1. Introduction
Groundnut (Arachis hypogaea L.) is an important oilseed crop in India, China, USA, and several African countries. It is a leguminous crop that is valued for its quality fodder, protein, resveratrol, vitamin, minerals, and other antioxidant molecules content, preserved in its seed. The total production of groundnut pod is ∼27.66 million tons/year worldwide that costs about 34.6 billion USD, as on November 2017 [1]. The cultivated form of groundnut is an amphidiploid with 2n = 4x = 40 chromosomes. It is believed that this crop was originated in the Southern Bolivian region, resulting from a natural cross between A. duranensisand A. ipaensis, followed by chromosome duplication [2]. As a consequence, a ploidy barrier was identified between the cultivated and the wild species, and hence, insertion of stress related gene from the diploid progenitor becomes complex. Although substantial phenotypic variability for yield related traits are present in this crop, yet the variability for vitamin (mainly vitamin E) and micronutrient (especially Fe and Zn) contents, resistance to aflatoxin, insect, and pathogen is very less in the cultivated form. This low variability of the above traits limits the genetic improvement of groundnut via conventional and marker-assisted breeding. Genes for Fe and Zn biofortification have already been identified in various plant species and the same have been exploited for transgenic research in model plants [3], [4], [5], [6], [7]. Several candidate genes, responsible for the enrichment of vitamin E content, have been identified in other crop species or microorganisms [8], [9], [10], [11], [12]. Candidate genes have been identified with the application of gene expression and microarray analysis in groundnut, Medicago sp., and maize inbred lines for Aspergillus flavusresistance [13], [14], [15].
Transgenic approaches would help to introduce those genes in groundnut for better mineral content, high vitamin E content, and aflatoxin resistance. Till now, different forms of transgenic groundnut were developed at various laboratories across the world. The exploitation of such transgenic lines is hindered due to their poor yield, poor acclimatization in field, and non-acceptance by the consumers and policy makers. Although, several scientific papers on efficient regeneration from various explants have been available in groundnut [15], [16], [17], [18], [19], [20], yet very limited success (with consistent genetic transformation) in cultivated groundnut has been achieved so far. Lack of efficient protocol for regeneration, genotype dependency of protocol for regeneration, and inadequate facility to handle a large number of regenerants or transformed plants, accounted towards this paucity. Recent advancement in genome sequencing, genomics, gene-editing, high-throughput screening technologies and other biotechnological tools would certainly aid in to address this problem. In this review a glimpse of concept, various approaches, bottlenecks, and future perspective of transgenic research in groundnut have been documented.
2. Development of transgenic plant: basic concept and method
2.1. Establishment of intended cell, tissue or organ culture
Totipotency of plant cell simplifies the usage of any plant part as explant for transgenic research in general. However, organogenesis and transformation efficiency varies from tissue to tissue as well as genotype to genotype [20], [21], [22]. For transgenic research in groundnut, cotyledon, cotyledonary node, de-embryonated cotyledon, embryogenic callus, embryonic axes, axillary bud, zygotic embryo, immature leaf, shoot tip, mesocotyl were used as explants [20], [23], [24]. Out of these explants, de-embryonated cotyledons were most frequently used for groundnut transformation [25]. In fact, higher frequency of organogenesis (shoot induction) was recorded in vertically split de-embryonated cotyledons in a modified regenerating medium containing 20 µM 6-benzyladenine and 10 µM 2,4-dichlorophenoxy acetic acid [18]. In another report, Chen et al. [26] found that mesocotyl-derived explants gave higher transformation efficiency than the efficiency obtained with cotyledon-derived explants.
Nowadays, in planta transformation is a method of choice for groundnut transformation [27], [28]. It is a non-tissue culture-based genotype-independent protocol for developing transgenic groundnut. However, it requires high-throughput screening methodology for the identification of positive transformants [24], [29].
2.2. Approaches of gene insertion
2.2.1. Agrobacterium mediated transformation
Disarmed Agrobacterium (LBA4404, EHA105, EHA101, C58, A281, GV2260 strains)-mediated plant transformation was mostly used in groundnut due to its versatility, genotype independency, stability of transformants, and integration of foreign DNA in single copy approach, and also due to rare transgene rearrangement. Octopine, nopaline and succinamopine producing strains were used in groundnut for successful transformation [30], [31], [32], [33]. Agrobacterium-mediated transformation has another advantage since it can also be used for in planta transformation, which was otherwise not possible through biolistic method. Sometimes over production of secondary metabolites (like resveratrol synthesis) and elucidation of root nodule infection mechanism were tried in groundnut roots by the A. rhizogenes-mediated transformation [34], [35]. Geng et al. [36] also reported A. rhizogenes-mediated Cry8Ea1 transgene overexpression in groundnut roots that imparted biotic resistance against groundnut root beetle (Holotrichia parallela).
2.2.2. Biolistic approach and whisker mediated transformation
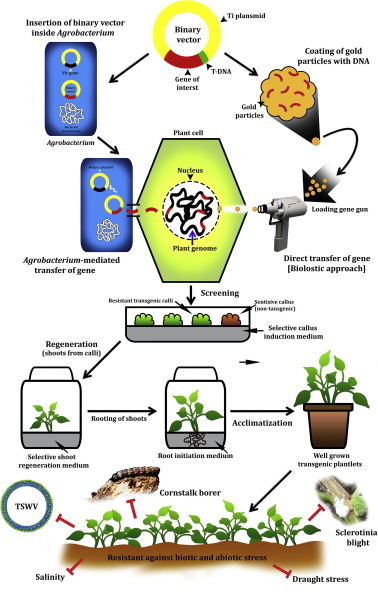
Biolistic method is a genotype-independent technique that can be utilized for inserting transgenes in plants. But, this method imparts a low transformation efficiency and high copy number of integrated transgene [37], [38]. Of the various explants used for biolistic approach in groundnut, mature zygotic embryo was successfully used for introduction of transgene without any regenerated plants [39], [40]. In a report from China, immature cotyledon from developing seeds of cv. Luhua 9 and YueYou 116 were exposed to biolistic approach and the resultant transgenic plants were developed from an improved somatic embryogenesis system [41]. This biolistic method was also used in groundnut by Yang et al. [42] for transforming embryogenic culture with mercuric ion reductaseA (merA) gene. The same method was used for introducing gene of interest in the embryogenic tissue of cv. Georgia Green as well [38]. The different dimensions of genetic transformation approaches are highlighted in Fig. 1.

Fig. 1. A brief account on different modes of genetic transformation and its ensuing resistance/tolerance against biotic and abiotic stresses.
Source: Diagram made by Saikat Gantait.Another direct plant transformation is whisker-based direct DNA delivery into the explants. This method was successfully used in maize and rice wherein either silicon carbide or aluminum borate-based whiskers were used to deliver the binary vector plasmids into the plant cells [43], [44]. This method was also used in groundnut transformation for inserting chitinase gene into epicotyl explants for the purpose generating leaf spot disease resistant plants [45].
2.3. Construction of vector and co-integration of gene
2.3.1. Construction of promoter
A promoter element is obligatory to achieve constitutive or tissue specific or stress-inducible expression of transgene in plant system. In earlier cases of plant transformation, promoters from Agrobacterium nopaline synthase or octopine synthase have been widely used. In groundnut, constitutive expression is achieved mostly by the usage of 35S-CaMV promoter or double 35S-CaMV [46]. To procure a strong constitutive expression of transgene, 35S promoter from figwort mosaic virus (FMV) was used [15]. A comparative study reported rice actin-2 promoter to exhibit a higher expression of reporter gene than the CaMV promoter, in groundnut [42]. Promoter plays an important role in tissue-specific expression of transgene. For example, a promoter vspB from soybean gave higher levels of gene expression in the leaves and stem over roots in groundnut [47]. In another case, stress inducible promoter (RD29A) was used in groundnut transformation for time-regulated expression of transgene during water limiting condition or drought stress [48], [49]. Construction of promoter for seed-specific expression is equally important for molecular farming and enriching micronutrient and other bioactive compounds in groundnut seed. In a recent study, a groundnut gene AhLPAT2 was over-expressed in Arabidopsis for increasing seed oil content by using napin promoter from rapeseed [26]. A groundnut seed promoter (GSP), which includes putative promoter regions of the groundnut gene 8A4R19G1, was characterized and tested for its control over tissue-specific expression of a transgene in tobacco [50]. It is absolutely essential to keep such seed-specific promoter secluded in order to avoid pod/seed infection by Aspergillus flavus in future. An interesting development on seed-tissue-specific expression of reporter genes (GUS and GFP) in groundnut was observed, when Ara h 2.02 promoter element of the groundnut plant itself was used [51].
2.3.2. Insertion of selectable marker
A selectable marker is a gene that is introduced into a cell, especially a bacterium or a plant cell that confers a trait, which is suitable for artificial selection. A selectable marker gene system must encourage the selective growth and differentiation of the transformed tissue in addition to providing resistance to a substrate. This substrate can be an antibiotic, a herbicide, an anti-metabolite, or a specific carbon source etc. Based on different criteria used for selection, selectable markers can be broadly divided into three categories, namely: (a) conditional positive selection, (b) non-conditional positive selection, and (c) conditional negative selection [52]. In most of the reports on groundnut transformation, application of antibiotic resistant genes nptII(neomycin phosphotransferase II) and hpt (hygromycin phosphotransferase) was considered as conditional positive selection [18], [19], [38]. A binary vector pPZP200 carrying spectinomycin resistance gene was used for developing a transgenic groundnut plant that exhibited resistance to Aspergillus flavus [15]. The use of bar (bialophos resistance) gene as a selectable marker, was first reported in groundnut by Brar et al. [53]. With the help of molecular biological techniques Bhatnagar-Mathur et al. [54] developed a simple, easy, and fast transformation protocol without using selectable marker in groundnut, for generating transgenic plants.
2.3.3. Insertion of reporter gene
Reporter genes are equally important in genetic transformation for confirming transgenic events during the selection of transformed explants or calli. Reporter gene should be used to improve the transformation system and the efficiency of recovering transgenic plant [52]. Such genes (particularly gfp and luc) also aid in to regulate different cellular process, protein localization, and intracellular protein trafficking. Till now, very few reporter gene systems were used in plant transformation. Of them, reporter genes like uidA/gusA (β-glucuronidase) [20], [41], [53], luc (luciferase) [55], and gfp (green fluorescent protein) [51] were substantially used in groundnut transformation studies, including other plant species as well. Use of gfp as reporter gene has its benefits throughout the process of non-destructive screening for positive transformed cells/calli in groundnut [56]. The same GFP reporter system has been used for monitoring successful Agrobacterium rhizogenes-mediated transformation of groundnut petiole [57].
2.4. Screening of transformed plants
Transgenic plants need continuous efforts to regenerate infected explants or calli in different media, and to execute the screening process in selective media, based on the type of selectable marker gene in binary vector. The most favored selection media for groundnut transformation is either kanamycin or hygromycin, containing callus proliferation and regeneration medium [19], [20], [53], [57]. Following the screening of transgenics on antibiotic- or herbicide-containing media plates (based on type of selectable marker gene), the regenerated explants are subjected to screening yet again to assess the activity of protein/enzyme product from reporter gene. Screening for reporter gene is essential to confirm the transgene expression and its localization either through biochemical assay (for GUS gene) [19], [47] or through microscopic observation (GFP gene) [57], or in some cases, via the ELISA [58] method. After getting the confirmation both from selectable marker and reporter gene, the explants should be used to validate the integration of transgene based on southern hybridization [39], [40] or PCR [20] or gene expression [40] or western blotting[42], in groundnut.
2.5. Expression of target gene in transgenic plants
Expression of target gene is the most important and ultimate step for the development of a transgenic plant. After selecting transformed explants with the aid of selectable marker and reporter gene, the integration of transgene is examined using the above-mentioned technique. Presently, over-expression of transgene is being checked via real time PCR technique [15]. But, often the gene may fail to express due to errors in the site of integration or due to gene silencing. Therefore, the transgenic plant should be screened via western blotting technique, provided the antibody for the transgene product is available. Otherwise, the transgene expression can be evaluated based on different bioassays and/or field evaluation for the desirable trait(s). Based on the gene of interest, scientists must develop an easy and high throughput bioassay technique for the purpose of screening large number of transformed plants. Bhatnagar-Mathur et al. [59] and Sarkar et al. [60] screened the DREB1Aexpressing groundnut plants by taking several physiological parameters and pod yield in limited soil moisture field conditions and laboratory conditions, respectively. Whereas IPT (isopentenyltransferase) over-expressed plants were screened at reduced irrigated field condition [61]. The transformed and genetically stable plants with overexpressed Cry1AcF gene were screened for insect resistance based on reduced damage to the leaves and increased larval mortality in a larval feeding assay in vitro [62]. High-throughput phenotyping facility can also be used for screening transgenic plant for biotic and abiotic stress tolerance in near future.
3. Successful enhancement of characters through transgenic approach in groundnut
There are scores of essential traits, which were introgressed in cultivated groundnut through genetic transformation approach. The cultivated groundnut faces major yield loss owing to the unavoidable biotic and abiotic stresses. Depending on the region of groundnut cultivation, the type and intensity of virus or soil/foliar fungal pathogen infestation may vary. On the other hand, the abiotic stresses could be considered as the prime reason for yield loss around the globe, since it causes 50% yield loss of the majority of the crops [63]. To combat these adverse biotic and abiotic stresses, significant efforts were put in via the genetic transformation of groundnut and by developing resistant genotypes against such stress factors (see Table 1).
Table 1. List of genes, their origin and use in enhanced biotic and abiotic stress tolerance in groundnut.
| Gene | Gene origin | Mode of gene transformation | Enhanced tolerance or resistance of transgenic to | Ref. (in chronological order) |
|---|---|---|---|---|
| TPWVN | TSWV-L | Agrobacterium-mediated | Tomato spotted wilt virus | [66] |
| crylA(c) | Bacillus thuringiensis | Microprojectile bombardment | Lesser cornstalk borer | [76] |
| TPWVN | Tomato spotted wilt virus | Microprojectile bombardment | Tomato spotted wilt virus | [17] |
| TPWVN | TSWV-L | Microprojectile bombardment | Tomato spotted wilt virus | [67] |
| AGLUI | Medicago sativa | Microprojectile bombardment | Sclerotinia minor | [64] |
| merA | Arabidopsis thaliana | Microprojectile bombardment | Mercury stress | [42] |
| CP | Peanut stripe virus | Microprojectile bombardment | Peanut stripe potyvirus | [68] |
| Barley oxalate oxidase gene | Hordeum vulgare | Microprojectile bombardment | Sclerotinia minor | [40] |
| AtDREB1A | A. thaliana | Agrobacterium-mediated | Drought stress | [48] |
| AtDREB1A | A. thaliana | Agrobacterium-mediated | Drought stress | [81] |
| cry1EC | B. thuringiensis | Agrobacterium-mediated | Spodoptera litura | [25] |
| AtNHX1 | A. thaliana | Agrobacterium-mediated | Salinity and drought stress | [85] |
| IPT | Agrobacterium tumefaciens | Agrobacterium-mediated | Drought stress | [61] |
| AtNHX1 | A. thaliana | Agrobacterium-mediated | Salinity and drought stress | [90] |
| cry1AcF | B. thuringiensis | Agrobacterium-mediated (in planta) | Spodoptera litura | [62] |
| AtDREB1A | A. thaliana | Agrobacterium-mediated | Drought stress | [82] |
| AtDREB1A | A. thaliana | Agrobacterium-mediated | Drought stress | [59] |
| mtlD | Escherichia coli | Agrobacterium-mediated | Salinity and drought stress | [86] |
| PDH45 | Pisum sativum | Agrobacterium-mediated (in planta) | Drought stress | [88] |
| MuNAC4 | Macrotyloma uniflorum | Agrobacterium-mediated | Drought stress | [84] |
| AtNAC2(ANAC092) | A. thaliana | Agrobacterium-mediated (in planta) | Salinity and drought stress | [83] |
| AtDREB2A, AtHB7 and AtABF3 | A. thaliana | Agrobacterium-mediated | Salinity and drought stress | [91] |
| AtDREB1A | A. thaliana | Agrobacterium-mediated | Salinity and drought stress | [60] |
| SbpPAX | Salicornia brachiata | Agrobacterium-mediated | Salinity stress | [92] |
| SGT1 | Arachis diogoi | Agrobacterium-mediated (in planta) | Induces cell death and enhanced disease resistance | [28] |
| SbASR-1 | S. brachiata | Agrobacterium-mediated | Salinity and drought stress | [93] |
| Alfin1, PgHSF4, and PDH45 | Alfalfa, Pennisetum glaucum, Pea | Agrobacterium-mediated (in planta) | Drought stress | [95] |
| AtDREB1A | A. thaliana | Agrobacterium-mediated | Drought stress | [96] |
| BjNPR1 and Tfgd | Brassica juncea and Trigonella foenum-graecum | Agrobacterium-mediated (in planta) | Aspergillus flavus and Cercospora arachidicola | [97] |
| AtHDG11 | A. thaliana | Agrobacterium-mediated | Salinity and drought stress | [98] |
3.1. Biotic stress resistance
3.1.1. Resistance to viral disease
Among some noxious viruses, the infection caused by Tospovirus, affects majority of the groundnut growing regions throughout the globe. Typically, this infection is triggered by some analogous viruses, which are serologically different [for instance, groundnut bud necrosis virus, groundnut ringspot virus, groundnut stripe virus (GStV) and tomato spotted wilt virus (TSWV)]. A number of efforts have been exerted to utilize pathogen-derived resistance over tospoviruses to attain in vivo resistance, till date. Yang et al. [17] and Chenault et al. [64] reported that the development of transgenic groundnut through particle bombing [comprising TPWV nucleocapsid protein (TPWVNP) gene] ensued the revival of genetically transformed plants comprising one copy of the TPWVNPgene, which exhibited its proteomic expression. Yang et al. [17] confirmed the expression of the transgene to be constant throughout the generations, which supported the Mendelian pattern of genetic inheritance. During subsequent ex vitro evaluations, Yang et al. [65] found that such distinct transgenic line exhibited a higher tolerance against viral disease in comparison to control lines. Superior function of the genetically transformed lines over Georgia Green, the most extensively adopted TSWV-tolerant groundnut cultivar was detected when the disease pressure was high. A similar degree of tolerance to TSWV was displayed along with the simultaneous expression of TSWVNP gene by the genetically transformed groundnut lines, developed via Agrobacterium-mediated transformation [66]. In this report, the genetically transformed plants merely showed localized contamination following physical infestation, while either the non-transgenic plants or transgenic plants carrying only the reporter genes were completely infested and exhibited post-inoculation limited growth and development. Magbanua et al. [67] transferred TSWVNP gene in an antisense direction into groundnut; consequently, the Mendelian inheritance of the TSWVNP gene was duly exhibited by the generative transgenic groundnut plants, and during continuous observation for four months it was detected that the transgenic lines exhibited better TSWV-resistance in contrast to the control ones. Outcomes of these reports conclude that the development of disease symptom could be deferred by the expression of sense or antisense TSWVNPgene, however, such gene expression failed to offer a comprehensive disease resistance. Even considering this drawback, Yang et al. [65] concluded that the resistance of genetically transformed groundnut plant following rigorous field-trials could be counted as superior in comparison to commonly cultivated lines of groundnut.
In the large groundnut growing regions of Asia and Africa, several viruses (other than TSWV) are also important causal organism of groundnut diseases. Indian groundnut clump virus and GStV are two such instances that are being studied to unlock the mechanism of pathogen-derived resistance in groundnut [15], [68]. Till now, usual resistance to PStV has not been discovered in the groundnut gene pool, and the virus can be transmitted through seeds. Advancements were made to develop resistance against PStV through genetic transformation, wherein the non-translatable or yet translatable truncated forms of the coat protein gene were inoculated via particle bombing [68]. Introduction by physical means in glasshouse environments confirmed that the resistance is offered by both the genes, however, the same can result in a rapid or an interrupted recovery from disease signs. The physical inoculation system was efficient since all the control plants were infested. Since no genetically transformed groundnut plant showed any proteomic expression of transgene, hence, the resistance was counted as ‘RNA-mediated’. The evidence showing the presence of small interfering RNAs (siRNAs) homologous to the viral transgene supports this concept of ‘RNA-mediated’ resistance. Such siRNAs are specific to ‘post-transcriptional gene silencing’ process [69].
3.1.2. Resistance to fungal disease
An array of potential antifungal genes was investigated to assess their efficiency mainly against Aspergillus and other fungal diseases borne from soil [40], [63], [70], [71], [72], [73]. Such genes express the antimicrobial peptides, anti-apoptotic proteins, oxalate oxidase, chitinase, glucanase as well as ribosome deactivating protein. Chenault et al. [70] used particle bombing as an aid to insert the chitinase and glucanase genes in Okrun, a Spanish bunch type groundnut variety. Chenault et al. [71] then executed ex vitro trials (for a span of three years) with 32 genetically transformed groundnut lines that individually carried one copy of the transgene. The resultant disease scores exhibited the following result: 14 out of the 32 lines exhibited considerably higher resistance to Sclerotinia blight in comparison to Okrun. One of the 14 genetically transformed lines showed no occurrence of disease throughout that three-year span. However, it is yet to be established whether the expression of the inserted chitinase transgene is the sole reason for such excess level of resistance. Inadvertently, the same line also revealed an unanticipated vertical growth habit with open canopy that is comparable with the feature of the resistant genotype (SW Runner). It was considered that this variation in growth pattern might be due to the introduction of transgene that distorted the expression of a gene that governs that pattern of growth. Livingstone et al. [40] reported another gene, barley oxalate oxidase, which was found to be highly efficient against Sclerotinia blight. This fungus exudes oxalic acid that triggers cellular injury of the diseased plants. Oxalate oxidase-expressing plants are competent to destroy the oxalic acid secreted by this fungus, thus significantly boosting the resistance against fungal pathogen.
3.1.3. Resistance to insect pests
Majority of the groundnut pests are comparatively easy to control with the help of contact insecticides. The exceptions are those that burrow underground to feed on injured roots and pods. According to Lynch and Mack [74], application of integrated management approach comprising host plant resistance is necessary to regulate the insect pests in groundnut. Cornstalk borer (CSB), a lepidopteran insect causing major concern in the southeastern region of USA, feeds on growing groundnut shoots and pods. However, Moar et al. [75], during in vitro feeding experiments, observed that CSB is vulnerable to the cryIA(c)toxin, secreted by Bacillus thuringiensis. Insertion of cryIA(c) gene into groundnut by particle bombing and its consequent expression in genetically transformed plants might offer the resistance against CSB [76]. Lynch and Wilson [77] and later, Bowen and Mack [78] reported that the CSB injury is linked with the infection caused by Aspergillus flavus or A. parasiticus and consequent aflatoxin synthesis. Keeping this in view, Ozias-Akins et al. [79]intended to remove aflatoxin with the development of insect resistance, in groundnut.
3.2. Resistance to abiotic stress
3.2.1. Resistance to water stress
Scarcity of water is the most prominent abiotic stress, which firmly reduces the yield of groundnut; in so doing it minimizes the prospects of livelihood advancement of poor farmers who belongs to the semi-arid tropical region. Persistent and continuous attempts to evolve superior drought tolerant groundnut via traditional breeding approaches resulted in inadequate success mainly due to deficiency in determining the fundamental physiological activity as well as absence of adequate divergence for drought tolerance-associated characters. In this backdrop, transgenic-based methods have been employed to hasten the process of molecular introgression of putatively favorable genes for rapid development of stress-tolerant groundnut [80].
Bhatnagar-Mathur et al. [48] inserted DREB1A, an ABA-independent transcription factor, in JL-24 (a groundnut variety) and detected that DREB1Aprovided water-economizing facility in the genetically transformed plants in comparison to their non-transgenic parents. In the same year, Vadez et al. [81]substantiated that DREB1A distinctly stimulated the response of groundnut root when exposed to water scarcity, wherein, the stimulated roots grew significantly longer, especially in the deeper layers of soil. The same group [82]later identified that the influence of DREB1A on groundnut (genetically transformed with DREB1A directed by rd29 promoter) root could alter the distribution of the rooting system in a consistent manner throughout the soil profile and hence, root length density was increased to facilitate the water extraction frequency. Such improved tolerance against water stress, by the inducible expression of DREB1A in genetically transformed groundnut, made it capable to enhanced pod yield, yield components and harvest index. Bhatnagar-Mathur et al. [58] confirmed the yield advantage of transgenic groundnut under water stress using the DREB family of transcription factors, over non-transgenic plants. Genetically transformed plants considerably improved seed filling and checked 20–30% pod yield loss in comparison to their non-transgenic counterpart.
Considering the accumulation of free oxygen radicals to be harmful to groundnut during water stress, Bhatnagar-Mathur et al. [49] studied the aspects of the oxidative injury, the antioxidant configuration, and the osmoprotection (under increasing water stress), to be introduced in genetically transformed groundnut plants through overexpression of AtDREB1A transgene, directed by Atrd29A, a stress-inducible promoter. However, they reported that the over-expression of AtDREB1A transcription factor or modification in antioxidative mechanism doesn't bring about any improvement in transpiration efficiency in transgenic groundnut. Later on, Sarkar et al. [59] reported an improved function of transgenic AtDREB1A groundnut at early growth phase, owing to its overexpression, accompanied with potential up-regulation of several stress-inducible downstream genes in the signal transduction pathway.
AtNAC2, a gene associated with the plants’ response to stress hormone signals (like ABA), proved to be quite efficient in the development of transgenic groundnut, tolerant against water stress. Patil et al. [83] reported that the overexpression of AtNAC2 showed an improved tolerance in limited water condition. Their experiment confirmed that AtNAC2 functioned as an efficient candidate gene to enhance the water stress tolerance via genetic transformation method. MuNAC4, another horsegram (Macrotyloma uniflorum)-derived representative of NAC transcription factor, has been reported to develop tolerance against water stress. Pandurangaiah et al. [84] developed transgenic groundnut lines with the aid of recombinant MuNAC4 binary vector transformation method. The transgenic lines, following steady insertion and subsequent overexpression of MuNAC4 gene, performed a substantial part in successful water stress tolerance by lessening any injury to membrane structures, enhancing osmotic and antioxidative enzyme regulation. On a similar note, the efficiency of AtNHX1 gene (a vacuolar type Na+/H+ anti-porter gene driven by 35S promoter) was recorded to induce tolerance against water stress and high salinity. Asif et al. [85] reported that expression of AtNHX1 in the transgenic groundnut made them more resistant to water stress situation than the non-transgenic plants. Influence of mannitol accumulation with the increasing water deficiency is a well-established fact. Bhauso et al. [86] assessed groundnut plants transformed with mtlD (Escherichia coli derived), against water stress and reported that transgenic plants displayed enhanced mannitol synthesis and improved tolerance owing to over-expression of mtlD gene.
Such advancements have the potential to bring in significant benefits in developing groundnut lines, resistant to water stress. In addition, detection of additional physiological attributes associated with water stress tolerance is necessary to generate clear perceptions, regarding the system of water-stress tolerance in groundnut.
3.2.2. Resistance to salinity
Resistance against salinity in plants can be developed through the detection of factors responsible for retaining the ion homeostasis under high salinity. In fact, salinity stress causes a reduced synthesis of protein via regulation of gene expression, exhibiting the association of complex synthesis of nucleic acid. According to Tuteja et al. [87], a motor of protein (Helicases) that is an ATP-based enzyme, possesses the ability to unbound DNA, RNA or DNA–RNA amalgam via a control mechanism involving “replication, transcription, translation, repair/recombination and ribosome biogenesis”. In any kind of stress situation, it was observed that the helicases retained the plant growth equilibrium via transforming the abiotic stress-triggered pathways to adapt resistance/tolerance against extreme saline condition. The potential of helicase gene to generate salinity tolerance in transgenic groundnut was evaluated in multiple instances. Such as, PDH45 gene (pea DNA helicase), when overexpressed in genetically transformed groundnut, exhibited around 10% enhanced yield under salinity stress. In addition, steady chlorophyll content along with reduced PEG-triggered desiccation was evident in the genetically transformed groundnut lines. Consequently, the insertion of helicase gene confirmed the possibility of pyramiding the yield and salinity-tolerant attributes in groundnut via triggering the tolerance at cellular level [88]. Furthermore, the offspring of genetically transformed groundnut lines displayed better root growth frequency under drought situation. Hence, the direct function of helicase gene generates a potential scope to explore resistance against saline environment in other plants as well.
Greater levels of salts in soil involve water stress situations resulting in Na+ and Cl− accumulation, unfavorable to groundnut. According to Krishna et al. [46], elimination process of these ions offers a defense mechanism to the plants against salinity, merely at low levels of the solvent, ensuing in the inhibition of vital metabolic pathways and associated growth in high salinity. Overexpression of AtNHX1 gene led to the invasion of Na+ ion inside vacuole via the action of Na+/H+ antiporter that induced tolerance against salinity in the transgenic groundnut line [89]. Correspondingly, the level of proline content in the leaves of groundnut (genetically transformed with AtNHX1 gene) was increased due to the stimulation of the tolerance factors [85]. Assessment of Na+/H+ antiporter gene in genetically transformed groundnut, under saline conditions, established minimum injury, boosted frequency of biomass production, higher chlorophyll content, high photosynthesis rate, and stomatal conductance, along with increased transpiration and CO2 absorption [90]. An effort was built by Pruthvi et al. [91] to introduce salinity tolerance in TMV2 cultivar of groundnut via simultaneously expressing the stress-reactive transcription factors linked with downstream gene expression. The genetically transformed groundnut lines following co-expression of transcription factors like AtDREB2A, AtHB7, and AtABF3, exhibited improved tolerance to salinity with plant biomass proliferation, in comparison to the non-transformed lines. In addition, owing to greater ROS scavenging and osmotic regulation by proline synthesis under salinity stress, the genetically transformed lines demonstrated superior stability of membrane and chlorophyll.
The peroxisomal ascorbate peroxidase genes (SbpAPX), copied from an acute halophytic plant species [Salicornia brachiata Roxb (Amaranthaceae)], which is an important salt responsive gene source, was transferred by Singh et al. [92] in groundnut lines for their evaluation against high saline environment. The outcome of their study revealed the ectopic gene expression, which substantiated the confirmed superior tolerance to salinity, wherein, transformed groundnut plants were grown green but the wild plants become pale green to yellow. Another gene, SbASR-1, cloned from the same halophyte (S. brachiate), translates a plant-specific stress-reactive protein. Tiwari et al. [93]introduced this SbASR-1 gene that functioned as a transcription factor, therefore acquiring the ability to thrive in saline condition. The leaves of genetically transformed groundnut displayed lesser electrolyte leakage, and higher chlorophyll content, and higher relative water level, when compared to non-transformed plants. A brief account of genetic transformation and its ensuing resistance/tolerance against biotic and abiotic stresses has been presented in Fig. 1.