1. Introduction
1.1. Background and motivation
Oil recovery from the petroleum reservoirs can be achieved by primary, secondary and tertiary oil recovery methods. Primary and secondary recovery methods, depending on the reservoir characteristics, can only recover about 30–50% of the original oil in place (Alhomadhi et al., 2014, Muggeridge et al., 2014, Alyousef et al., 2017). Hence, the remaining oil in the petroleum reservoir remains the target of any enhanced oil recovery (EOR) operations such as chemical injection, gas injection, thermal oil recovery and microbial enhanced oil recovery. During enhanced oil recovery process, there is an improvement in the oil displacement and volumetric sweep efficiencies. This can be achieved through reduction of oil viscosity, capillary forces, interfacial tension and the development of a favorable mobility ratio between the displacing and the displaced fluid (Wei et al., 2014). This results in the eventual mobilization and the production of a substantial portion of the trapped residual oil in the reservoir at minimum cost (Payatakes, 1982). Gas injection with about 39% contributions to world's EOR remains one of the most commonly used EOR methods in the fields (Almajid and Kovscek, 2016, Alyousef et al., 2017).
During gas injection, hydrocarbon and non-hydrocarbon gases like methane, air, carbon dioxide, natural gas and nitrogen are injected into the reservoirs for the recovery of residual oil (Liu et al., 2011). Carbon dioxide gas injection contributes an estimate of 38% of US EOR production (Singh and Mohanty, 2017a, Singh and Mohanty, 2017b). Gas injection can either be miscible or an immiscible gas flooding. In miscible gas flooding, the gas is injected either at minimum miscibility pressure (MMP) or beyond. Oil recovery is enhanced by the reduction of viscosity and interfacial tension as the injected gas mixes completely with the oil. In immiscible flooding, the injected gas does not mix with the reservoir oil. Reservoir pressure is maintained as the gas injection takes place below the minimum miscibility pressure (MMP) (Shokrollahi et al., 2013). However, any gas enhanced oil recovery process suffers from low areal and vertical sweep efficiencies (poor macroscopic sweep efficiency) because of gas higher mobility and lower density compared to oil (Rossen et al., 2010). This results in gas segregation, gravity override, viscous fingering and severe channeling of the injected gas through the high permeability streaks during gas injection EOR process (Andrianov et al., 2012).
Foamed-gas injection became popular in 1950s in order to mitigate the limitations of gas injection and improved the mobility of injected gas during gas injection EOR (Holbrook, 1958, Sun et al., 2014). Foam can be produced when a foaming agent containing liquid comes into contact with gases such as carbon dioxide, air, nitrogen, and sufficient mechanical energy is supplied that can cause the liquid to foam (Green and Willhite, 1998). Foam in porous media was defined by Falls et al. (1988) as dispersions of gas in liquid such that the liquid phase is continuous and some part of the gas phase is made discontinuous by thin liquid films called lamellae. Foam controls gas mobility by increasing the apparent viscosity of the displacing fluid and reducing the relative permeabilityof the gas phase. The displacing fluid apparent viscosity is increased by drag forces exerted by the moving bubbles on the pore walls while gas relative permeability is reduced by gas trapping. Results of some previous experimental studies revealed that the fraction of the trapped gas in the porous media can be as high as between 50% and 100% (Bernard and Jacobs, 1965, Friedmann et al., 1991, Nguyen et al., 2009). Foams apparent viscosities were also reported to be up to 1000 times higher than that of their constituent phases in some cases (Zhu et al., 2004, Liu et al., 2005). In heterogeneous porous media, foam helps to divert the injected fluid from the high permeability regions to the low permeability un-swept areas by lowering the gas mobility in the high permeability zones (Kovscek and Bertin, 2002, Skauge et al., 2002, Blaker et al., 2002).
Despite these advantages, foams are unstable due to rapid collapse of their thin liquid interfacial films and will require surface active and stabilizing agents for continuous generation and long-time stability (Rio et al., 2014). For enhanced oil recovery (EOR) applications, the generated lamellae should be long-lasting and should be able to translate from pore to pore in the reservoir in the presence of resident brines, oils and at high temperatures (Zhu et al., 2004, Falls et al., 1988, Kam and Rossen, 2003). Stable aqueous foams generation has been achieved using surfactants, polymer and proteins (for food foams) as the conventional foaming and stabilizing agents for several decades (Romero et al., 2002, Murray and Ettelaie, 2004, Romero-Zerón et al., 2010, Samin et al., 2017). It has been demonstrated experimentally that gaseous bubbles can be prevented from coalescing by the adsorption of surfactant, polymers and protein molecules at foam air-water interface (Rossen, 1996, Bournival et al., 2014, Zhang et al., 2015). Polymers can also increase the continuous-phase viscosity and formed a chain-network between droplets (Xu et al., 2017).
However, at reservoir conditions, especially as the foam contacts the resident brines and crude oils in porous media, surfactant-stabilized foam, polymer enhanced foam and protein foams become unstable due to the faster rate of collapse of the thin liquid films at the gas-liquid interface (Zhu et al., 2004, Yekeen et al., 2017b). The stabilizing species possess high tendency to degrade in the reservoir in presence of oil and at high salinity and temperatures and may modify the physical properties of the reservoir rocks (Yusuf et al., 2013, Rafati et al., 2016). For polymer enhanced foam, there is high tendency for the polymer to loss its viscosity-enhancing properties at high temperature and salinity (Kutay and Schramm, 2004). Polymer molecules can also increase up to 10 to 15 times of their original concentration in the aqueous phase in the formation, causing pore throats blockage and formation damage (Emrani and Nasr-El-Din, 2017).
The foamability and stability of the conventional foams has been investigated in previous experimental studies through bulk-scale stability experiments (Farzaneh and Sohrabi, 2015), bubble-scale stability experiments (Osei-Bonsu et al., 2015), and pore scale visualization experiments (Almajid and Kovscek, 2016). Results of these studies showed that oil was very destructive to the static and dynamic stability of surfactant foam irrespective of the surfactant type. In a more complex system when oil is present in porous media, Almajid and Kovscek (2016) found that the snap-off of oil close to the pore throat in the micromodelshindered effective generation of lamellae. This phenomenon was termed “hindered generation”. As a result of high coalescence and rapid destabilizationof the conventional foams. The cost of the foam EOR projects are usually prohibitively expensive and the projects are likely to be uneconomical for large scale applications (Nguyen et al., 2014). The effectiveness of surfactant-stabilized foam is also greatly affected by the high rate of adsorption of surfactant molecules on reservoir minerals and rock surfaces (Lee et al., 2015, Yekeen et al., 2017c).
The principal mechanisms of lamellae destruction and aging process are liquid drainage, coalescence, and coarsening (Fameau and Salonen, 2014, Krzan et al., 2013). The instability of the inter-bubbles films results in bubble breakage and merging of the two smaller bubbles to form larger bubbles due to rupture of liquid films between bubbles (Bubbles coalescence) (Briceño-Ahumada et al., 2016, Langevin, 2017). According to Krzan et al. (2013), foam drainage is the major mechanism of lamellae destruction in aqueous foams due to the influence of gravitational acceleration, viscous force and capillary pressurebetween the adjacent bubbles. In foam coarsening, there is diffusion of gas from smaller bubbles to bigger bubbles because of the higher Laplace pressure (the pressure difference between the inside and outside of any bubble) on smaller bubbles (Saint-Jalmes, 2006, Martinez et al., 2008). Hence, the smaller bubbles vanish with time resulting in an increase in average bubble size (Hilgenfeldt et al., 2001).
Due to the limitations of surfactant-stabilized foam, polymer enhanced foam and protein foams, the use of nanoparticles as foam stabilizing species for EOR applications has recently attracted prodigious attention. The presence of the foam stabilizers (nanoparticles) at the gas-liquid interface of the foam aids in mitigating the limitations of the conventional foams. The advantages of nanoparticles as foam stabilizers has been identified from previous studies as high and sustained stability at reservoir conditions (Khajehpour et al., 2016, Singh and Mohanty, 2015, Singh and Mohanty, 2017a, Maestro et al., 2014). This can be attributed to the irreversible adsorption and aggregation (accumulation) of nanoparticles at the gas-liquid interface of the foams and Plateau borders. The adsorbed nanoparticles improved the foam stability by reducing the direct contact between the fluids to prevents liquid drainage, gas diffusion, and the rate of film rupture and bubbles coarsening (Maestro et al., 2014, Karakashev et al., 2011, Yu et al., 2012a, Li et al., 2016). Compared to surfactants, nanoparticles are less prone to adsorption on reservoir rocks and clay minerals during migration. The results of previous experimental studies showed that nanoparticles can be transported with little retention in porous media and without causing core plugging in the pore throats due to their small sizes (Yu et al., 2012b). Yu et al. (2012b) demonstrated that the equilibrium adsorption of nanoparticles in sandstone, limestone, and dolomite porous media is very low. Their results further showed that the core permeability was not changed during the coreflooding. Their nano-sizes can aid their easy passage through the reservoir pore channels (Zhang et al., 2016a, Zhang et al., 2016b). This will prevent the trapping of nanoparticles in porous media during the flow process of nanoparticles-stabilized foam (Jikich, 2012).
Nanoparticles as foam stabilizing species are solids, hence, the nanoparticles-stabilized foams are expected to be durable and highly resistant to unfavorable reservoir conditions of high salinity, high temperatures and oil presence (Zhang et al., 2009, Yusuf et al., 2013). Nanoparticles can be grafted with different functional groups to improved aqueous stability and obtained the desired wettability for optimum foam generation (Panthi et al., 2017, Singh and Mohanty, 2017b). Nanoparticles surface coating can also be designed to give a better CO2 solvation capability than that of surfactants and their higher adhesion energy to the fluid interface enables them to produce long-lasting foams. Likewise, the possibility of producing nanoparticles from inexpensive and economically viable raw materials like fly ash, sand, limestone and silicaconferred a great economic advantage on use of nanoparticles stabilized foam for enhanced oil recovery (Paul et al., 2007, Worthen et al., 2013a, Singh and Mohanty, 2014). In carbonate and sandstone porous media, the addition of 0.5 wt% nanoparticles to a surfactant solution was found to be effective in enhancing oil recovery (Roebroeks et al., 2015). This was achieved mainly by reducing foam drainage and increased foam apparent viscosity in porous media.
The idea of foams and emulsions stabilization by particles was first conceived by Ramsden in 1903 and Pickering in 1907. Ramsden (1903) and Pickering (1907) found that durable emulsions and foams can be formed by the adsorption and accumulation of colloidal particles at the liquid-liquid interface of macro-emulsions and gas-liquid interface of foams. Since then, particles-stabilized foams has found extensive applications in minerals floatation, the pharmaceutical industry, fire-fighting as fire retardants, food and beverages industries (Murray et al., 2011). However, until the advent of nanoparticles, the application of particles-stabilized foams for enhanced oil recovery has been limited. This can be attributed to the fact that most colloidal particles are in micron-sizes and are not appropriate as stabilizing species for foams applications in the oil producing reservoirs. The tendency of the micron-sized particles to be retained within the pore bodies and pore throats, causing core plugging and blocking the pore throats in the oil-producing reservoir is very high (Eftekhari et al., 2015).
1.2. The objective of the review and its novelty
A number of review papers on particles-stabilized foams has been written within the last decades. Hunter et al. (2008) investigated the functions of particles in conventional foam stability. Their review shows that particles-stabilized foam stability is governed by particle-interface and particle-surfactant interactions. Fameau and Salonen (2014) reviewed the influence of particles on foam stability and the mechanisms of foam aging process. They reported that the presence of solid particles independently and in presence of surfactants, proteins, and polymers can helps in creation of long lasting foam stability by their aggregation at the foam Plateau border or by fluid viscositymodifications. The mechanism of foam lamellae stabilization by solid particles was discussed by Kruglyakov et al. (2011). They found that foam stability is influenced by changing particles contact angle, shear stress and surface tension. Murray and Ettelaie (2004) reviewed the influence of different nanoparticles types and protein on foam stability. They concluded that the competing influence of non-protein nanoparticles and protein nanoparticles on foam stability is not yet explicit.
Despite these previous reviews, and the availability of several research articles on experimental studies of nanoparticles-stabilized foam for EOR applications, no previous attempts has been made for a comprehensive review of the laboratory experiments of nanoparticles-stabilized foam for EOR applications. This is the first attempt to summarize the existing research articles on experimental studies of nanoparticles-stabilized foam for EOR applications. This review provides information on the current status of nanoparticles application for foam stabilization, highlighting the findings, the challenges and directions for future studies. An in-depth review of previous studies on the experimental methods and parameters influencing the foam static stability and dynamic performance in porous media has been conducted. Authors ideas based on the results of their experimental studies are also discussed, compared and contrasted with the results in literature. The manuscript proceeds with the review of experimental techniques that have been used to study nanoparticles-stabilized foam static and dynamics stability followed by a an overview of the mechanisms of foam stabilization by nanoparticles. We then reviewed the influence of different parameters on the foam static and dynamic generation, stability and propagation in porous media. Recent modelling works and challenges of field applications of nanoparticles-stabilized foam were also discussed. A critical assessment of the findings, the challenges and limitations of the previous and present study are presented. Areas that has not been extensively studied are highlighted while the directions for further research are suggested.
2. Experimental techniques
Three major techniques are normally used for the experimental study of conventional foams for mobility control and enhanced oil recovery applications. These techniques includes: bulk and bubble-scale stability experiments, macroscopic experiments using consolidated and unconsolidated porous mediaand the pore scale visualization experiments. Similar methods have been adapted for laboratory research of nanoparticles-stabilized foams. An extensive review of these techniques for the nanoparticles-stabilized foams, highlighting the process, their advantages and limitations have been conducted in this section.
2.1. Bulk-scale stability experiments
The bulk static stability experiments are normally conducted with graduated transparent cylinder and calibrated foam column equipments (with different sizes of porous stone as gas diffuser), foamscan instrument, laboratory blender and high pressure viewing cell. The foam is produced as the nanoparticlesdispersion comes into contact with gases such as carbon dioxide, air, nitrogen, and sufficient mechanical energy is supplied. The energy can be supplied in form of shearing and shaking (Alargova et al., 2004, Cui et al., 2010, Stocco et al., 2011b, Singh and Mohanty, 2015, Eftekhari et al., 2015, Maestro et al., 2014, Li et al., 2016, Lee et al., 2015) stirring (Sun et al., 2014) and bubbling by gas sparging (Carn et al., 2009, Karakashev et al., 2011, Singh and Mohanty, 2017a). The foamability is taken as the height of the foam in the transparent foam column while the foam stability can be determined from the foam decay height as a function of time and the foam half-life. The foam half-life is the time taken to reach half of the foam original height after generation. The foam stability is also evaluated from drainage half-life and the rate of liquid drainage, that is, the time taken from the liquid to drain from the foam or the amount of liquid that drained per time. The time taken for half of the water to drain from the foam is the drainage half-life (Yang et al., 2017b).
The normalized foam height (expressed in equation (1)) is normally used to complement the results of foam stability determination from the foam half-life (Zhang et al., 2008a, Yekeen et al., 2017a)(1)
Foams were generated in Plexiglas column in Carn et al. (2009) studies by bubbling nitrogen gas through a porous glass disk with varying porosity ranging from 40 to 90 μm, 90–150 μm and 150–200 μm. The foams for the static stability experiments were generated by Sun et al. (2014) and Li et al. (2016) by stirring SiO2/SDS dispersions in a warning blender for 3 min at 8 000 rpm.
Yang et al. (2017b) generated AlOOH/SDS stabilized nitrogen foam by stirring the foaming dispersions in Waring blender at 6 000 rpm for 3 min. Singh and Mohanty (2015) generated foam through vigorous shaking techniques (10 times handshaking) of SiO2/AOS dispersions while Eftekhari et al. (2015) generated foam through vigorous shaking of nano-ash-AOS suspension in test tubes. In Singh and Mohanty (2017a) studies, foam was generated in a graduated acrylic cylinder by gas sparging through a stainless steel porous frit. Similar methods of static foam generation was adopted in our studies as reported in Yekeen et al., 2017a, Yekeen et al., 2017b. Foams were generated in a tempered glass column by gas sparging through a porous filter plate (40–100 . The foamability and stability were studied with a computer controlled Krüss dynamic foam analyzer.
2.2. Applications and limitations of the bulk-scale stability experiments
The bulk-scale stability experiments are static stability tests used to screen different foaming/stabilizing agents in order to choose the optimum foaming/stabilizing schemes for maximum foam generation, propagation and stability in porous media. The experiments provide useful information on the influence of various critical parameters such as oil presence, salinity, nanoparticles concentration on foam static generation and stability. However, the results of static stability experiments are not appropriate for describing foam flow in porous media since the structure, lamellae thickness and aging process of bulk foams are not the same as that of foam in porous media. Foam generation in presence of nanoparticles required higher energy inputs. This has been attributed to the need to overcome the adsorption barriers during the production of nanoparticles-stabilized foams (Espinoza et al., 2010, Stocco et al., 2011a, Stocco et al., 2011b). Hence, there exists a critical shear rate that should be exceeded for the generation of nanoparticles-stabilized foams. A flowratehigher than this critical shear rate is required for successful foam generation. This high energy inputs are normally supply in form of energetic shaking and turbulent mixing during bulk stability experiments. A table summary of the required shear rate for successful generation of nanoparticles-stabilized foam from the results of previous studies are presented in Table 1. The static and dynamic stability experiments are usually conducted at constant shear rate (expresses in form of combined flowrate of gas and the foaming dispersions). The use of constant flow rate can affects the accuracy of the experimental results since the critical shear rate is expected to increase as the experiments progresses due to the aggregation of the nanoparticles which can block the pore spaces of the foam generator during the experiments. The attainment of this threshold shear rate in the oilfield is also very challenging. The threshold shear rate required for nanoparticles-stabilized foam generation may be higher than the practical injection rates during pilot and oilfield trials (Shamsijazeyi et al., 2014).
Table 1. Summary of previous studies showing used and investigated shear rate/total flowrate for foam generation.
| Source | Size (nm) |
Nanoparticles Type (Surface modifications or surfactants) |
Conditions (T,P, Salinity, Quality) |
Shear viscosity (Shear rate or Total flowrate) |
|---|---|---|---|---|
| Espinoza et al., 2010 | 5 nm | SiO2 (PEG-coated) | 95 °C, 1 350 psia, 2 and 4% NaCl | 1300 S−1 |
| Yu et al., 2012a |
20 nm (100 nm after Dispersion) |
SiO2 |
25 °C, 1 500 psig DI water, NaCl |
1419S−1 to 3312S−1 (foam generation occurred above 1419S−1) |
| Worthen et al., 2013a |
5–30 nm (196 ± 19 nm HDD in DI water) |
SiO2 (50% SiOH) | 50 °C, 2 800 psi, DI water, Q = 0.75 | 7.25 cP (1 200 S−1) |
| Worthen et al., 2013a |
5 nm (5.5 ± 0.13 nm HDD in DI water) |
SiO2 (3% Short-chain PEG-coated) | 50 °C, 3 000 psi, DI water, Q = 0.90 | 15.7 cP (580 S−1) |
| Worthen et al., 2013b | 28 nm |
1% SiO2 (0.5% CAPB) |
50 °C, 2 800 psi, 1% NaCl, Q = .75 |
40 cP (580 S−1) |
| Xiao et al., 2016 | NP1 (15–20 nm) NP2 (60–70 nm) |
0.5 wt % SiO2 (0.01 wt % ANS) |
40 °C, 1 140 ± 20 psig,0, 2 and 5% NaCl, Q = 0.50, 0.60, 0.70 and 0.80 |
5 950 S−1, 8 925 S−1 11900 S−1, 14875 S−1, 17850 S−1 |
| San et al., 2017 | 17-20 nm | SiO2 | 25 °C, 40 °C and 65 °C 1 500 psi, NaCl (1, 5 and 10%) CaCl2(0.1, 0.3, 0.5 and 1.0%) | 800 cm3/h |
*Polyethylene glycol (PEG), caprylamidopropyl betaine (CAPB), hydrodynamic diameter (HDD), nanoparticles (NP), proprietary anionic surfactant (ANS).
2.3. Bubble-scale stability experiments
In some cases, especially in presence of oil, the foam decay process can be erratic that the observed foam height might not be a true representative of the total foam height (Osei-Bonsu et al., 2015). Hence, the bubble-scale stability experiments are usually conducted to complement the results of the static-stability experiments. For nanoparticles-stabilized foam, the bubble-scale stability experiments are also conducted to understand the mechanisms of foam generation and stability from the bubble-size distribution and the bubble-scale dynamics. The results of the bubble-scale stability experiments are compared with the bulk-scale stability results in order to fully understand the physical phenomena and governing mechanisms of nanoparticles-stabilized foam generation, stability and decay. In several cases, the foam micro-bubbles were allowed to stabilize and the bubble suspension were placed on a microscope slide, the foam texture, bubbles morphology and the thin liquid films were then observed. The foam texture is described by the number of lamellae per unit volume (Alyousef et al., 2018). The bubble size distribution and average diameter are usually measured with the image analysis software (Image-J). The micrographs are taken using different microscopes such as Nikon microscope equipped with a high-resolution camera (Singh and Mohanty, 2015), an Olympus BX-61 microscope equipped with long working distance objectives (Alargova et al., 2004), Motic BA400 microscope system (Cui et al., 2010), confocal laser scanning microscopy (Singh and Mohanty, 2017a) and Olympus microscope (Maestro et al., 2014). In some experiments, the nanoparticles-stabilized foam morphology and bubble size distribution were also investigated with the aid of Cryo-TEM and optical microscope (Martinez et al., 2008), high pressure and high temperature visual cell (Khajehpour et al., 2016, Li et al., 2016) and sapphire observation tube (Yu et al., 2014). Leica EZ4 HD stereo microscope was used to observe the foam morphology and the bubble size distributions in our previous studies (Yekeen et al., 2017b) while (Alyousef et al., 2018) study the nanoparticles-surfactant stabilized N2-foam bubble-size distribution using Nikon Stereo Photomicroscope.
Xue et al. (2016) introduced the concept of “Sauter mean diameter” and “Polydispersity” to quantify nanoparticles-stabilized foam bubble size distribution. The foam was generated in the glass bead pack and the micro-bubbles were observed on the microscopy cell (at the outlet of the glass bead pack). The bubbles morphology was captured with camera and the images were analyzed using Image J. Sauter mean diameter was calculated with equation (2)while polydispersity was calculated with equation (3)(2)(3)Where and represents Sauter mean diameter and Polydispersityrespectively. is the diameter of the foam bubble while stands for the median of the volume averaged bubble diameter in the foam. The bubble size and size distribution were averaged over at least 100 bubbles in Xue et al. (2016) studies. The smaller Sauter mean diameter signifies high foam stability and low rate of bubble coalescence and coarsening. The insignificant change in bubbles polydispersity showed that the most of the nanoparticles-stabilized bubbles shape remain uniform with time compared to the polyhedral shape of the LAPB foam.
2.4. Applications and limitations of the bubble-scale stability experiments
Generally, it is difficult to observe the nanoparticles adsorption and accumulation at the lamellae of the foam since the resolution of most microscopes is in the order of microns. It was also difficult to quantify the rate of liquid drainage from the micro-bubbles on the microscope slides. Hence, the bubbles morphology as function of liquid drainage could not be determined in several cases. Likewise, the foam generation and coalesce rate of the foam under the microscope might not be a true representation of the process taking place in the bulk foam since the portion on the micro-slide is just a small representative of the whole foam structure. However, using a confocal laser scanning microscopy, Singh and Mohanty (2017a) observed the presence of nanoparticles at the thin liquid films between air bubbles which enhanced the foam stability in presence of nanoparticles. In our studies (Yekeen et al., 2017b), the foam morphology under the Leica EZ4 HD stereo microscope revealed that the lamella width of the nanoparticles-surfactant stabilized was thicker than that of the surfactant stabilized foam. In consistent with Xue et al. (2016)studies, the shape of the SiO2/SDS foam were uniform and mainly exists in form of regular sphere or circle while that of the SDS-stabilized bubbles are irregular and are mainly in form of polyhedral (Sun et al., 2014, Yekeen et al., 2017b).
2.5. Macroscopic experiments
To obtain quantitative information about nanoparticles-stabilized foam performance in porous media, various kind of porous media such as glass bead packs, sand packs, sandstone and carbonate reservoir cores have been employed. The porous media were either consolidated or unconsolidated and were packed in either a see-through or opaque tubes. The experiments were conducted at room conditions or at different reservoir and simulated field conditions. The foams can be pre-generated or in-situ generated through the generation process of co-injection (simultaneous injection of gas and foaming agent solution) or alternative injection of gas and slugs of foaming agents’ dispersion. Foam propagation, stability and mobility were estimated from the oil recovery and pressure drop across the porous media determined with the aid of different kinds of pressure and differential pressure transducers. The pressure build up along the portions of the porous media indicates foam generation and extent of reductions in gas mobility (Khajehpour et al., 2016). Higher pressure drop signifies viscous foam and considerable resistance to gas mobility in porous media. Alyousef et al. (2017) obtained a steady state pressure drop of 100 psi during the flow process of nanoparticles-surfactant foam compared to 88.5psi obtained during the flow process of surfactant-stabilized foam.
The pressure drop data can be used for calculating foam mobility, apparent viscosity, normalized viscosity and mobility reduction factor (Aroonsri et al., 2013, Khajehpour et al., 2016, Yu et al., 2014, Alyousef et al., 2017). Apparent viscosity during foam flow in the bead pack tube is calculated from Darcy's Law, equation (4) (Aroonsri et al., 2013, Yu et al., 2014). Apparent viscosity during foam flow in the capillary tube is calculated using Hagen-Poiseuille equation for a Newtonian fluid in laminar flow, equation (5), (Xue et al., 2015). Apparent viscosity during foam flow in the 2D Hele-Shaw cell is calculated using the plane-Poiseuille equation (6) (Yan et al., 2006, Osei-Bonsu et al., 2016, Yekeen et al., 2017b).(4)(5)(6)
In equations (4), (5), (6) represents the foam apparent viscosity, stands for permeability, is the pressure drop, is the length and A is the crossectional area while is the volumetric flow rate. For the 2D Hele-Shaw Cell, b is the gap thickness, is the pressure gradient across the Hele-shaw cell, is the superficial velocity of the foam while = represents permeability. The ratio of the pressure drop across the core owing to foam flow to the pressure drop due to single-phase gas flow is the mobility reduction factor (MRF) (Singh and Mohanty, 2015). For example, for the nanoparticles-stabilized CO2 foam, the MRF can be calculated from equation (7) (Aroonsri et al., 2013, Kim et al., 2016, Emrani and Nasr-El-Din, 2017):(7)Where stands for the experiment during foam flow while and stands for the experiment during the gas flow.
Another very important parameters that is used to describe nanoparticles-stabilized foam stability and performance in porous media is the interfacial viscoelastic modulus. The interfacial viscoelastic modulus describe the response of an interface to local contraction or expansion (Li et al., 2017). It signifies the ability of bubble to resist external disturbances, deformation and coalescenceduring film stretching. The interfacial viscoelastic modulus (in mN/m) is expressed as (Li et al., 2017).(8)Where is the surface tension, in mN/m and A is surface area, in m2. The interfacial elasticity of the liquid film is directly proportional to the interfacial viscoelastic modulus of the liquid. Stable foams are formed at high interfacial elastic modulus due to the high mechanical strength and high elasticity of the liquid film (Li et al., 2017). Previous studies show that the nanoparticles-stabilized bubbles were able to maintain and recovered their spherical shape after being stretched through the pore channels in porous media due to their enhanced films interfacial viscoelasticity (Ravera et al., 2006, Wang et al., 2008, Sun et al., 2014, Lv et al., 2015, Yekeen et al., 2017d).
2.5.1. Literature results
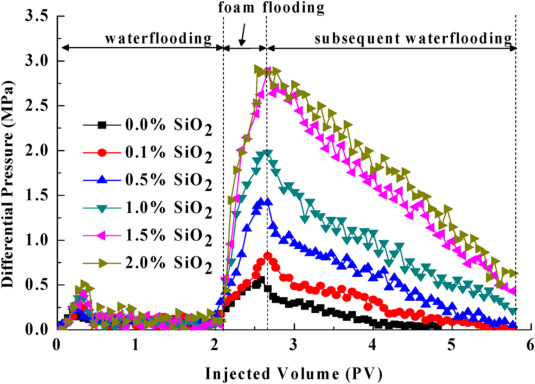
Sun et al. (2014) used sand pack models (packed with silica sands of different permeability) for their macroscopic studies of SiO2/SDS foam performance and stability in porous media. The foam was pre-generated in silica sand pack model of diameters 70 μm−100 μm. The foam performance was estimated as function of differential pressure increase and profile control effect. The plots of the pressure drop with respect to the pore volume of the displacing fluid injected is shown in Fig. 1. One can see that the pressure drop increases in presence of nanoparticles and with increasing nanoparticles concentrations signifying reduction in mobility of the injected fluid. Subsequent injection of water after foam flooding resulted in diversion of the injected water into oil trapped un-swept zone. In the presence of nanoparticles, more oil was recovered from the low permeability layer compared to the high permeability layer as shown in Fig. 2. Nanoparticles-surfactant foams were generated in Singh and Mohanty, 2014, Singh and Mohanty, 2015 studies by co-injection of the foaming dispersions and nitrogen gas through a Berea sandstone cores at a fixed foam quality. The mobility reduction factors (MRF) determined from the measured pressure drop showed that foam stability in absence and presence of crude oil was promoted by nanoparticles. In the absence of oil, there was an increased in mobility reduction by a factor of 2 in presence of 0.3 wt % nanoparticles concentration.
 Fig. 1. Plots of pressure difference as a function of nanoparticles concentration during SiO2/SDS foam flooding showing increasing pressure drop in presence of nanoparticles (Sun et al., 2014).
Fig. 1. Plots of pressure difference as a function of nanoparticles concentration during SiO2/SDS foam flooding showing increasing pressure drop in presence of nanoparticles (Sun et al., 2014). Fig. 2. Improvement in oil recovery in presence of nanoparticles and the recovery of oil from the high and low permeability layer by SiO2/SDS foam (1.5 wt% SiO2 and 0.5 wt% SDS concentration was used) (Sun et al., 2014).
Fig. 2. Improvement in oil recovery in presence of nanoparticles and the recovery of oil from the high and low permeability layer by SiO2/SDS foam (1.5 wt% SiO2 and 0.5 wt% SDS concentration was used) (Sun et al., 2014).In sand pack models, Li et al. (2016) observed an increase in pressure difference and improved oil recovery after waterflooding at optimum SDS/SiO2concentration ratio of 0.17 during the SDS/SiO2 foam flow process. In Worthen et al., 2013a, Worthen et al., 2013b studies, the nanoparticles-stabilized CO2foam was pre-generated in spherical glass beads packed model (180 μm). The differential pressure across the capillary tube was determined as the generated foam flow through the capillary tube and the foam apparent viscosity was calculated using the Hagen– Poiseuille equation (equation (5)). Similarly, Yu et al. (2014) observed a significant reduction in CO2 gas mobility in presence of nanoparticles. The CO2 foam apparent viscosity was calculated from the pressured drop during the foam flow process in the capillary tube. Nanoparticles-stabilized CO2 foam was generated by Aroonsri et al. (2013) by shearing the aqueous nanoparticle dispersion and the CO2 gas in bead pack filled tubes. Foam flow through fractured and unfractured Boise and Berea sandstones cores and Indiana limestone cores was monitored. Pressure drop across the cores and capillary tube was determined to calculate the foam apparent viscosity and mobility reduction factor (MRF). Their results showed that there is no significant trapping of nanoparticles during the nanoparticles-stabilized CO2 foam flow process in fractured and unfractured Boise sandstone cores. They also observed the existence of threshold shear rate for foam generation in unfractured and fractured Boise sandstone cores and the diversion of flow from the fracture into the matrix zone when foam is generated in both zones. The calculated MRF was found to increase with the increasing shear rate.
Eftekhari et al. (2015) found that the nano-ash-stabilized foam demonstrated higher apparent viscosity in Bentheimer sandstone core compared to the conventional foams. Alyousef et al. (2017) recently investigated the performance of SiO2 nanoparticles and anionic surfactant foam in Bentheimer sandstone porous media and found that the nanoparticles addition into the foaming solution resulted in recovery of 49.05% of OOIP compared to 41.45% of the OOIP recovered in absence of the nanoparticles. Recently too, attempts have been made to use transparent front plate glass bead packs and sand pack models to visualize the flow diversion mechanisms by nanoparticles-stabilized foams in a layered heterogeneous porous media (Singh and Mohanty, 2017a, Singh and Mohanty, 2017b). Using a transparent two-layer heterogeneous 2D sandpack model, the flow diversion and displacement mechanisms by SiO2-AOS foam was studied by Singh and Mohanty, 2017a, Singh and Mohanty, 2017binvestigated the performance of foams stabilized by PEG-coated and GLYMO-coated nanoparticles and surfactant at high temperature and high salinity conditions.
The foam stability in terms of its potential to divert the accompany fluids to low permeability un-swept layer was observed in Singh and Mohanty, 2017a, Singh and Mohanty, 2017b experiments. The permeability contrast of the layered model was calculated as 6:1. Singh and Mohanty (2017a) results showed a higher sweep efficiency by the SiO2-AOS foam (Fig. 3) in terms of the injected pore volume, and an improvement in the foam resistance factor by a factor of 1.95 compared to the surfactant-stabilized foam. The dominant mechanisms controlling the crude oil displacement process during the foam flow process in the layered model were identified as: the occurrence of foam flow diversion from high-permeability layer to the low permeability layer, attributed to the increased foam strength in the high permeability layer (Fig. 4a), and the flow of oil from the low-permeability layer to the high-permeability layer (Fig. 4b). They proposed that these two-major cross flow characteristics should be capture during simulation studies. However, in Singh and Mohanty (2017b)study, cross-flow of oil from the low-permeability layer to the high-permeability layer was only observed during surfactant-stabilized foam flooding. There was no cross-flow during the flow-process of GLYMO-coated nanoparticles/surfactant foam. This can be attributed to the effective plugging and total blocking of the high permeability layer by the foam due to their superior stability. The summary of some selected literature on the macroscopic studies of nanoparticles-stabilized foams is presented in Table 2.
 Fig. 3. Plots of % sweep efficiency versus injected pore volume showing the high sweep efficiency of SiO2-AOS foam compared to the AOS-foam and waterflooding (Singh and Mohanty, 2017a).
Fig. 3. Plots of % sweep efficiency versus injected pore volume showing the high sweep efficiency of SiO2-AOS foam compared to the AOS-foam and waterflooding (Singh and Mohanty, 2017a). Fig. 4. Occurrence of (a) diversion of foam from high-permeability layer to low-permeability layer and (b) the flow of oil from the low-permeability layer to the high-permeability layer during SiO2-AOS foam flow in a layered, heterogeneous porous media (Singh and Mohanty, 2017a).
Fig. 4. Occurrence of (a) diversion of foam from high-permeability layer to low-permeability layer and (b) the flow of oil from the low-permeability layer to the high-permeability layer during SiO2-AOS foam flow in a layered, heterogeneous porous media (Singh and Mohanty, 2017a).Table 2. Summary of previous macroscopic studies of foam performance in presence of nanoparticles.
| Nano-particles types |
Surface modification/surfactant |
Sizes (nm) | Experimental conditions | Foam generator/Porous Media Details | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| temperature | pressure | salinity | Types | K | |||||
| SiO2 | PEG coated | SiO2 |
50 °C, 75 °C 90 °C, 95 °C |
1 350–1 400 | NaCl | glass beads pack, | – | – | Espinoza et al. (2010)/SPE129925 |
| Psia. | (2–4%) | Capillary tube | |||||||
| SiO2 | – | 12 nm, |
25 °C 60 °C |
1 200- 2000 psia |
NaCl (0.5%, 2.0%, 5.0%) |
Sapphire observation tube | – | – |
Yu et al. (2012c) CMTC 150849 |
| 100- | |||||||||
| 150 nm | |||||||||
| SiO2 | – | 17 nm | 25 °C | 1,200psig, | NaCl | Berea sandstone core |
33. 01md |
17. | Mo et al. (2012) |
| 1 500 psig | (2.0%) | 36% | SPE 159282 | ||||||
| SiO2 | 50% DCDMS capped, Tergitol™ 15-S-20 | 100-200 nm | 35 and | 1 200-3000psia | – | Glassbead pack, | – | – | Worthen et al. (2012)/SPE 154285 |
| PEG coated | 6 nm,5 nm | 50 °C | Capillary tube | ||||||
| SiO2 | – | 20 nm | 25 °C | 1500psia | NaCl |
Glassbead pack, Capillary tube |
– | – | Yu et al. (2012a) |
| 100 nm | Sapphire cells | SPE 153336 | |||||||
| SiO2 | PEG coated | 5 nm | 50 °C | 2000 Psia. | NaCl brine | Glass bead pack | 1800md | 22-29% | Aroonsri et al. (2013)/SPE 166319 |
| (3M-PEG) | 10 nm | 2 800 psia | Boise Sandstone | 200 md | |||||
|
Proprietary coated (EOR-5XS and EOR-12) |
Berea sandstone Indiana limestone | 7 md | |||||||
| SiO2 | – | 17-20 nm | 20 °C | 1200 psig. | NaCl (2%) | Berea sandstone core | 33 md | 0.174 | Yu et al. (2013) |
| 270 md | 0.205 | SPE 164074 | |||||||
| SiO2 | DCDMS capped | 5-30 nm, | 50 °C | 1 200-3000psia | NaCl | Bead pack model | 22.5d | 0.34, | Worthen et al. (2013a) |
| PEG coated |
(5,5nm 165- 196nm) |
(0, 1 & 8%) |
Capillary tube View cell |
– | 1 | ||||
| SiO2 | CAPB | 28 nm | 50 °C | 19.4mpa | NaCl | Bead Pack model | 22.5d | 0.34 | Worthen et al. (2013b) |
| (0–3%) | Capillary tube |
000d |
|||||||
| SiO2 | DCDMS capped | 12 nm,10 nm?0 nm | 25 °C | 1200psig | NaCl | glass beads column, | 25.04d | 36.82% | Yu et al. (2014) |
| 2% | capillary tube | ||||||||
| SiO2 | – | 17-20 nm | 25 °C -60 °C | 1 200- 2500psi | NaCl | Sandstone core | 270md, | 0.205 |
Mo et al. (2014) SPE-169110-MS |
| (2%) |
Limestone core Dolomite core |
106md, 295 md |
0.184 0.185 |
||||||
| TTFA nanoparticles |
Anionic, cationic & non-ionic |
80 nm. | Room temperature |
100 psi (backpressure) |
NaCl (1%) |
Berea sandstone core | 300 md | 18.9% | Singh et al. (2015) |
| SPE-175057-MS | |||||||||
| SiO2 | PEG coated | 10 nm (20 nm) | Room temperature | 100 psi | NaCl (1, 2, 4, | Berea sandstone cores | 357 md | 0.235 | Singh and Mohanty (2015) |
| /AOS | (backpressure) | 6, 8, & 10 | Foam flow exp. | 313 md | 0.223 | ||||
| wt%) | Retention exp. | 383 md | 0.213 | ||||||
|
core flood 1 core flood 2 |
315 md | 0.229 | |||||||
| SiO2 | AOS (0.5 wt%) | SiO2 | Room temperature | – |
NaCl (2 wt%) |
sand packs models | Manan et al. (2015) | ||
| Al2O3 | (20–30 nm) | ||||||||
| CuO | Al2O3,CuO | ||||||||
| TiO2 | (40 nm) | ||||||||
|
TiO2 (10–30 nm) |
|||||||||
|
Fly ash nano- particles |
AOS | 100-200 nm | – | – | NaCl (0.5,1&5 wt % | Bentheimer sandstone core | 2.0 d | – | Eftekhari et al. (2015) |
|
CaCl2(0.9,1.9 9.5 %) |
|||||||||
| SiO2 | – | 5, 12, 25, and 80 nm | 70 °C | 2 200 psi. |
NaCl (8%) & CaCl2 (2%) |
Boise sandstone Sandpacks |
2.6–4.8 d, 55d |
0.275–.317 0.42. |
Kim et al. (2016) |
| Alumina-coated silica nanoparticles | Triton CG-110, | 20 nm | Room temperature |
100 psi (backpressure) |
– | Berea sandstone cores | 606 md | 22% | Singh and Mohanty (2016) |
| AOS | foam flow | 442 md | 20% | ||||||
| core flood 1 | 585 md | 22% | |||||||
|
core flood 2 core flood 3 |
125 md | 18% | |||||||
| colloidal SiO2 |
LAPB AOS |
– | 50 °C | 3 000 psia | KCl (0–3%) |
glass bead pack capillary tube viscometer |
23 d | – | Xue et al. (2016) |
| SiO2 | Surface | 5 nm | 70 °C | 2200psi | NaCl (8.53%) | Boise sandstones | 4676md | 0.32 | Lotfollahi et al. (2016) |
| treated | CaCl2 | Experiment 1 | 3301md | 0.30 | SPE-179664-MS | ||||
| (EOR-5XS) | (1.11%) | Experiment 2 | 2800md | 0.30 | |||||
|
Experiment 3 Experiment 4 |
2599md | 0.29 | |||||||
| Bare SiO2 | Hexylamine | 14–20 nm | 25 °C | – | – | sandpack models | 1968md | 38.1% | Sun et al. (2017) |
| Model no 1 | 1931md | 37.8% | |||||||
| Model no 2 | 1890md | 41.2% | |||||||
| Model no 3 | 1901md | 36.7% | |||||||
|
Model no 4 Model no 5 |
1958md | 40.4% | |||||||
| PECNP | Surfonic N120 | 140.47- | 40 °C | 1 300 psi and | KCl | Indiana limestone | 292 md | 0.19 | Kalyanaraman et al. (2017) |
| 442 nm | 1800 psi, | (2 wt %) | Core #1 | 356 md | 0.16 | ||||
|
Core #2 Core #3 |
387 md | 0.17 | |||||||
| SiO2 | AOS/PEG coating | 10 nm (20 nm) | Ambient conditions | – | NaCl | 2D heterogeneous layered sandpack model | 14 d | 30%, | Singh and Mohanty, 2017a, Singh and Mohanty, 2017b |
| 55 °C & 75 °C | (1, 2, 4, 8 wt %) | 22.6 d (top layered) | |||||||
| (static stability) | 3.8 d (bottom layer) | ||||||||
| SiO2 | – | 7–20 nm. | 25° C, 40° C, and 65° C | 1500 psi. | NaCl | Berea Sandstone 1 | 203 md | 0.195 | San et al. (2017)SPE 179628 |
| 1-10% | Berea Sandstone 2 | 200 md | 0.193 | ||||||
|
CaCl2 0.1–1.0% |
Berea Sandstone 3 | 192 md | 0.191 | ||||||
|
SiO2 and Fe2O3 |
AOS | SiO2 (100 nm & | 77 oF- 250 °F | Atmospheric to 800 psi | NaCl | Buff Berea sandstone cores | 120 md | 20% | ) (SPE-185551-MS and SPE 174254) |
| 140 nm) | (1–10 wt %) | −170md | |||||||
|
Fe2O3 (50 nm) |
|||||||||
| SiO2 | Ligands grafted proprietary. | <50 nm | 25, 60, and 70 °C 80 °C | 3 000 psig | 15% TDS brine (8.5% NaCl, 4.3% CaCl2, and 2.2% | Berea and Boise sandstones | 281md | 0.26 | Alzobaidi et al. (2017) |
| Experiment 1 | 4500md | 0.35 | |||||||
| Experiment 2 | 2200md | 0.32 | |||||||
|
Experiment 3 Experiment 4 |
1500md | 0.32 | |||||||
|
colloidal SiO2 |
silane modified/.AOS | 23.3 nm |
Room temperature |
Room pressure | – | water-wet Bentheimer sandstone | 1.4–2.5 D, | 20%–24% | (Rognmo et al., 2017) |
| multi walled carbon nanotube (MWCNT) | Tergitol 15-s-40, anionic alpha olefin sulfonate |
OD 10 nm, ID 4 nm, and tube length of 2 nm |
25 °C | – | 3% salinity (NaCl, 2.4 wt% CaCl2, 0.6 wt %). | Ottawa sandpack | 4 D | 37.5% | Wang et al. (2017) |
|
AlOOH nano- particles |
SDS CTAB |
around 100 nm |
22 ± 2 °C 60 °C. |
– | – | Sandpacks | 2.7 ± 0.2 D | 35.5 ± 0.2% | Yang et al., 2017a, Yang et al., 2017b |
| surface modified SiO2 |
alpha olefin sulfonate, isopropyl alcohol & citrus terpenes |
30 nm. | 25 °C and 50 °C. | 1 200 psi | 1% NaCl | Bentheimer sandstone |
1.61D 1.47D 1.55D 1.69D |
22.08% 20.79% 21.84% 20.55% |
Alyousef et al. (2017) |
| Surface modified SiO2 | PEG and | 20 nm | 25 °C, 60 °C and 80 °C | 110psi | 1,2,4,6 and 8% NaCl | 2D Heterogeneous sandpack | 22.6 D (top layer) | 31% | Singh and Mohanty, 2017a, Singh and Mohanty, 2017b/SPE-187165-MS |
| GLYMO | 12 nm | API brine (8% NaCl and 2% CaCl2) | 3.8 D (bottom layer) | ||||||
| 15.7 D combined | |||||||||
* Dichlorodimethylsilane (DCDMS), polyethylene glycol (PEG), Thermally-treated fly ash nanoparticles (TTFA), polyelectrolyte complex nanoparticles (PECNP), laurylamidopropyl betaine (LAPB) surfactant, dodecyltrimethylammonium bromide (DTAB) and caprylamidopropyl betaine (CAPB). (3-Glycidyloxypropyl) trimethoxysilane (GLYMO), cetyltrimethylammonium bromide (CTAB).
2.5.2. Results of our macroscopic experiments
In our macroscopic experiments, flow diversion and oil displacement mechanisms of SiO2-SDS foam was investigated in visual layered glass bead pack models. The layered model was filled with two different sizes of glass beads (90–150 μm) for the lower permeability layer and (600–850 μm) for the higher permeability layer which resulted in a permeability contrast of 8:1. The foam injection and fluid injection into the model was conducted at flowrate of 3ml/min-6ml/min. The experiment was conducted to observe the fluid diversion process by SiO2-SDS foam in crude oil-filled macroscopic model. Fig. 5a shows the image of the macroscopic model fully saturated with the Malaysian crude oil (with viscosity of 10.016 cp and density of 0.8283 g/cm3). Waterflooding (0.5 wt % NaCl) was conducted to recover the residual oil from the macroscopic model. Fig. 5b shows that the upper layer of the model was only efficiently swept after the injection of several pore volume of water. However most of the residual oil was still trapped in the lower permeability layer of the model with the breakthrough of injected water at the production outlet.
 Fig. 5. aCrude oil saturated high and lower permeability layer of the macroscopic layered model, b. Waterflooding of the model resulted in recovery of oil from the upper permeability layer only, and c. SiO2-SDS foam flooding of the layered macroscopic model. The oil is dark brown, the foam is white while the water is colored blue for visual observation (result of our macroscopic experiments). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5. aCrude oil saturated high and lower permeability layer of the macroscopic layered model, b. Waterflooding of the model resulted in recovery of oil from the upper permeability layer only, and c. SiO2-SDS foam flooding of the layered macroscopic model. The oil is dark brown, the foam is white while the water is colored blue for visual observation (result of our macroscopic experiments). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)Fig. 5c shows that with the injection of pre-generated SiO2-SDS foam into the macroscopic model after water breakthrough. Most of the water and the incoming foams into the upper permeability layer was diverted into the lower permeability layer where the oil saturation was higher. This resulted in improved sweep efficiency during the flow process of the SiO2-SDS foam. The results is consistent with the findings of Singh and Mohanty (2017a) that, there is a cross-flow of foam from the high permeability layer to low permeability layer during the flow process of nanoparticles-stabilized foam due to the high stability of the foam. The cross-flow observed in our study was so effective that the rate of oil recovery from the low permeability layer was almost better than the high permeability layer. The SiO2-SDS foam flow favorably through high permeability layer of the layered macroscopic model where they are trapped and their entrapment results in diversion of subsequent injected foams. The foam completely blocked the main flow path in the high permeable zones to divert the injected fluid and subsequent incoming foams into the lower permeable region of the micromodel with high oil saturation.
2.5.3. Applications and limitations of macroscopic experiments
The advantages and limitations of the macroscopic experiments are well documented in literature. The macroscopic experiments are “black box” experiments that determine foam performance and mobility mainly through oil recovery, measurement of pressure drop across the porous media, saturation profiles, foam textures and other indirect properties of foam flow in porous media (Emadi et al., 2012). In some cases, X-ray computed tomography (CT) and magnetic resonance imaging (MRI) have been employed in addition to the use of transparent layer models to monitor foam propagation and stability and for determining in-situ fluid saturations during foam flow in consolidated and unconsolidated porous media (Nguyen et al., 2007, Lv et al., 2013). Despite the use of these techniques, the macroscopic models are not appropriate for investigating the pore scale mechanisms of nanoparticles-stabilized foam flow and oil displacement mechanisms in porous media (Manlowe and Radke, 1990, Emadi et al., 2013). Foam texture cannot be vividly observed with X-ray computed tomography (CT) and magnetic resonance imaging (MRI). The foam lamellae appeared very thick and the bubbles are not distinctively separated (Hou et al., 2013).
Another major challenge of the macroscopic studies of nanoparticles-stabilized foam flow is the inability to observe foam generation, coalescence and foam propagation in porous media. For the pre-generated foam, although it is possible to visually observe the foam structures in the foam generator before injection into the porous media, there is possibility of faster bubble coalesce and coarsening at the entry point of foam into the porous media. Hence, the pre-generated foam may not be suitable for the real oilfield applications due to the increasing cost and difficulty of injection into the reservoir (Shamsijazeyi et al., 2014). For the in-situ generated foam, we have to depend on the pressure drop as indication of foam generations and mobility in porous media. Higher pressure drop is expected during the propagation of stronger foam as a result of resistance to gas flow (Alyousef et al., 2018). Although the pressure drop is a strong indication of foam mobility and propagation in porous media, it is difficult to conclude that the observed pressure difference is entirely due to foam formation and propagation in porous media. The foaming dispersions depending on the concentration and viscosity may also induced a significant pressure drop in porous media. Consequently, the liquid components of the bubbles can also be mistakenly judged as foam during the flow experiments in macroscopic models.
Generally, accurate predictions of pressure drop behaviors during nanoparticles-stabilized foam flow in porous media should be given utmost considerations in the design of macroscopic experiments. In some previous studies, the porous media were sectionalized to create multiple measuring points and multiple pressure taps were installed along the various sections of the core (Chen et al., 2010, Hou et al., 2013 Singh and Mohanty, 2015, Khajehpour et al., 2016). Through this method, fractional pressure drop (pressure gradient) can be obtained at different time and positions. It is also possible to capture the local inlet and outlet effects and the frictional pressure drop across the porous media. With this method, Hou et al. (2013) successfully determines the pressure changes and foam texture at different periods and sections of the core during nitrogen foam core-flooding. The use of multiple pressure taps can assist in determining if the generated lamellae were actually mobilized across the porous media or if foams needs to be generated at new sites in porous media for a successful propagation. If the pressure drop increased occurred evidently at the different sections of the porous media and not in any one segment only. It can be inferred that stable and stronger foam generation and propagation is possible within the porous media (Gauglitz et al., 2002).
The minimum velocity for stable foam generation in porous media is another critical area that should be investigated in future macroscopic studies. The possibilities of foam propagation in actual reservoir and oilfield conditions at low velocities and low pressure gradients as foam travelled father from the wellbore is still debatable. The existence of minimum velocity or pressure gradient (∇p) that needs to be exceeded for stable foam generation has been reported in various literature (Ransohoff and Radke, 1988, Tanzil et al., 2002Gauglitz et al., 2002). However, recent experimental and modelling study by Lotfollahi et al. (2016) shows that for nanoparticles-stabilized CO2 foam generated at high pressure gradients close to the wellbore, the foam stability and propagation can be sustained at low injection rates (low velocities) and low pressure drop at distance far away from the wellbore in the reservoir. In future studies, the design of the macroscopic experiments should mimic the pressure and flow characteristics of the bubble flowing in the realistic porous media. It should be able to capture the pressure difference across the various sections of the porous media and predict the foam behaviors at changing velocities and pressure drops at distance far and near the wellbore (Ma et al., 2016).
The experimental conditions are also major limitations of previous macroscopic studies of nanoparticles-stabilized foams. In most of the previous studies, the flowrate that provides the shear rate above the threshold shear rate, the temperatures at which the experiments are conducted, the permeability and porosity of macroscopic models are not true representative of the reservoir conditions. The use of Hagen-Poiseuille equation for a Newtonian fluid in laminar flow, and plane-Poiseuille equation for apparent viscosity calculations of foam flow in capillary tube and the 2D Hele-Shaw cell, can constitute serious limitations to the quantitative estimation of foam mobility reduction during macroscopic studies. These equations are mainly used for Newtonian fluids flow while results of some recent studies have shown that foams are non-Newtonian, nonlinear rheological complex fluids, that does not conform to the Newtonian postulate of the linear relationship between shear stress and shear rate (Xiao et al., 2016, Xiao et al., 2017). Nanoparticles dispersions in form of nanofluids were also observed to demonstrate non-Newtonian properties (Wang et al., 2017). Xiao et al., 2016, Xiao et al., 2017 employed the power law model to describe the rheological properties of nanoparticles-stabilized CO2-foam due to their non-Newtonian behaviors. Further study of rheological properties of nanoparticles-stabilized foam is recommended for future studies.
2.6. Microscopic studies
Micromodels are used for pore scale investigation and visualization study of the mechanisms of gas mobility control by foam and foam flow process in porous media. They can be made by etching or through lithography using transparent materials made from glass, silicon, quartz, various microfluidic devices or even polymeric materials. A micromodel is an artificial (idealized) 2D representation of a porous medium made of transparent materials that enable visual and the pore level observation of multiphase flow phenomenon and behavior in porous media (Sayegh and Fisher, 2009, Karadimitriou and Hassanizadeh, 2012). The porous system is a network of connected pores in tens of microns. To build a micromodel, it is necessary to obtain a high contrast diagram of flow network patterns of the model. Different kinds of methods have been used by researchers for generating network patterns used in micromodels. The design pattern is called a perfectly regular patterns when all pores geometry are the same. That is, the width and the depth of the pore and the distance between the pores are constant throughout the entire network. In irregular patterns models network, there is no spatial correlation in the pores pattern and its geometry. The pores are randomly distributed in the whole network (Sandnes et al., 2007). Generally, the flow network patterns are etched onto a glass plate to produce an imprint of the design on the micromodel. This plate can then be sandwiched with another flat plate to seal the channels. There is an inlet and an outlet ports for the introduction and removal of the wetting and non-wetting phases. The distribution of phases in micromodels can be visualized using microscopes, digital cameras, or their combinations.