1. From pyrethrum to the pyrethroids: The beginning of green chemistry
1.1. Chemistry
Pyrethrum is a mixture of six natural lipophilic pyrethrin esters extracted from the flower heads of the chrysanthemum plant, Chrysanthemum cinerariaefolium, and is arguably the most successful and effective botanically derived natural insecticide. The insecticidal properties of pyrethrum have been known since the middle ages, and it has been widely used to protect people from fleas, lice, and ticks, which vector various human diseases that have killed millions. Its use, however, was largely restricted to indoor/personal usages because the natural pyrethrins rapidly break down to non-insecticidal products in air, light, water, and soil. During the 1960s and 1970s, a systematic approach was taken to modify photolabile structures within the natural pyrethrins, while retaining their fast and potent insecticide action and low mammalian toxicity. Most significant were the replacement of the methyl groups on the vinyl moiety of the acid grouping of the natural esters with halogens, such as bromine, chlorine, and fluorine, and the replacement of the natural alcohol moieties with photostable alcohols, such as 3-phenoxybenzyl alcohol. These investigations resulted in more than 20 synthetic pyrethrins (the pyrethroids) being synthesized and commercialized for widespread and intensive use in all aspects of insect control, including agriculture, vector control, and household and pet insecticide formulations, making human exposure almost a certainty [1]. Two of the most successful pyrethroids are the type I pyrethroid, permethrin (which has no alpha-cyano grouping on the methylene carbon of the 3-phenoxybenzyl alcohol), and the type II pyrethroid, deltamethrin (which has the alpha-cyano grouping).
1.2. Site and mode of action
The natural pyrethrins, the synthetic pyrethroids, and p,p′-dichlorodiphenyltrichloroethane (DDT) share a common major target site in nerve tissue: voltage-sensitive sodium channels (VSSCs). These insecticides all cause a slowing of VSSC inactivation and a prolongation of deactivation, resulting in increased sodium ion influx and membrane depolarization. In the case of type I pyrethroids, such as permethrin, depolarization leads to depolarizing after-potentials, which ultimately results in repetitive firing of the nerve cell and the tremor syndrome (T-syndrome) of intoxication. In the case of type II pyrethroids, such as deltamethrin, deactivation is greatly extended, leading to substantial membrane depolarization and causing choreoathetosis with the salivation syndrome (CS-syndrome) of intoxication.
1.3. The Food Quality Protection Act and the United States Environmental Protection Agency/Risk Cup analysis
In 1996, the United States Congress passed the Food Quality Protection Act (FQPA) and instructed the United States Environmental Protection Agency (USEPA) to reassess pesticide tolerances in term of aggregate exposure and cumulative risk. As set forth in the FQPA, the risks associated with those pesticides that share a common mechanism of action are to be added and, if the total risk exceeds a set amount (the Risk Cup), the group can be banned from use. Because the pyrethroids share a common mechanism of action at VSSC and have a widespread and intensive use pattern, the USEPA began a data review. In 2010, the USEPA notified registrants that testing failed to address the possibility of age-related differences in the pyrethroid sensitivity of juveniles versus adults at lower doses more relevant for human health risk assessment. In response, the Council for the Advancement of Pyrethroid Human Risk Assessment (CAPHRA) was formed in 2011 to represent the interests of pyrethroid and pyrethrin insecticide registrants and to address the question: Are young children (i.e., hand-to-mouth toddlers) more sensitive to the neurotoxicity of pyrethroids than adults?
To address this question, CAPHRA developed a research program to systematically examine various aspects of the toxicokinetics (i.e., absorption, distribution, metabolism, and excretion) and toxicodynamics (receptor–ligand interactions establishing potency and efficacy) associated with pyrethroid neurotoxicity and to distinguish whether the sensitivity described in neonatal rats is relevant to infants and children. In 2011, CAPHRA accepted a proposal from the Neurolemma Group, a consortium of researchers from the University of Massachusetts Amherst, Salve Regina University, and Precision Bioassay, Inc., to address the in vitro/ex vivo assessment of potential age-related toxicodynamic differences of pyrethroids. The following is a summarization of the development of an innovative ex vivo approach to address this aspect by improving a microtransplantation assay based on rat brain neurolemma-injected Xenopus laevis oocytes and high-throughput electrophysiology techniques.
2. The problem to be addressed and a plausible solution
2.1. The problem: There is no such thing as “the” VSSC
In order to study the effect of neurotoxic insecticides, such as pyrethroids, on ion channels, a variety of electrophysiological approaches have been used, including external cell recordings, whole-cell patch clamps, and the heterologous expression of cloned channels by injecting complementary RNAs (cRNAs) into Xenopus laevis oocytes [2]. Electrophysiology allows rapid data collection that matches the speed of the gating processes of ion channels, and is the method of choice for the direct determination of the action of neurotoxicants on the kinetics of channel opening and closing events. However, many of the most common preparations used to measure native channel activities are non-neural [3] and/or non-mammalian [4], [5]. In addition, voltage-sensitive ion channels, including VSSCs, are assembled in mammals from multiple subunit isoforms, which have undergone alternative splicing, RNA editing, and post-translational modifications, including glycosylations, methylations, and phosphorylations. The ramification of this assembly process is that there is no such thing as “the VSSC”; rather, there is a vast variety of different channels with unique kinetic and pharmacological properties. Because of this, the actual channel isoform make-up expressed in a tissue is not usually known, nor is it known how their expression varies with time or location. Thus, the determination of the effect of neurotoxicants, such as the pyrethroids, on individual VSSC, one by one, becomes a difficult if not impossible task. Additional problems also result from using heterologously expressed channels, as many of the genomic, transcriptomic, and post-translational modification events simply do not take place.
2.2. A solution: Microtransplantation of neurolemma fragments into Xenopus oocytes
An alternative method exists that couples the merits of an intact biochemical system with the sensitivity and rapid data acquisition of electrophysiology and minimizes many of the issues associated with the protocols summarized above. A robust literature exists [6], [7], [8], [9], [10], [11], [12], [13] that has established a method that uses nerve membrane fragments (i.e., neurolemma) injected into Xenopus laevis oocytes. This approach has been validated using nerve fragments from a number of different organisms and allows the direct measurement of ion currents flowing through intact ion channels, imbedded in their endogenous lipids, with proper subunit make-up and post-translational modifications, using standard two-electrode voltage-clamp (TEVC) electrophysiological approaches. One of the major issues with this technique, however, is the variability of neurolemma fragment incorporation by the oocyte [9], [14].
3. Our approach: Neurolemma-injected Xenopus oocytes
3.1. Strengths and weaknesses of this approach
The microtransplantation approach allows nerve membrane fragments from rat brain, which contain lipids and proteins in their native state, to be inserted into the plasma membrane of Xenopus laevis oocytes and function. The advantages of this procedure include the following: ① Expressed proteins exist in their endogenous state and are surrounded by their natural lipids, mimicking the state found in the rat brain; ② a number of neural targets, including voltage-sensitive ion channels, are present for study; ③ ion currents can be rapidly studied 1–2 h post-injection; ④ injection of nanograms of tissue is sufficient for measuring ion currents; ⑤ both fresh and frozen brain tissue can be used; ⑥ the procedure is amenable to study differences across species, ages, and brain regions; ⑦ specific ion currents can be isolated electronically and/or pharmacologically; and ⑧ data acquisition is rapid using standard electrophysiological approaches and is amenable to high-throughput systems (e.g., Robocyte2®) [15]. The disadvantages associated with the method can include: ① target proteins that are uncharacterized in many tissues and species; ② the occurrence of complex currents due to the expression of multiple target proteins; and ③ a variable level and orientation of nerve fragment incorporation [9].
3.2. Initial neurolemma-injected oocyte assay
We began a systematic characterization of using tissue fragments from juvenile (post-natal day 15, PND15) and adult (post-natal day 90, PND90) rat brains microtransplanted into Xenopus laevis oocytes to investigate the age-related differences in the expression pattern, functionality, and sensitivity to a type I pyrethroid (permethrin) and type II pyrethroid (deltamethrin) using automated western blotting and TEVC electrophysiology techniques [16], [17], [18].
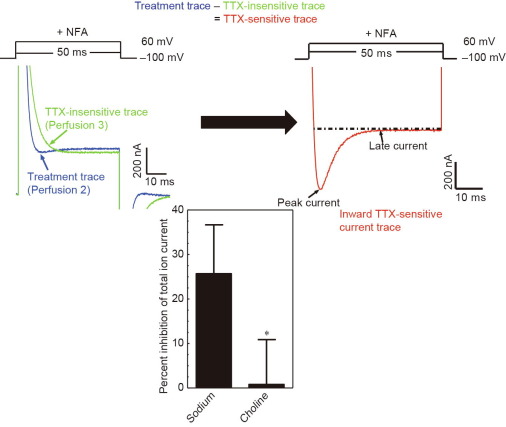
From these initial studies, we established that the adult rat brain neurolemma inserted itself into the plasma membrane of oocytes, physically contained all nine VSSC isoforms found in rat brain, and, upon membrane depolarization, elicited a complex outward ion current, a portion of which was sensitive to tetrodotoxin (TTX, a specific VSSC blocker) and was abolished when sodium ions were replaced in the recording buffer by non-permeant choline ions [16]. Furthermore, it was found that the majority of the outward current seen upon depolarization was due to a calcium-active chloride current, which could be substantially blocked using niflumic acid (NFA), revealing an inward TTX-sensitive current (Fig. 1).
 Fig. 1
Fig. 1This inward TTX-sensitive current was increased in a concentration-dependent manner by DDT (a neurotoxic insecticide that also acts on the VSSC; but not by dichlorodiphenyldichloroethylene (DDE), a non-neurotoxic metabolite), permethrin, and deltamethrin, as measured by the area under the curve (AUC) following a pulsed depolarization [17], [18]. Even though the TTX-sensitive AUC values fit the sigmoidal, pyrethroid-dependent, concentration–response curves generated using the Hill equation reasonably well, variation as judged by standard error bars was large and reduced the usefulness of this approach for regulatory issues (Fig. 2, Fig. 3).
 Fig. 2
Fig. 2 Fig. 3
Fig. 34. Improving the precision and assay performance
A collection of innovative changes to the ① assay protocols, ② data acquisition criteria, and ③ data analysis methods resulted in substantially improved precision and, hence, assay performance [18], establishing this procedure as a toxicologically relevant ex vivo assay. In the remainder of this review, we systematically examine these improvements, outlining a general approach that can be used to improve the performance of similar assays using other target sites and other animals/tissues.
4.1. Changes made to assay protocols improving overall oocyte health
In order to improve the neurolemma microtransplantation assay, sources of variation were identified by fitting permethrin responses to the linear mixed model with random effects associated with neurolemma preparation, oocyte preparation, and individual oocyte. The results of these fits included an estimated variance component associated with each source (neurolemma preparation, oocyte preparation, and oocyte). The results indicated that the variance components associated with neurolemma preparations were very much smaller than those for oocyte preparations and individual oocytes [18]. With this information, we undertook a systematic evaluation of ① variation in the viability among oocytes from a single frog within a plate (intraplate variation); and ② procedures to adjust/minimize the variability in neurolemma incorporation into the plasma membrane among groups of oocytes taken from different frogs (interplate variation).
Under the assay setup protocols used for the original method, it became apparent that overall oocyte health and viability declined over the time it took to complete a full concentration-dependent response curve (CDRC), resulting in only 30%–50% of the oocytes providing useful data (e.g., TTX-sensitive inward current). To minimize the decline in oocyte health, the following protocol changes were implemented: ① After surgically removing the oocytes, only healthy oocytes that were stages V and VI were defollicated and selected for injection; ② the 96 oocytes in a full assay microplate were injected with 0.5 mg·mL−1 neurolemma at staggered time points (every 6 h) that ensured a more consistent incorporation time; ③ oocytes were recorded by being placed in recording buffer on the assay plate only for the duration of the electrophysiological recording protocol; and ④ the time interval to electrophysiologically record an oocyte was reduced from 15 to 11 min. In addition, the treatment of oocytes within a row with pyrethroids was alternated between different microplates, with odd value concentrations being applied to rows 3–5 and even concentrations to rows 6–8. This order was reversed in the next assay microplate, and so forth, minimizing any potential plate effect.
4.2. Changes in data acquisition criteria
A four-step perfusion protocol was developed in which four individual electrophysiological scans were made on each oocyte in order to select neurolemma-injected oocytes that all had a TTX-sensitive inward current prior to data analysis (TTX-washout protocol). Oocytes were perfused first with 1× ND96 medium and 10−4 mol·L−1 NFA for 2 min, followed by an electrophysiological recording (scan 1). Oocytes were then perfused with 1× ND96 medium and 10−4 mol·L−1 NFA containing 10−5 mol·L−1 TTX for 2 min, followed by a second recording (scan 2). TTX was washed out using a 1 min perfusion with 1× ND96 medium and 10−4 mol·L−1 NFA. To examine the effect of pyrethroid treatment, either permethrin or deltamethrin (5 × 10−9 to 1 × 10−6 mol·L−1 in 1.0% dimethyl sulfoxide (DMSO)) in 1× ND96 medium and 10−4 mol·L−1 NFA was perfused for 5 min and another electrophysiological measurement was recorded (scan 3). Lastly, each oocyte was perfused with 10−5 mol·L−1 TTX in 1× ND96 medium and 10−4 mol·L−1 NFA for another 2 min and recorded (scan 4). Each perfusion was delivered through separate tubing using isolated automated valves at a rate of 0.5 mL·min−1. After each of the four perfusions, current traces (scans 1–4) were recorded and subjected to a leak subtraction protocol. The leak subtraction resulted in the production of a single current trace that reflected the effect of the treatment in the perfusion. The trace was a result of a step (pulse) depolarization from −100 mV holding potential to +60 mV for 50 ms, as previously described [16], [19]. A TTX-sensitive inward current was determined by subtracting the TTX-insensitive current (+TTX) from the total current (−TTX), as previously described [20]. To determine if an “inward” TTX-sensitive current was found in the presence of NFA, scan 2 was subtracted from scan 1. Only oocytes that produced a TTX-sensitive inward current (as measured by the AUC) of at least −100 nA but less than −4000 nA were used in data analysis.
These intraplate protocol changes increased the percentage of oocytes that produced data that could be used in the analysis on each plate from 30%–50% to over 80%, reflecting a substantial improvement in the overall health of the neurolemma-injected oocytes. Although the use of oocyte-selection criteria and protocols that improved oocyte health resulted in less variability on an intra-assay plate basis, the data obtained were still not consistent enough for regulatory evaluations.
4.3. Changes in data analysis reducing assay variability
In order to reduce variability on an interplate basis, AUC values were converted to proportion of control response values using Eq. (1).where VTR mean AUC is the mean of the AUCs obtained for each assay plate for oocytes treated with 70 μmol·L−1 veratridine (VTR, a persistent VSSC activator that binds to a site different from the pyrethroids and acts as a positive control); control mean AUC is the average of the AUCs obtained for each assay plate for oocytes receiving no pyrethroid or VTR treatment (negative control); treatment mean AUC is the AUC obtained by subtracting scan 3 from scan 4 in the presence of pyrethroid treatments.
By this process, the assay-specific response range is bounded by the means of the AUCs from oocytes untreated with pyrethroid or VTR (negative control) and from oocytes treated with 70 μmol·L−1 VTR (positive control, no pyrethroids). In the absence of pyrethroid treatment, the proportion of control response is expected to be 0. As pyrethroid concentration increases, the proportion of control response increases as the AUCs of the pyrethroid-treated oocytes increases, approaching a maximum value of 1.0. With the data now bounded from 0 to 1, the individual assay data were fit directly to a logit-log (generalized) linear regression.
5. Concluding remarks
Initial attempts using rat brain neurolemma fragments microtransplanted into Xenopus oocytes to study the effects of pyrethroid insecticides on native VSSC resulted in the detection of a depolarization-dependent, TTX-sensitive, inward current in the presence of NFA, which increased when challenged with increasing concentrations of pyrethroids. These innovative findings supported the contention that this technique is a useful and toxicologically relevant ex vivo approach to study the effect of nerve toxins and toxicant on their target sites in their native state. Although the CDRCs fit well to the Hill equation used to generate them, the variation as determined by standard error was large and diminished their usefulness in a regulatory situation. Using an iterative approach, we systematically examined changes to the assay protocols to increase overall oocyte health and improve data acquisition and data analysis, as summarized in Table 1.
Table 1. Steps to reduce variability in the neurolemma-injected oocyte assay in order to obtain more consistent CDRCs generated with increasing concentrations of pyrethroids.
| Reduction of intra-assay plate variability | Reduction of inter-assay plate variability |
|---|---|
|
|
Adoption of these changes resulted in CDRCs with substantially reduced variation, as judged by their standard error bars and improved overall R2 values (Fig. 4), and yielded a substantially improved assay precision and, hence, assay performance. Using this modified approach, we are currently evaluating potential age-related differences in the toxicodynamics of pyrethroids on native VSSC from juvenile versus adult rat brain neurolemma.
 Fig. 4
Fig. 46. Prospects for future development
Microtransplantation of neurolemma fragments into the plasma membrane of Xenopus laevis oocytes is an ex vivo method that has been used to study a variety of channels and receptors in their native state using standard electrophysiological approaches [6], [7], [8], [9], [10], [11], [12], [13], [14]. Unfortunately, variation in the incorporation of neurolemma limited its use to qualitative analyses. With the use of specific blockers (e.g., TTX and NFA), we were able to isolate a depolarizing-dependent, TTX-sensitive, inward sodium ion current, which was increased by pyrethroid and DDT insecticides in a concentration-dependent fashion, mimicking their action on individual VSSC heterologously expressed in oocytes by prolonging the inactivation process of the channel’s gating kinetics, thus validating the neurolemma-based approach. Furthermore, the addition of a positive control using VTR, a specific activator of VSSC, allowed us to normalize neurolemma incorporation between different batches of oocytes, reducing variability and allowing the assay to be useful in a quantitative fashion. In this manner, the collection of innovative changes presented above outlines a general approach that can be used to improve the performance of similar assays using other target sites and other tissues/animals. Importantly, there is a rich and increasing availability of selective agonists and antagonists for a wide variety of voltage- and ligand-gated channels and receptors, which can be used as outlined above to increase assay performance. In addition, the assay has been demonstrated to function using human brain fragments obtained from cadaver tissue samples [11]; this allows the direct comparison of toxicants on human target sites versus non-human animal model target sites, thereby validating the usefulness of our current animal models. Although this is speculative at the moment, there are many non-neural tissues (adipose, liver, muscle, etc.) that possess the same target sites as nerve (e.g., VSSC, nicotinic acetylcholine receptors, gamma-aminobutyric acid (GABA)–chloride channels, glycine–chloride channels, etc.) and that could be studied using the above assay protocols. In particular, the action of commonly occurring environmental contaminants, such as polyfluoroalkyl substances (PFASs) and pesticides, on such non-target tissues has not been studied in detail, although their role in metabolic syndrome, including increased occurrence of obesity and type 2 diabetes, has been widely suggested.
Acknowledgements
This work was supported by the Council of the Advancement of Pyrethroid Human Risk Assessment (CAPHRA) (#S17110000000004).
Compliance with ethics guidelines
The sponsor had no role in the study design, in the collection, analysis or interpretation of the data; in writing the article; or in the decision to submit the article for publication.
John Clark and Steve Symington declare that they have no conflict of interest or financial conflicts to disclose.