which increases ductility and fracture toughness. It is found in the annealed condition as well as the beta annealed condition sometimes. Grade 23 has good corrosion resistance as well as great strength and toughness.
Ti-6Al-4 V is a titanium alloy that is commonly used in biomedical applications such as orthopaedic implants [44]. Originally intended for aerospace applications, this alloy has found its way into the biomedical industry because of its excellent biocompatibility and corrosion resistance [54]. Its tensile strength is exceptional (it can reach up to 1100 MPa), it has a low specific density, low Young's modulus, high hardness of 36 HRC, a high fatigue strength, enhanced corrosion resistance, a high fracture toughness, and superior biocompatibility. It also has good weldability and creep resistance up to 300 °C [39]. These characteristics make this alloy an excellent choice for biomedical implants [25,54,67]. High-strength prosthetic implants [39], dental implants, cardiac implants, hip and knee prostheses, artificial knee joints and bone plates, etc. are made from Ti-6Al-4 V [38,68].
According to new research, implants consisting of Ti-6Al-4 V alloys have been related to long-term health difficulties like Alzheimer's disease, neuropathy, and osteomalacia [34]. The release of aluminium and vanadium ions from Ti-6Al-4 V implants is thought to be the cause of these complications [34]. Vanadium is a cytotoxic element [69], while neurotoxicity and neurodegenerative diseases have been associated with the presence of aluminium in titanium-aluminium-based alloys [70]. The modulus of the Ti-6Al-4 V alloy is higher than that of human bone tissue, and this is a matter of concern because bone resorption and implant failure can occur as a result of the stress-shielding effects induced by modulus mismatch [51].
Ti-6Al-7Nb (TAN)
In biomedical applications, Ti-6Al-7Nb (IMI-367) is a novel titanium alloy that is generally substituted for Ti-6Al-4 V alloy [71,72]. It was developed in the 1970s as an implant without vanadium to mitigate the cytotoxicity associated with vanadium [69]. This alloy is available in milled forms such as rods, bars, billets, extrusions, etc. [39] and is described by the ISO 5832–11 standard [59]. It is a ternary vanadium-free α+β titanium alloy used as an implant material and has improved biocompatibility, mechanical characteristics, and corrosion resistance [73].
Due to its microstructure, where Al stabilized the α-phase and Nb stabilized the β-phase, TAN alloy has a high strength that is practically identical to Ti-6Al-4 V [63,72]. It has a tensile strength of 862 MPa and an elongation of 10% less than the Ti-6Al-4 V alloy [73]. The niobium in TAN is non-toxic, and its Young's modulus is lower and closer to that of human bone tissue, reducing stress-shielding around the implant [71]. Its wear rate is comparable to that of CP-Ti but lower than that of Ti-6Al-4 V alloy [72]. Its resistance to corrosion and bio-tolerance is higher compared to Ti-6Al-4 V [50,63]. This alloy was developed as a wrought material for orthopaedic applications and has been investigated as a new alloy for whole hip prostheses [73]. It's a high-strength alloy with good biocompatibility, so it's employed as a surgical implant alloy [39] in dental implants and femoral stem prostheses [71].
Nickel-titanium (Ni-Ti) alloy
Nickel-titanium alloys, also known as nitinol, are shape memory alloys (SMA) that have been widely employed in a variety of applications, including biomedical, microelectromechanical systems, actuators, aerospace, and automotive devices [74]. Because of its remarkable features such as shape memory effect, super-elasticity, biocompatibility, and corrosion resistance, nitinol is the most extensively used SMA amongst other types of SMAs such as CuZnAl, and CuAlNi [75,76]. The shape memory effect is the process where an initially deformed low-temperature material recovers plastic strains through austenite-martensite phase transformation [75,77]. Due to its atomic structure, Ni-Ti can exist in two phases: austenite and martensite, depending on its temperature [78]. When the atomic lattice undergoes martensitic transformation between austenite and martensite phases, the space within it changes, allowing it to alter its shape. This behaviour is known as "super-elasticity" or "pseudo-elasticity" [77]. Super-elastic materials can regain their initial shape after the removal of the deformational stress, and they will subsequently recover their deformation strain [3]. This unique behaviour can be utilized for biomedical applications at varying temperatures [78,79].
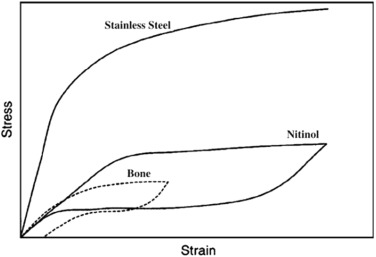
Ni-Ti alloys have been identified as appropriate materials for medical implantsdue to their excellent biocompatibility, mechanical properties, and corrosion and wear resistance [80]. A protective Ti-based oxide surface layer on Ni-Ti products improves the corrosion resistance of the alloy. They feature a high strength-to-weight ratio with an ultimate tensile strength of 1240 MPa [24]. They also have an elastic modulus of roughly 48 GPa, which is virtually comparable to bone's elastic modulus [25,76,81], making them ideal for long-term orthopaedic implants [82]. The recoverable strain in Ni-Ti is roughly 8%, whereas it is more than 1% in bone [76,79]. The same behaviour of Ni-Ti and bone deformation in the body demonstrates the biomimetic behaviour of Ni-Ti implants under loading and unloading conditions [76]. The stress-strain curves in Fig. 3.1 depict the comparison amongst Ni-Ti, stainless steel, and human bone.
 Fig. 3.1. Stress-strain curves for stainless steel, Ni-Ti and the human bone [76].
Fig. 3.1. Stress-strain curves for stainless steel, Ni-Ti and the human bone [76].Because of the good qualities of the Ni-Ti alloys, they have been used for orthopaedics, cardiovascular, and orthodontic arch wires, guide wires, minimally invasive surgical instruments, and stents [83]. It has a shape-memory effect [34], which causes compressive stresses that help damaged bones knit together [79]. It has also been found to dilate blood vessels, hence its use as a self-expanding cardiovascular stent, thus increasing the flow of blood to vital organs [28,79]. Flexible self-expanding NiTi stents are currently used as a replacement for urethrotomies in current urethral strictures, keeping the biliary duct lumen open, minimizing dysphagia and helping in the restoration of swelling and nutritional intake for patients with malignant strictures [84,85]. The drawback of Ni-Ti is weak interfacial bonds for using these metallic implants in orthopaedic surgery [76]. In some studies [25], Ni concentrations above a certain level have also been observed to produce severe local tissue irritation, necrosis, and toxic reactions. The amount of Ni released by implants, on the other hand, is insufficient to elicit these effects [25].
Titanium-zirconium (TiZr) alloy
Because of the issues with the first generation of titanium alloys, new second-generation titanium alloys have been researched, manufactured, and brought onto the market. Beta stabilisers like zirconium (Zr), niobium (Nb), tin (Sn), palladium (Pd), tantalum (Ta), and indium (In) are used as alloying elements in these innovative Ti alloys and are deemed extremely biocompatible and relatively harmless (i.e. non-toxic) when compared to aluminium and vanadium [6,56,72,86]. Titanium alloys in this category include binary titanium alloys, near β-type and β-type titanium alloys [57] and were developed to avoid the "stress shielding" phenomenon caused by the modulus difference between human bone and implant [56, 87]. These new varieties of titanium alloys include TiTa, TiHf, TiZr, TiNb [36], Ti-Nb-Zr, Ti-Mo-Zr-Fe, Ti-6Al-7Nb, Ti-Nb-Zr-Ta alloys etc. have been developed [71]. They offer excellent workability and mechanical properties and are alloyed with inert elements [71]. In terms of biocompatibility, corrosion resistance, modulus of elasticity, and wear resistance, innovative beta-type titanium alloys are regarded to be more favourable for biomaterial applications [25].
The TiZr alloy was created by Roxolid, Institut Straumann AG in Basel, Switzerland. TiZr is formed when zirconium is alloyed with titanium. From a metallurgical perspective, Zr is easier to alloy with Ti [36]. Originally, it was made by mixing Ti with 13–15% Zr. Ti and Zr are both transition metals that belong to the same periodic table group and have similar chemical characteristics. Zirconium is a biocompatible, bio-inert material and has high resistance to corrosion with a good biological response [88]. In TiZr, there is no limit to how much Zr can dissolve in the titanium matrix since the Zr element forms a complete solid solution with both α and β Ti phases [89]. Zirconium is added to Ti to enhance its mechanical strength, plasticity, and hardness and decrease its elongation, hence ensuring fine processing properties [72]. It lowers the fusion temperature of the titanium (1670 °C) as its amount increases, thus enhancing the castability process of titanium alloys [90]. When compared to other titanium alloys, most investigations have demonstrated that the Ti-Zr alloy has greater corrosion resistance and biocompatibility [91]. A binary TiZr alloy does not suffer from corrosion problems after long exposure to body fluids [88]. It has a tensile strength of more than twice that of the Ti alloy or even of Zr [89].
TiZr alloy is used for medical implants, especially as dental prostheses, although its use to its full potential is yet to be realised [72,92]. It was developed specifically for smaller diameter dental implants (≤3.5 mm) [72] because TiZr had increased fatigue strength and maintains the biocompatibility and osseointegration properties the same as for cpTi, the gold standard [72]. Saulacic et al. [93] explained that cpTi and TiZr implants have faster osseointegration than Ti6Al4V implants. Lee et al. [92] discovered that the Ti-Zr alloy composition influenced the in vitro biological response. They discovered that Ti-Zr with 30 mol% had the best physiologic response and the strongest strength.
Ti-13Nb-13Zr (TNZ)
Ti-13Nb-13Zr (TNZ) alloy is amongst various near β-type titanium alloys [38,94] and was developed by Davidson and Kovacs in the early 1990s [32]. It possesses a beta-phase stabiliser in the form of niobium, and zirconium is isomorphous with titanium's alpha and beta phases [95]. It consists of hcp martensite in water-quenched conditions and submicroscopic bcc beta precipitates as it ages [32]. It has a lower elastic modulus (65–79 GPa) [94], great biocompatibility, and strong corrosion resistance than standard grade 5 titanium alloy [96] due to its alloying components. It's a superior Ti alloy because both niobium, Nb and zirconium, Zr are recognized as non-toxic [94,97], biocompatible and non-allergenic elements with no adverse effects on the human body [54,67]. Its tensile strength is estimated to be over 1300 MPa [95] and it has been proven that when Nb is alloyed with titanium at a specific amount, it lowers Young's modulus [37].
The American Society for Testing and Materials (ASTM) ASTM F1713–96 standard specifies the TNZ and it has already been certified for use as an orthopaedic implant material by ASTM and the US Food and Drug Administration (FDA) [94]. The TNZ alloy is an attractive alternative alloy for hard tissue implants due to the strengthening of the TiO2 passive coating by Nb2O5 and ZrO2 oxides [67]. Hard tissue implants made of Ti–13Nb–13Zr alloy are also intriguing because they have been established to have some antibacterial effect against Gram-negative bacteria [67]. ZrO2 is not cytotoxic, carcinogenic, teratogenic, or genotoxic oxide and it is harmful to different kinds of bacteria [98]. Because of Nb2O5’s good lubricating qualities, titanium alloys with a high Nb concentration have better wear resistance [5]. Ti alloys with a high Nb content have a thin passive layer on the surface that lasts longer than Ti alloys with a low Nb content [25]. When compared to Ti-6Al-4 V alloy, Ti-13Nb-13Zr alloy has been established to have higher corrosion resistance [50].
According to Lee et al. [38], warm-rolled TZN has higher mechanical qualities in comparison to other types of TZN. It has a tensile stress of 1050 MPa, compared to 850 MPa for both hot rolled alloy and ASTM TZN [99]. It also has a higher hardness of HV 335±5 than the hot-rolled alloy and the solution-treated and cold-rolled alloy with hardness of HV 215±4 and HV 285±7, respectively [54]. It also has enhanced corrosion resistance, and it is attributed to its sub-microcrystalline structure [35].
Ti–15Zr– 4Nb–4Ta (Ti-15–4–4)
It is a titanium alloy created in Japan for long-term biomedical applications [100] and has been standardized in Japan with the Japanese Industrial Standard JIST 7401–4. Because the amount of released metallic ions into the medium for Ti, Zr, Ta, and Nb particle extractions is modest (0.3 mg/L), the alloy's constituents are biocompatible [51,59]. When compared to the Ti–6Al–4 V alloy, this alloy has a higher mechanical strength [101], a lower elastic modulus, and greater corrosion resistance due to the addition of Zr [64] and at 1 × 108 cycles, it has a fatigue strength of around 730 MPa [102]. This alloy is free of cytotoxic components and has a high ability to create apatite [103]. In comparison to Ti-6Al-4 V and Ti-6Al-7Nb, Ti-15–4–4 releases the fewest metal ions in various physiological solutions [104]. In their investigation, Okazaki [59] found that cells in Ti-15–4–4 grow faster than in Ti-6Al-4 V. Ti-15–4–4 has been successfully used to fabricate bone plates, artificial hip joints, and tooth implants [65]. Ti-15–4–4 is predicted to be the favoured titanium alloy for future biomedical applications because of its exceptional corrosion resistance [65].