1. Introduction
1.1. Robot-Assisted Surgery
As the term implies, ‘robot-assisted surgery’ can be defined as a surgical procedure where a robotic system assists a surgeon in executing the complex, invasive surgical steps on a human body with increased control and flexibility enabling high accuracy and precision when compared to the traditional techniques [1]. It is often associated with minimally invasive surgery (MIS), unless an open surgery, but fundamentally meaning that the robotic surgical procedure can be executed with small and minimum number of incisions, significantly benefitting the patient. It has the potential to reduce trauma and recovery period after the surgery when compared to the traditional surgery [2]. The field of robot-assisted-surgery has greatly benefited with the improvement in computing power, associated technologies, and advent of imaging techniques such as Ultrasound (US), Computed Tomography (CT) and Magnetic Resonance Imaging (MRI). Termed as Computer-Integrated-Surgery (CIS), it has revolutionized the way surgeries are planned and executed, taking advantage of patient specific information, pre-operative and intra-operative imaging, and state-of-the-arm robotic-assisted surgical technologies [3,4]. One of the primary ways of classifying a robotic surgical system is categorizing them based on intended anatomical application, as also followed in this article. Before we discuss these systems in more detail in later sections based on target anatomy, it is also useful and important to understand their classification based on the technical approach taken towards system design viz. tele surgical robots, image guided robots and hand-on or cooperative surgical robots, as explained by Takács et al. [4]. Tele surgical robots take advantage of fundamental teleoperation mode where a master console is used to control the slave robot to control the end effector which in this case can be an endoscope or orthopedic tool or any other surgical tools [5]. The advancements in haptic technologies, elimination of hand tremors etc. has made the tele surgical robotic surgeries increasingly more effective, although time delay is an area where much more research and development is required, particularly where distances between the master and slave site are large e.g., space or intercontinental applications. Another significant advantage is the capability of reducing the exposure to surgeons in case of radiation intensive environment. Image guided robots take advantage of pre-operative and intra-operative imaging modalities such as US, CT, or MRI to execute surgical tasks such as needle insertion [6] or bone drilling or milling. The aspect of imaging feedback fundamentally differentiates this approach from tele surgery in the sense that surgical tasks can be executed autonomously or semi-autonomously without the need for human operator. Cooperative surgical robots are hands-on robotic systems where the surgeon is in direct control of the device eliminating several challenges such as loss of feedback due to sensors observed in tele surgical systems. These systems take advantage of both, sensory technologies and the direct human feedback to execute complex tasks [4]. One of the additional methods of classifying robots in general is the Level of Autonomy (LoA). Although not necessary, it would be helpful to have a background in it while discussing individual surgical robotic systems throughout the article. Haidegger proposed a LoA scale of 0 to 5 for classifying surgical robotics with ‘LoA 0’ representing absence of any robotic feature and ‘LoA 5’ as fully autonomous, a system with no human involvement, currently non-existent [7]. Between both, ‘LoA 1’ represents a system performing a low level function such as executing minor safety protocols, ‘LoA 2’ enables the system to perform a certain task autonomously for a short time such as executing bone milling under image guidance, ‘LoA 3’ would be a system where a major portion of the procedure is executed including taking certain low-level cognitive decision while broadly working under human supervision and ‘LoA 4’ would represent a highly autonomous system with whole procedure being executed by the system with only the control of emergency stop with the human. In essence, these classifications become extremely handy and aid both developers, and/or surgeons to work together from the concept stage to the final product in the journey of designing and developing a robotic surgical system.
1.2. A brief history of robot-assisted surgical systems
The last four decades have seen a total transformation in robotics applied to the field of surgical treatments. In the year 1983 the world witnessed the development of the first robotic system in history for assistance during treatment on a human patient, called Arthrobot. It was used for the first time in 1984 at UBC Hospital in British Columbia followed by 60 arthroscopic procedures next year [4]. Arthrobot assisted a surgeon in manipulation and positioning of patient's leg using voice command [8]. The first robot that was used for direct surgical support on a patient dates to 1985 when an industrial Puma robot was used to perform biopsy on brain using CT [9]. Later in 1988 another version of Puma robot was used to perform robotic transurethral resection [10]. To simulate the prostate tissue for the resection, researchers used an acrylic box that contained a potato while the box was connected to an artificial penis. This robot had a specially designed cutting assembly. An axial cut was performed when the robot was in forward stroke while a diathermic cauterization was performed on return stroke [11]. However, later, manufacturer of the Puma robot refused to allow its use in surgery citing that it is unsafe since it was originally intended to be used inside a barrier to avoid human contact [12]. In 1991, Imperial College London developed ProBot, a robot for transurethral resection of the prostate [13,14]. In the same year, an orthopedic robot prototype ROBODOC was developed by IBM for high-speed milling and to assist with implant positioning by utilizing preoperative CT imaging [15]. Five years later, in 1996, Automated Endoscopic System for Optimal Positioning (AESOP) was developed by Wang et al. for assisting surgeons to hold a camera [16,17]. The camera was controlled by a foot pedal or a hand remote control [18]. It was one of the first FDA approved robotic system for surgical applications [19]. In that year, ZEUS robotic system was also introduced that comprised of AESOP system and two laparoscopic instruments with 6-Degrees of Freedom (DOF) [20]. It received FDA approval in 2001 and in the same year the robot was used to perform the first transatlantic surgery from New York on a patient located in France [21]. In the year 2000, Intuitive Surgical, which had been developing robotic surgical system for past few years, received FDA approval for its da Vinci surgical system which became the first FDA approved surgical robot for general laparoscopic surgery [22]. These technological developments were the early days for surgical robotics. In the current era, the advancements in medical imaging, haptics, 3D vision, and miniaturization are helping engineers to build compact and more effective surgical robots. Improvements in sensory technologies have provided a more holistic view on the surgical approach and it is significantly contributing to the advancement of medical robotics. These sensors provide a better understanding of the interaction between the robotic tool and targeted organs. The percentage of general robotic surgeries performed in 2012 as a portion of total number of surgeries was as low as 1.8 %. This number jumped to over 15% by the year 2020 [23]. The market size for robotic surgery is projected to be $19 billion by 2027 [24,25].
1.3. Purpose, scope, and arrangement of the article
Considering the recent developments and the significant potential surgical robotics market holds in the coming future we realized the need for a comprehensive review of the existing commercial surgical systems. To our knowledge, there has been no recent review article directed exclusively towards commercially available robot-assisted surgical systems that cover all anatomical regions. Therefore, our objective through this article is to review robotic systems that exist in the market. This will enable the developers and researchers including those just getting started in the field of surgical robotics to understand the current trends and identify the existing gaps and the requirements for the development of next generation of surgical robots. Since the focus of this review article is commercial robots our primary source of information were the websites of developing companies. Also, whenever required, peer reviewed articles have been included that were available through the search of the corresponding robot name in google scholar and PubMed. The scope of this article includes all robotic systems that are invasive, and we have excluded non-invasive therapeutic robotic devices. Systems that existed in the past but have been discontinued are not included in this study. Additionally, when available we have added brief information from relevant clinical studies or trials to get familiarized with the clinical results for a specific surgical system.
The robotic systems reviewed in this article have been primarily classified based on the type of tissue the device operates on with subclassification based on anatomical region. A reader can also place them under several different categories as described by other methods of classification under section 1.1, but for the purposes of this article we have followed the target tissue and anatomy as the basis. Section 2 “Robotic surgical systems for osseous tissue” discusses systems operating on bones, or those where instruments pass through the bones. Section 3 “Robotic surgical system for soft tissue” discusses systems that operate on soft tissues. The sub sections for each of these sections are based on specific anatomical region. Section 4 “Robotic systems for microsurgery” discusses the robots developed and applied for microsurgery. Section 5 “Generic robotic surgical systems” discusses the systems that do not fall under any single specific surgical area rather have been designed to operate on multiple anatomical applications. In Section 6 “Discussion and Conclusion” we discuss our observation and current trends in the field of surgical robotics. We end the article with a table that acts as a quick reference for a reader to review the robot-assisted surgical systems discussed in this article.
2. Robotic surgical systems for osseous tissue
2.1. Hip and Knee Surgery
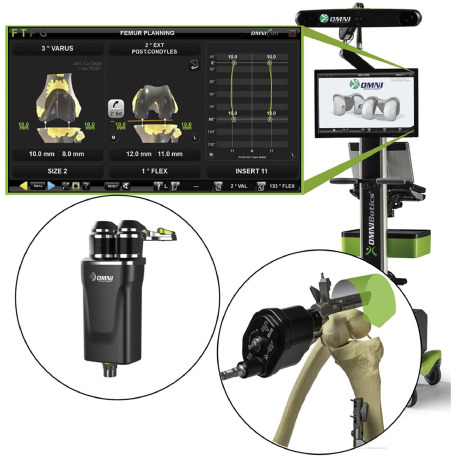
Stryker developed Mako [26], an Unites States Food and Drug Administration (FDA), approved robotic system to assist surgeons in total hip replacement (THR), total knee replacement (TKR) and partial knee replacement (PKR) surgery. It also received Conformite Europeenne (CE) mark for TKA, total hip arthroplasty (THA) and Unicondylar Knee Replacement (UKR). The basic system consists of a robotic arm, a camera stand and a guidance module while the attachments including the cutting instruments vary as per specific application along with the software module. A surgeon uses a preoperative image of the patient's anatomy of interest and preplans the surgical procedure. Based on this preplanning the robotic arm creates a virtual boundary while operating on the patient using orthopedic tools. The position of the patient and the robot during the operation is tracked using an optical tracker. This system has been widely used commercially. As of May 2021, it has been utilized for more than 500,000 procedures (THR, TKR & PKR) by surgeons across the world. [27]. One of the studies that compared 40 manual total knee arthroplasty (TKA) procedures with 40 Mako TKA procedures showed that robotic procedure led to decrease in post-operative pain, requirement of analgesics and physical therapy along with 26% reduction in hospital stay [28]. TSolution One developed by THINK surgical is a CE marked and an FDA approved TKR or TKA surgical platform [29]. It consists of two subsystems viz. TPLAN, a 3D preoperative planning workstation and TCAT, a computer-assisted tool. TPLAN enables a surgeon to plan positioning of the implants with respect to the 3D anatomy model of the patient. The other subsystem, TCAT, consists of an electromechanical arm and an arm base. The base consists of controller, monitor, software, pedant control and other tools [29]. During surgery the surgeon registers the anatomical position with autonomous TCAT robotics system, and it performs the bone cutting actively under the supervision of the surgeon. As of early 2020, more than 550 TKA procedures had been performed globally using TSolution One system [30]. Think Surgical in partnership with Sagentia Innovation developed TMINI miniature robotic system, one of the most recent FDA approved orthopedic systems for TKA surgery [31,32]. It consists of TPLAN, TNav, an optical tracking navigation control and TMini, a handheld, wireless robotic device. TPLAN aids a surgeon in implantation of knee components by enabling creation of 3D models using CT images. The system automatically compensates the surgeon's hand movements for locating bone pins using optical tracking. Following this, the mechanical cutting guides are connected to pins mounted on bones allowing the resection of bones subsequently. Corin developed Omnibotics [33,34], an FDA approved, and CE marked robotic system for TKR or TKA procedure. It consists of a workstation, an OMNIBot cutting guide and a robotic ligament balancer called BalanceBot (Fig. 1). Before starting the surgical procedure, the surgeon checks the range of motion by attaching several sensors that are tracked by the tracker on the workstation. The BalanceBot enables the surgeon to measure tension in the soft tissue to better plan the implant placement. The system has imageless bone morphing technology that aids in building a 3D representation of the joint. Based on this information, the computer calculates the sizing and alignment of the implant. Once the plan is ready, based on this pre-operative plan bone resection is performed by surgeon using the robotic OMNIBot cutting guide which aligns itself automatically, post which tibia is reshaped. Once all bones are prepared, implants are positioned. After the procedure is completed, the surgeon uses the BalanaceBot again to measure the balance of the ligaments and check whether the joint is stable or not. The sensors are used again to check the range of motion. As of March 2020, 25000 TKA procedures have been performed using OMNIBotics which includes 5000 cases where BalanceBot was used for ligament balancing [35]. As per several multicenter studies performed in Europe for TKR, it was observed that Omnibotics group had higher Knee Society Scores (KSS) than manual arthroplasty. Significant improvements were also observed for WOMAC (Western Ontario and McMaster Universities Osteoarthritis) Pain, Stiffness, and Function and SF-12 (Short Form Survey) Physical Scores [36]. Zimmer Biomet developed ROSA Knee system [37], an FDA approved robotic platform for resection of bones and to assist in positioning of implant during TKA. It consists of a robotic unit that has a robotic arm and an optical unit. Each unit consists of a touch screen. Surgeons use software to plan the positioning of an implant and its size. Both units are located on each side of the operating table. Based on the preoperative plan, robotic arms are positioned in a way to fix the location of a jig at the appropriate location and then pinned to the bone [38]. Once it is fixed the surgeon performs the resection. Zimmer Biomet also developed a similar system ROSA Partial Knee [39] system for partial knee arthroplasty (PKA) and ROSA Hip system [40] for THA. As per multiple studies comparing ROSA knee TKA with manual procedure, it was shown that at six and twelve months interval group that underwent robotic procedure had higher Forgotten Knee and Oxford Knee scores along with less pain [41]. Smith and Nephew developed Cori Surgical System [42], a handheld surgical robotic instrument for TKA and Unicompartmental Knee Arthroplasty (UKA). It does not require preoperative image rather an optically tracked tool paints over the anatomy to generate a real time 3D image of the bone. Optically tracked jigs are used to hold the anatomical site for accurate tracking. Once the anatomical area is mapped a surgeon can utilize Cori, an optically tracked burring tool (i.e., a precision milling tool) to remove bone from the target site. The burring tool tip has a retractable sleeve which protects the bone and comes out and covers the burring tool tip to avoid accidental damage to bones. Virtual boundary tracked by the optical tracker relative to the anatomical site facilitates this procedure. Navio [43], a handheld surgical system, similar to the Cori, is another CE marked and FDA approved robot developed by Smith and Nephew for TKA, Patellofemoral Arthroplasty (PFA) and UKR. A randomized controlled study by Adamska et al. [44] compared robotic TKA by CORI and NAVIO surgical system with manual TKA and concluded that both procedures reported satisfactory results, in turn implying that robotic method does not necessarily have compelling enough advantage compared to manual, particularly considering the cost incurred and the total surgical time. The orthopedics company of Johnson & Johnson, DePuy Synthes developed VELYS [45], an FDA approved robotic assisted solution for knee replacement surgery. It consists of a satellite station and a base station. Satellite station consists of a robotic assisted device, touch screen and a transfer mechanism. The base station consists of a camera, touchscreen, and footswitch and consoles that run various applications and drive the robotic device. The camera tracks the position of the bones, the robotic device, and instruments through optical markers. Prior to procedure the transfer mechanism holds the robotic device while during the surgery the robotic device is positioned along the operating table rail by the satellite station. The surgical robot aligns the cutting tool real time using optical markers mounted on anatomical site and the robot. Currently, for the VELYS system, a non-randomized clinical trial of 200 participants is underway in United States, results of which are expected by end of 2023 [46]. In a separate study, Clatworthy presented data from a limited cohort for one-year results [47]. The limited data showed high satisfaction by patients and favorable outcome scores. Monogram Orthopaedics [48] developed a robotic system for aiding surgeons during knee replacement surgery. It consists of a 7-DOF robotic arm with a reach of 1.3 meters. It enables the surgeon to perform complex tasks while using its flexible motion feature. The robot has integrated force and torque sensors for safety purposes and collision detection. The system comprises of a high efficiency rotary cutting system that can reach tiny areas. It can be tracked in real time due to its closed loop design. A navigation system, part of the overall robotic system offers real time imaging while following a pre-scanned analysis of the knee.
 Figure 1. OMNIBot robotic system includes a workstation, an OMNIBot cutting guide and a robotic ligament balancer BalanceBot [34]
Figure 1. OMNIBot robotic system includes a workstation, an OMNIBot cutting guide and a robotic ligament balancer BalanceBot [34]2.2. Neurosurgery
Medtronic developed Mazor X Stealth Edition [49,50], an FDA approved robotic surgical guidance system for spine surgery (Fig. 2). It consists of a workstation that includes a touchscreen control panel, system hardware components and a storage system for robotic guidance system. The guidance system is comprised of a table mounted arm, a control panel for the surgeon and a navigation system. A plan is prepared by the surgeon based on the prior CT scan or an O-arm scan during the surgery. The navigation system maps the operating field. Further, patient scan is performed, and each vertebral body is registered. The robotic arm moves according to the trajectory based on prior plan. Once position is fixed the required procedure is performed using the instruments. Stealth navigation, part of the system, allows real time visualization of implants and instruments. Several clinical studies performed using Mazor system showed high accuracy, increased reproducibility & predictability, significant reduction in time used between pedicle screw placements, and on an average 2.6 days shorter stay compared to open hand procedures [51]. The Mazor X platform also received CE mark for the European market in 2017 [52]. ExcelsiusGPS [53], by Globus Medical is an FDA approved and CE marked robotic system developed to assist with the spine surgery. The system utilizes preoperative & intraoperative CT and fluoroscopic images of the patients including a dynamic reference frame and camera for real time guidance of pedicle screw positioning. It consists of a foot pedal controlled robotic arm that is activated by the surgeon and positioned based on the pre-planned trajectory [54]. Once the arm position is fixed, the screws are manually placed by the surgeon. The system optically tracks the robot and the patient position using optical trackers mounted on them. Vardiman et al. [54] reported the data from first 56 cases of pedicle screw placement using ExcelsiusGPS system and 97.7% accuracy was observed. In these 56 cases, 348 screws were placed using this system, out of which only 9 had to be repositioned implying a success rate of 97.4%. ExcelsiusGPS Cranial Solution [55] also has an FDA approved robotic solution for cranial surgery. It can perform common and complex stereotactic procedures such as biopsies, stereoelectroencephalography (SEEG). This system is broadly similar to the spine robot. One of the advantages with cranial solution is that preoperative MRI and CT are automatically merged for planning and navigation, unlike the spine robot which is based on CT. CUVIS-spine [56] is an FDA and MFDS (Ministry of Food and Drug Safety), South Korea, approved, CE marked spine surgical robot system developed by CUREXO, a South Korean company. It consists of three parts viz. a robotic arm, a main console, and an optional staff console. The robotic system guides pedicle screw according to plan. Preoperative plan is generated based on pre surgical images for the target point, entry point and the route of the pedicle screw. A robotic arm acts as the positioning device for the pedicle screw insertion during the surgical procedure. An optical tracker tracks the patient and the surgical arm in real time for dynamic tracking. Both ExcelsiusGPS and CUVIS-spine have a force-controlled motion enabled controller to position the robotic arm and display the instrument position in real time [57]. ROSA ONE Brain [58] by Zimmer Biomet is an FDA approved surgical robot used for brain biopsy, SEEG, Deep Brain Stimulation (DBS) and endoscopy. The ROSA robotic arm acts as a gross positioning system and can be used for multiple minimally invasive brain interventions. The system uses a fixed fixture with the patient head and registers its position. Generally, the skull has a section removed so that surgeons can place electrodes in the brain, but ROSA burrs small holes for precise placement of instruments. It generates a 3D model of the patient's brain from multiple CT scans stacked together to create a preoperative plan. It uses another extension to support the patient's head with a stereotactic frame that has optical markers. The 6-dof robotic arm provides guidance, depth control, and speed control for the operating tool. ROSA is equipped with haptic feedback and will restrict placement of tools according to the preoperative plan. A standard master device communicates the preoperative plan through tele-manipulation; however, the robotic arm is equipped with force sensors to allow surgeons to adjust the arm manually. Several clinical studies have been performed and published using ROSA ONE Brain system since its introduction. One such study by Liu et al. evaluated the accuracy of lead placement in DBS between 2012 and 2016 for 144 patients. The methodology involved measurement of radial error (RE) and it was shown that no statistical difference was observed. It was shown that submillimeter accuracy was achieved for robot-assisted DBS [59]. ROSA One Spine [60] is Zimmer Biomet's FDA approved spine surgical assistive device, where the robotic arm facilitates the surgeons to perform thoracolumbar minimally invasive and complex spine procedure by utilizing the robotic arm as a positioning device for the surgical tools. Chenin et al. [61] evaluated the accuracy of pedicle screw placement using ROSA Spine under the guidance of fpCT and it was observed that out of 110 screws, 91.8% were placed completely within the pedicle. Monteris developed Neuroblate [62], an FDA approved minimally invasive robotic system for Medical Resonance Imaging (MRI)-guided laser ablation for brain. The system primarily consists of laser probes, robotic probe driver, platform for attachment to MRI table, and a control workstation and is used with stereotaxic frames and systems for patient stabilization. It begins with a preoperative plan to determine the size and location of brain tissue that needs to be ablated. The stereotactic head frame is integrated with the patient carrier so that the patient can be transferred from the Operating Room (OR) to the MRI scan room. The NeuroBlate driver and probe are attached to the patient's skull and MRI scans provide intraoperative guidance of the laser probe during ablation. LAANTERN (Laser Ablation of Abnormal Neurological Tissue Using Robotic NeuroBlate System Trial) is one of the largest studies that has been sponsored by Monteris where up to 3000 patients were enrolled at multiple centers over five-year period, starting 2015 [63]. One of the first studies published through this by Groot et al. [64] studied “efficacy of LITT (laser interstitial thermal therapy) for newly diagnosed and recurrent IDH wild-type glioblastoma” with final inclusion of 29 patients for new and 60 for recurrent. It was observed that in the newly diagnosed category median OS (Overall Survival) was similar to conventional surgical resection. It establishes viable solutions for patients who have tumor that either can't be operated upon or resected. Neuromate [65], an FDA approved robot by RENISHAW is used for DBS, SEEG, Neuro endoscopy, biopsy. Preoperative MRI and CT data is registered to the patient using the Neuromate registration module and intraoperative CT scan. Neuromate can perform stereotactic frame-based surgeries as well as frameless surgery. A laser tool is used to mark entry points on the patient's skull and once marking has been completed the laser tool can be replaced with the tool holder on the robotic arm. Drilling alignment is performed with Neuromate acting as a guide. Guide tubes and stylets can then be inserted, and perioperative scanning can confirm depth and location before moving onto electrode insertion. The robotic arm operates semi-autonomously while following a preoperative plan to mark entry points and aid surgeons in alignment of tools. Yasin et al. [66] reported their experience from 102 frameless stereotactic biopsies performed using Neuromate between March 2013 and April 2018. It was shown that Neuromate robot-assisted frameless stereotactic biopsies had a diagnostic yield similar to frame-based while encountering complications at comparable rates. Based on this data, the institution where these procedures were performed use Neuromate for all stereotactic neurosurgeries. AiM Medical Robotics [67] developed a portable, MRI compatible, intraoperative robotic device for neurosurgery to address functional brain disorders such as Parkinson's, epilepsy and cancer. It can provide precise positioning of tools for neuro specific surgeries under MRI environment, letting surgeons take the advantage of imaging for targeting the site. Remebot [68] is China's first home-grown minimally invasive neurosurgical robot approved by National Medical Products Administration (NMPA), China, for deep brain surgery [69]. It's a six-axis robotic arm that provides enhanced precision and flexibility of motion. It decreases the trauma caused due to brain surgeries, thereby faster recovery time for patients. The robot is equipped with MRI/CT compatible imaging system and is able to provide automatic positioning based on the imaging data. This feature helps surgeons to locate the diseased site. Through AI technology, the robot is capable of image identification, image processing, mapping accuracy and thereby provides an enhanced and optimized plan for surgery. Brain biopsy, implanting electrodes, operating for brain hematoma are examples of surgeries performed using Remebot. Li et al. [70]performed a retrospective study of 33 patients, divided into groups, and compared the safety, efficacy and accuracy of frameless brain tumor biopsy performed using Remobot with the frame-based procedures. No significant difference between diagnostic yield and complication rate was observed. It was concluded that both methods are safe and efficacious with Remobot having the advantage of shorter total time for procedures along with its wider application in young pediatric patients. Brainlab developed a 7-DOF robotic positioning arm Cirq [31,71] to assist surgeons during Spinal and Cranial surgery. It is mounted on the OR table and controlled through a foot pedal. The arm can be manually moved as long as power is provided to the system. Buttons on the device can be used to freeze the position of the arm when holding and positioning tools are used for non-motorized instruments. Software such as Kick and Curve can be used for navigation with a tracking attachment module as well as Airo for intraoperative imaging. The foot pedal is a custom master device with alignment controlled by a small dial, and advancement and retraction of the end tool controlled by large buttons. It is an FDA approved and CE marked system. Chesney et al. [72] performed a retrospective study of 84 patients who underwent lumbar fusion surgery where placement of 714 transpedicular screws was performed using Cirq arm. The authors concluded that the Cirq robotic system demonstrated efficacy and safety for these procedures, but it still requires other comparative studies.
 Figure 2. Mazor X Stealth Edition Robot [50]
Figure 2. Mazor X Stealth Edition Robot [50]2.3. Dental surgery
Yomi [73] by NEOCIS is an FDA approved robotic system for dental surgery. At the start of the procedure, Yomi constructs a preoperative plan from CT scans of the patient's mandible, maxilla, and teeth. Its software allows dynamic changes to the operation plan while Intra-operative tracking with an assistive robotic arm and real-time 3D graphics provides guidance for the surgery. The robotic arm also maintains alignment, position, and depth of operating drill. Yomi is equipped with haptic feedback to prevent deviation from the surgical plan and breaching the planned depth. Talib et al. [74] presented a report where they used and assessed the capabilities of Yomi by using the robotic system to position an implant in a 48-year-old woman who was missing maxillary left second premolar. The physicians were able to place the implant without any issues and observed quick healing. The patient also reported absence of any discomfort during the procedure demonstrating the advantage of haptic robotic procedure for placement of dental implants.
2.4. Generic Osteotomy
CARLO [75] by AOT is an autonomous robotic surgical device for Cold Ablation Robot-guided Laser Osteotomy (Fig. 3). It is the world's first CE certified robotic surgical device that can cut bone with laser technology. The system includes an Er:YAG laser head with a tactile robotic arm (LBR Med from KUKA), a 3D navigation system and software for preoperative planning and intraoperative use. Carlo can perform cold ablation and cut bones in any desired shape. Due to the system's ability to use cold ablation method, the ablated bone structure stays porous and functional and as a result the tissue grows back and heals quicker [76]. The laser head is mounted on the LBR med tactile medical grade robotic arm which ensures safe movement compatible for collaboratively working with human [77]. Holzinger et al. [78] assessed CARLO for clinical application and reported the results from orthognathic procedure on 14 patients for midface osteotomy. Surgeons reported that they did not encounter any intraoperative complications. Also, all osteotomy procedures were within 0.80 mm average deviation with mean time of 4.6 minutes for robot setup at the start of procedure. Tinvai Medical technology, a China based medical company developed two robotic devices for trauma and spine surgeries – TiRobot [79] and TiRobot-II [80]. Both devices can operate on spine, pelvis, femoral neck, shoulder ankle and hand and have a robotic arm which acts as a gross positioning system for the orthopedic tools for surgical procedure. The robotic arm can move precisely according to the pre-planned position for accurate positioning of the tool. An optical tracker tracks the robotic arm and tool for precise positioning. It has been approved by National Medical Products Administration (NMPA), China. As per Tinavi website, by September 2022, over 30,000 orthopedic procedures have been performed using TiRobot system distributed across 150+ medical institutions in China. It enables submillimeter precision for all spine and trauma related minimally invasive surgeries [81].
 Figure 3. Cold Ablation Robot-guided Laser Osteotome (CARLO) comprising of a laser head with robotic arm and navigation system [76]
Figure 3. Cold Ablation Robot-guided Laser Osteotome (CARLO) comprising of a laser head with robotic arm and navigation system [76]3. Robotic surgical systems for soft tissue
3.1. Abdominal Surgery
Avatera medical developed Avatera [82], a CE marked robotic system for Minimally Invasive Surgery (MIS). It consists of two units: a surgical robot and a control unit. The surgical unit consists of four robotic arms that control instruments and endoscope. The control unit is comprised of flexible seat for the surgeon, an eyepiece, manual haptic input devices and footswitches. The eyepiece was designed so as to not obstruct the surgeon's mouth and ears to avoid any miscommunication between the surgeon and the team. The image displayed by the system exactly matches the human eye's natural field of view. In May 2022, Avatera medical reported the successful clinical application of the Avatera system in ten humans for removal of prostate and kidney tumors [83]. These procedures were performed at the University of Leipzig Medical Center. As a result of this success, the system is being installed at more number of medical centers across Europe. Asensus surgical developed Senhance Surgical System [84,85], an FDA approved and CE marked robotic platform for laparoscopic procedures. It consists of independent robotic arms with a base platform for each arm and a control station equipped with a monitor screen to allow the surgeon to operate in a relaxed position. The system is active as long as the foot pedal switch is pressed, and the system freezes otherwise. It uses haptic feedback and a custom master device that allows independent control of the slave devices. Eye tracking technology is used for endoscopic control and an open console design allows staff to observe the surgeon's actions and communicate during the operation. Several number publications have reported clinical cases that used the Senhance surgical system. Few applications include colorectal, gynecology, pediatric etc. [86]. As latest as 2022, Holzer et al. [87]reported application of the system in pediatric robotic pyeloplasty on a year and half old girl. It was one of several examples of successful application of the Sehance robotic system. There was no recurrence of ureteropelvic junction obstruction after 6 months and normal growth was observed for the patient. Canady Surgical system [88] developed by US medical innovation (USMI) is a laparoscopic surgical robot to assist with MIS. It has been designed such that it can also be used for open surgeries. The system is equipped with three robotic arms which act as a positioner for the laparoscopic tool and the endoscope and has a ‘voice command’ feature that frees up the surgeon and assistant to dedicate their resources towards surgical and other tasks. The company has also developed flexible laparoscopic tools such as FDA approved, Canady Flex RoboWrist for laparoscopic operation that can be utilized with this robot. Revo-i [89,90], developed by Meerecompany Inc., South Korea, is a surgical robot for laparoscopic procedures. It consists of a master console, operation cart and a vision cart. The master console consists of a 3D viewer, touch pad, control arm and foot pedal while the operation cart has of four arms where one of the arms is equipped with a 3D camera. The vision cart that has a touchscreen monitor enables visualization of the whole surgical procedure. The operating procedure is customizable due to several different end effector attachments. Revo-i has the benefit of haptic feedback. It has been approved by MFDS, South Korea. Lim et al. [91] studied the safety and efficacy of Revo-i for cholecystectomy through phase I clinical study performed on 15 patients. Intraoperative safety was the primary outcome evaluated while 30-day postoperative complication and patient satisfaction was the secondary outcome. The surgeons observed no intra or post-operative complications. Also, most patients reported satisfaction with the procedure performed using Revo-i. AutoLap [92,93], developed by MST, is an FDA approved and CE marked system designed for holding and maneuvering laparoscopes or endoscopes during MIS. It consists of a processing unit consisting of all electronic components, a base unit, mounted on an OR bed, and a laparoscopic unit. The surgeon controls the laparoscope through a command unit. It consists of an autonomous endoscope that tracks the surgeon's standard laparoscopic tools and maintains endoscope in the field of surgeon's view. Titan medical developed a master-slave robotic system Enos [94,95] that enables surgeons to perform the surgery through a single incision on the body. In the past it was referred to as Single Port Orifice Robotic Technology (SPORT) surgical system. The system consists of a surgeon workstation, an open console with a monitor, and a patient cart consisting of a single support boom holding a central unit (CU). During docking, a camera insertion tube (CIT) is connected to CU. CIT is made of 3D high-definition camera and a light source. The system has two multi-articulated instruments that are inserted through CIT. Micro Hand S [96,97] by Shandong Wego Surgical Robot Co. is a surgical robot developed by multiple universities in China in collaboration with their government. It is a master and slave type robot consisting of a surgeon console, a patient console, and other accessories. The surgeon console has several parts that include an image display device, left and right master arms, stand columns, armrest, and a control system. The control system processes the information received because of operation of the master console by the surgeon. The patient console has a suspension arm, lifting column, two slave arms, pedestal, and an electrical control system. The arms enable positioning of the laparoscopic tools and are inspired by origami folding style that require less space and have better dexterity. They are mounted on a single column movable base to consume less space. Yao et al. [97] published the results for feasibility and safety of Micro Hand S from a Phase I clinical study where 81 patients underwent general surgery. Surgeons concluded that the system was reliable and safe for clinical applications, but the study had limitations such as small sample size, short follow up time etc. Considering these limitations, it was concluded that large, prospective, randomized controlled studies are required to further confirm the results from this study. Hugo RAS system [98] is a CE marked, master-slave type, minimally invasive robotic assistive surgical system developed by Medtronic. A robotic arm equipped with custom end effector for laparoscopic tool manipulation is mounted on a single column on a cart with wheels underneath. The master can control up to four of these seven DOF arms and each arm can be equipped with a laparoscopic tool or an endoscope. The surgeons wear 3D glasses for the 3D view of the operation site and a tracker tracks 3D glass motion on the surgeon's head to track the video [99]. Medtronic announced the first clinical trial in United States using Hugo RAS system in December of 2022. This is a part of ongoing clinical trials named Expand URO where 122 patients will be enrolled with the aim to study and evaluate the safety and performance of the Hugo RAS system for urological procedures [100]. MIRA [101], a Robotic Assisted Surgical (RAS) device developed by Virtual Incision is a single port incision minimally invasive master and slave device. The slave device consists of two articulated miniaturized instrument arms and an integrated camera that can articulate to triangulate along with the arm. The master console has two monitors: one to view the surgical procedure and the other provides 3D visualization of MIRA. MIRA is going to be deployed in international space station (ISS) through a NASA funding to explore the possibility of utilizing such technologies in space. Virtual incision reported in May 2023 submission of its De Novo request to United States FDA. The purpose of this submission is to seek the authorization of MIRA surgical system for marketing for bowel resection procedures [102]. The data from the first clinical trials performed for the purpose of Investigational Device Exemption (IDE) were also reported in 2023 [103]. The system was used to perform right or left colectomy on 10 patients consisting of 7 males and 3 females. No adverse events and intraoperative or postoperative complications were observed, and it was concluded that the system is safe and effective for the intended procedure [104].Vicarious Surgical Robot [105] developed by Vicarious Surgical is a single port minimally invasive surgical robot which utilizes a single incision of 1.5 cm in the abdomen to perform laparoscopic surgery. The single port surgical device holds two human inspired arms and a camera. The arm has 9-DOF that is similar to DOF of upper body movement of a surgeon [106]. For this master and slave type device the camera follows the surgeon's head for better visibility inside the abdomen and displays it on the master console. The advanced stereoscopic camera can rotate 360 degrees in both directions for superior vison and 3D immersive experience. Vicarious Surgical also has a Virtual Reality (VR) headset for the surgeons which will be rolled out in the future version. The Anovo Surgical System [107] by Momentis surgical is a master-slave system and FDA approved for Transvaginal Hysterectomy procedures. It is designed to replicate the motion of a surgeon's arms with wrist, elbow, and shoulder joints. The miniature robotic arms have human level dexterity, flexibility and 360 degrees of articulation feature that provides optimal access, working angles and enables obstacle avoidance. It permits multiple instruments to be introduced to the body through a single port. Distalmotion developed an open platform robotic system, Dexter [108] for robotic suturing and dissection tasks in a laparoscopic surgery. It consists of an open surgeon console and two patient carts positioned beside the operating table. Each cart is installed with an arm. The robot has a modular design and permits quick removal and repositioning of the two robotic arms. Its open architecture allows the surgeon to promptly switch between robotic approach for suturing and traditional laparoscopic approach for dissection. The standing laparoscopic mode can be used for stapling and vessel sealing tasks at the start and end of surgery. One of the major advantages of this system is the ability to leverage existing technologies within a hospital to perform the required surgery due to its open platform design. Dexter robotic system is CE marked. Thillou et al. [109] reported the experience from ten robot-assisted radical prostatectomy and lymph node dissection procedures performed using the Dexter system. Surgeons were comfortable using the system even for those without any experience of robotic surgery in the past. They attributed this to the adaptability of the system to existing setups. The device performed as intended without any incident of complication during the surgery. SOLOASSIST II [110] by AKTORmed is an FDA approved and CE marked robotic endoscopic control system for MIS. It provides vibration free stable positioning within a unique range of movement and has manually adjustable axis on the distal arm that allows exceptional flexibility. Joystick feature of the system allows intuitive operation while voice control feature executes surgeon's commands with precision. It is compatible with most common operating tables and endoscopes. Another cost-effective version of the system by the same company is SOLOASSIST IIs [111], which is an entry level model especially suitable for unplanned emergency procedures. This system offers a set of sterile disposable components to enable immediate use of the system without any prior processing. SOLOASSIST IIs, a CE marked system, has voice control feature but does not have the intuitive joystick control feature. Ohmura et al. [112] studied the efficacy of joystick controlled SOLOASSIST system in laparoscopic surgery. Several factors such as operative time, setup time, number of participating surgeons etc. were investigated for laparoscopic cholecystectomy cases performed before and after introduction of the system. Overall, it was observed that the system is useful for both elective and emergency procedures. Further, shorter procedure times were recorded along with a smaller number of doctors that were required while no significant difference in blood loss and setup time was observed. Emaro [113] developed by RIVERFIELD is the world's first pneumatic endoscopic manipulator developed in Japan. The manipulator moves the endoscope based on the head motion of the surgeon to map their field of view. An Inertial Measuring Unit (IMU) is mounted on the surgeon's head to detect its motion. The endoscope can also be operated with a foot pedal, manual switch and console [114]. Riverfield also developed IvyA1 [115], a pneumatically operated robotic endoscope holder which can assist in laparoscopic surgical procedure. Yoshida et al. [116] reported their experience with EMARO during extraperitoneal inguinal hernia repair procedure on a 77-year-old man. Surgeons did not encounter any intraoperative complication and reported that the robotic procedure was comparable to the traditional manual surgery but has the advantage of enabling execution of solo surgery. Freehand developed two collaborative robots (cobots) – Vista [117] and Panorama [117], each for specific type of surgeries. Vista has been designed for urological, upper gastrointestinal and bariatric surgeries while Panorama for gynecological, colorectal, and thoracic surgeries. They are CE marked and FDA approved cobots. The primary aim is to eliminate the requirement of an assistant for holding a camera during the surgery. The camera is held through a 3-joint modular design arm which is a part of robotic motion assembly providing steady platform. Further, it consists of Instinctive Motion Control (IMC) for reliable and error free interface for the surgeon. IMC is comprised of a headset to choose the direction of motion based on surgeon's motion. The direction of motion appears on an indicator unit. To activate the motion the surgeon presses a footswitch. The basic structure of both Vista and Panorama is same except that they have different range of motion due to specific surgical requirements. EndoMaster Pte Ltd, based in Singapore, developed EndoMaster EASE system [118,119], a robot-assisted surgical system that allows surgeons to perform incision-less surgeries for minimally invasive endoscopic surgery. As a feature of the system a surgeon can control two robotic arms that act as an extension of surgeon's arms to perform surgical procedures with maneuverability and precision. This robotic endoscope's design was inspired by the crab claw. One of the primary clinical applications of this system has been identified as treatment of gastrointestinal cancer using endoscopic submucosal dissection (ESD) due to its less invasive nature. Owing to its complex shape, executing ESD in colorectum is technically challenging due to the risk of colonic wall perforation. Endomaster EASE system simplifies it due to the feature of tissue retraction and superior visualization. As per early clinical studies performed on 6 patients, all had successful colorectal ESD, and no adverse event of perforation was observed. These early results have proven the suitability of system for ESD. This surgical instrument can remove early-stage stomach cancer without leaving scars [120]. A Honk Kong based startup company, NISI, developed Novel Surgical Robotic System (NSRS) [121], [122], [123], a miniature surgical robot for natural orifice surgical procedure. This device can be inserted through natural orifice such as the rectum or the vagina to perform abdominal surgeries. It has two 10-DOF arms equipped with surgical tools folded inside its body, includes haptic feedback for the surgeon for precision sensing, and has a high definition 2D, 3D camera for precise workflow. It's a highly portable system that could also be mounted on a surgery table. The developers have defined surgeries related to abdominal and pelvic region as the target procedure for the NSRS system [123]. Medicaroid, a Japan based company developed hinotori [124] surgical robotic system for laparoscopic surgery. It has received the Japanese Ministry of Health, Labor and Welfare (MHLW) regulatory approval and is based on the concept of “co-existence of humans and robots”. The system consists of a surgeon cockpit, an operation unit, and a vision unit. Operation unit has several arms that have compact design, like human arms, with the objective of reducing interference between arms and an assistant or between the arms themselves. The surgeon unit has been designed ergonomically to maintain proper posture during surgeries and reduce the physical toll and stress taken by the surgeons. High-definition 3D images are available through the Vision unit on the stereoscopic viewer of the surgeon unit. Hinata et al. [125] reported the results from safety and efficacy study through preclinical and clinical evaluation performed using hinotori system. Preclinical studies were performed on porcine and cadavers. It was shown that complex procedures such as vesicourethral anastomosis in porcine and radical prostatectomy on cadavers took similar time as the daVinci system. Clinical studies were performed on 30 patients to evaluate the safety of the system through radical prostatectomy procedure. The system was shown to be safe as the adversarial events and device errors were below 15%, a preset threshold value.
3.2. Bronchoscopy
Monarch [126] platform is an FDA approved device by Ethicon, designed to perform therapeutic and diagnostic procedures for bronchoscopy. This teleoperated endoluminal robot can navigate inside the human body, take images, and treat without making any incision. It utilizes a custom controller allowing the surgeon to directly control the endoscope while they navigate the lungs. Visual feedback and image display is provided from the endoscope for navigation purposes. The feed rate and navigation can be controlled by the surgeon from the visual platform. Monarch platform has been used to treat more than 20,000 lung cancer patients by now. Recently, application of the platform to treat kidney issues due to stones is also being explored [127]. University of California, Irvine reported the first successful clinical trial for robot-assisted electromagnetic guided percutaneous access and mini-percutaneous nephrolithotomy using the Monarch platform.
Ion endoluminal system [128,129] by Intuitive is an FDA approved robot-assisted surgical platform for minimally invasive peripheral lung biopsy (Fig. 4). Ion is flexible with the ability to articulate 180 degrees in all directions allowing navigation far into the peripheral lung. This robotic bronchoscopy platform has an ultrathin and maneuverable catheter that provides precision by delivering accurate biopsy of hard-to-reach nodules even outside of the airways. With the use of fiber optic sensing technology, the system has unique sensing capability and stability. This technology allows maintaining the active robotic control of the catheter position also correcting the unwanted tip deflection. Simoff et al. [130] reported the results from a multicenter study performed using Ion endoluminal system to evaluate safety and procedural outcomes during robot-assisted bronchoscopy. 60 patients across multiple hospitals underwent biopsy and in total 67 nodules were targeted. 97% biopsy completion was recorded with no incident of pneumothorax or airway bleeding. These favorable results showed that the system can be used to drive catheters safely in proximity to the target for biopsy.
 Figure 4. Ion Endoluminal System consisting of a cart and a controller [129]
Figure 4. Ion Endoluminal System consisting of a cart and a controller [129]3.3. Cardiovascular Surgery
Niobe and Vdrive [131], FDA approved systems developed by Stereotaxis, have been designed for performing cardiac ablation. These are a combination of Stereotaxis’ products that work together to guide a catheter that ablates heart tissue which is causing arrhythmia. Niobe uses magnets on both sides of the body to navigate the Vdrive catheter up to the heart. The magnetic field controls the end of the catheter to precisely perform the ablation of the heart tissue. One of the advantages of the Stereotaxis system is that it reduces exposure of x-ray fluoroscopy to the patient and the staff compared to the manual ablation. Vdrive system consists of three different components viz. V-Sono for Intracardiac echocardiography (ICE) catheter manipulation, V-Loop Variable Loop Catheter Manipulator for circular mapping catheters (CMC) and V-CAS Catheter Advancement System for magnetic catheter body and fixed curve sheath manipulation. Nölker et al. [132] studied and compared clinical performance of V-Loop system for remote CMC manipulation during electrophysiology procedure with the conventional manual navigation. This study consisted of 120 patients who were supposed to undergo CMC study to be followed by pulmonary vein isolation (PVI). The patients were randomly allocated in 2:1 proportion representing remote:manual navigation. The effectiveness was compared based on successful navigation to the targeted PV and recordings of PV electrograms. It was shown that the robotic remote navigation performs similar as far as safety and effectiveness is concerned when compared to manual procedure. Vdrive has also received CE mark while Niobe has been approved by MHLW, Japan. Corpath GRX [133] by Corindus, an FDA approved, and CE marked system operates on Percutaneous Coronary Intervention (PCI) and Pulmonary Vein Isolation (PVI). It uses robotic control for guide catheter, guide wire, and balloon stent catheter to treat heart arrhythmia. A surgeon operates the device from the radiation shielded cockpit inside the OR after manually starting the intervention from the groin. The OR staff will position Corpath attached to an extended reach arm so the surgeon can attach the manual set up at the patient's groin. Guide wire can be advanced, retracted, or rotated from the joystick on the control console through tele-manipulation. Smitson et al. [134] reported the safety and efficacy of the Corpath GRX system through a clinical study that enrolled 40 patients. These patients had shown obstructive coronary artery disease and indications for PCI. The study was evaluated based on clinical success and device technical success. Clinical success of 97.5% along with 90.0% of technical success was achieved. It was concluded that the Corpath GRX system is safe and effective for robotic PCI and enables high clinical and technical success rates. Corindus also developed Corpath GRX Neurovascular Robotic System [135] for neurovascular interventions and is a CE marked system. Recently there has been a report from clinical trials performed at a global scale where 117 patients underwent robot-assisted neurovascular aneurysm embolization, claimed to be first such study in the world. It was performed using the Corpath GRX Neurovascular system where 94.0% technical success and 95.7% clinical success was achieved. This achievement has been noted as a transformative change in the field of neurovascular intervention [136]. R-One [137], a CE marked robotic platform developed by Robocath has been designed for interventional cardiology to treat coronary artery disease through PCI. It includes a guide wire feeding system that is connected to a robotic arm to manually position it. The robotic arm is integrated on a table platform that the patient rests on. The cardiologists manually prepare the patient's entry point at the groin and load the guide wire into the system. The surgeon sits at a shielded control console and navigates using two monitors. Once the lesion has been identified, the stent can be loaded and progressed along the guide wire with the joystick. The future versions will be further developed to treat peripheral artery disease through PCI and stroke using the mechanical thrombectomy treatment. In May 2022, Robocath presented the results from clinical trials named R-Evolution study where safety and efficacy of R-One was studied during coronary angioplasty on 62 patients spread across several centers. 100% clinical success was reported as there were no major complications reported at 30 day follow up. Further, more than 95.0% technical success and 84.5% reduction in radiation exposure was reported [138,139]. The Amigo Remote Catheter System (RCS) [140,141], robotic system developed by Catheter Precision, has been designed for heart surgery. It is attached to the OR table and includes a catheter system that sits on a track above the patient. The catheter advancement and retraction are done by linear actuation towards or away from the patient. An interatrial trans-septal puncture is performed to insert the catheter and once inserted it can be rotated along with end deflection to stir the catheter to the site of operation. Amigo RCS received de novo approval from FDA in 2014 for right-sided radiofrequency catheter ablations. It was shown through clinical studies that success rates for high acute and chronic ablation were similar to manual procedures with similar duration of fluoroscopy and procedure time. There was a significant advantage in terms of decrease in radiation exposure while using the Amigo system.
3.4. Hair restoration
VENUSCONCEPT developed ARTAS iX [142], an FDA approved and CE marked intelligent robotic platform for hair restoration. It consists of a 7-axis KUKA robotic arm, a 44-micron high-definition multi-camera stereoscopic vision system, an advanced Artificial Intelligence (AI) system and a touch screen user interface (UI). The system intelligently selects the grafts from donor area and upon accurate identification and after developing the recipients site implants the grafts. The AI system continuously tracks the position, angle, size, and orientation of each hair follicle based on patient motion. A proprietary dissecting punch installed on the arm that has a muti-faceted tip removes sections of healthy hair follicles and grafts the hair by inserting the plugs in the transplant location. The UI enables the physicians to prepare a pre-operative 3D plan for customization and prescribing the recipient area. Artas iX has also received CE mark and is claimed to be the world's first intelligent hair transplant platform [143].
3.5. Opthalmic surgery
Preceyes [144] developed by Preceyes BV, a Dutch company is a CE marked micro surgical robotic device to assist with vitreoretinal surgery. It acts like a gross positioning device for the surgical tool and the tool can rotate along the remote center of motion. The device is a master slave device where the surgeon operates a 4-DOF instrument manipulator. It improves the precision of the surgeon's hand by downscaling movement and filtering hand tremors. The robotic instrument is locked in place when the surgeon releases their grip on the controller. Faridpooya et al. [145] studied the safety and feasibility of using Preceyes surgical system to perform epiretinal membrane (ERM) peeling surgery. 15 patients were enrolled and randomized in ratio of 2:1 proportion (robotic:manual). Surgeons did not record any clinically adverse event satisfying the primary objective. As secondary objectives, overall duration of surgery, best corrected visual acuity (BCVA), central retinal thickness (CRT) and distance travelled by forceps during peeling was measured. It was observed that duration of surgery was more than double in robot-assisted compared to manual, but distance covered by forceps was shorter while improvement in BCVA and CRT was same.
3.6. Otolaryngology
Flex robotic system [146] by Novus assists with oropharynx, hypopharynx, and larynx surgery by accessing the body through the mouth i.e., transoral access (Fig. 5). It was also developed for transanal surgery for conditions which require rectal lesion removal and was claimed to be the first miniaturized flexible endoscopic robot with features that allows maneuvering beyond areas that were unreachable through existing endoscopic microsurgery tools. The system consists of two units, viz. a control console and a cart and base along with a flexible robotic endoscope. The control unit has a joystick type haptic controller for precise 3D motion. The base is carried over the cart and enables stable operation of Flex Drive that is controlled through control unit. The Flex Drive robotic arm has a miniaturized, 3D, high definition, reusable digital camera at the end that enables image-based navigation. Flex navigates the body using a flexible robotic scope comprised of an inner and outer mechanism. The instruments are inserted into the robotic system to allow end effectors to treat sites inside the body usually inaccessible. This is a CE marked and FDA approved system. Morino et al. [146] performed a prospective study on 26 patients which underwent rectal lesion excision with full thickness excision on 14 patients and submucosal excision on 12 patients. Median operating time of 115 min was observed. For six patients the procedure had to be converted to transanal endoscopic operation. Other clinical results such as 30-day morbidity and positive resection margins were recorded including 12 months follow-up. Overall, it was concluded that it's a promising system that needs further technical refinements to improve the clinical results.
 Figure 5. Flex robotic system with (a) console and Flex Drive, (b) Flex Cart and Base, and (c) Flex Drive [146]
Figure 5. Flex robotic system with (a) console and Flex Drive, (b) Flex Cart and Base, and (c) Flex Drive [146]3.7. Urology
Procept BIOROBOTICS developed Aquabeam robotic system [147,148], an image guided surgical system for heat-free waterjet ablation of prostate tissue called Aquablation therapy. It is the first FDA approved robotic surgical system for treating lower urinary tract symptoms (LUTS) caused by benign prostatic hyperplasia (BPH) by automatic resection of tissue. It uses a high velocity waterjet stream for ablation and tissue removal. The system consists of a console, robotic handpiece, and Conformal Planning Unit (CPU) [148]. To start with, patient is positioned in dorsal lithotomy position following which a trans-rectal US transducer is inserted. The handpiece, a flexible scope is used for trans-urethral access of the bladder. Once the handpiece is registered treatment planning is performed. Post planning, treatment is initiated by pressing a foot pedal. Once the pedal is activated, a pump delivers high-velocity waterjet stream. The depth of penetration can be controlled by adjusting flow rates. Procept BIOROBOTICS reported extensive clinical data on safety, efficacy, and durability of Aquablation therapy using the Aquabeam system. The results were recorded from a multi-year follow up data from WATER (Waterjet Ablation Therapy for Endoscopic Resection), WATER II and OPEN WATER studies which evaluated safety and efficacy of resecting prostates 30-80 mL, 80-150 mL and 20-150 mL respectively. Both with regards to safety and efficacy, low rates with irreversible complications and durable results were observed across the whole range of prostate shapes and sizes [149]. EDAP developed Focal One [150], an FDA approved, semi-robotic High Intensity Focused Ultrasound (HIFU) device for prostate ablation. It consists of computer hardware, image fusion software and trans-rectal US probe [151] . Based on prior MRI and the US imaging and the fusion of both, target region is identified after which 3D model of the prostate is created. Once the treatment plan is created, probe is inserted trans-rectally and ablation is performed. Surgeons can constantly examine the ablation process and intervene to update the treatment plan. Hardenberg et al. [151] reported clinical data from a series of studies on patients that underwent focal HIFU therapy for targeted ablation using the Focal One system. A total of twenty-four patients were enrolled where nineteen were treated using focal and five using zonal HIFU. The follow up involved control MRI/TRUS fusion biopsy, change in Prostate-Specific Antigen (PSA), outcomes and complications reported by patients. 20 patients had biopsy at 12 months out of which 8 had positive biopsy within ablation region. No significant reduction in urinary continence was observed but potency had reduced. The study showed that focal HIFU enables local ablation but has limitations. Further studies are needed with strict follow-up procedures, as described in article, to judge the procedure described for prostate cancer therapy. EDAP also developed Ablatherm [152], an FDA approved, fully robotic HIFU device for non-invasive prostate treatment. The treatment starts with MRI and ultrasound fused imaging using the trans-rectal probe. Treatment is planned based on the imaging data after which target area is robotically ablated. It consists of a treatment, a control module and a probe that's designed for both imaging and treatment. The system has automatic safety features such as rectal cooling, detection of patient movement and real time monitoring of rectal wall. Chaussy et al. [153] reported the outcomes from transrectal HIFU therapy performed using the Ablatherm system that involved 65 patients with incidental prostate cancer at 70 years of age. Initial PSA of 4.9 ng/mL was recorded and after therapy, at 1.8 month follow up, lowest value of 0.07 ng/mL of PSA was observed. At mean follow up of 48 months a median PSA of 0.13 ng/mL was recorded. The intraoperative and postoperative side effects were minimum along with mild long-term effects. Overall, the HIFU therapy by Ablatherm has shown great potential after several years of investigation.