1. Introduction
Titanium (Ti) and its alloys have been a raw material for the manufacture of biomaterials because of their biocompatibility and resistance to corrosion [1]. The passivation phenomenon contributes to these characteristics and makes Ti suitable for use in dental and orthopedic implants, without promoting adverse reactions locally or systemically. Importantly, this metal upon exposure to air or aqueous electrolytes forms a passive and stable layer of titanium dioxide (TiO2) which can reach a thickness of 2–10 nm on its surface in 1 s, providing resistance to the release of metal ions [2], [3]. TiO2 can also be used for the construction of biosensors, because as a semiconductor it allows for the rapid transport of electrons from reactions on its surface to the Ti substrate, improving the performance of these important tools for the diagnosis of diseases [4].
According to the dimensions of the surface characteristics, the roughness of the surface of the implants can be of macro- (varies from millimeters to microns), micro- (1–10 μm) or nano- (1–100 nm) scales [5]. Nanoscale surface topographyis preferred for implant making. The fact that bone tissue presents nanoscale structures, such as collagen, allows this nanotopography, with surface energy higher than the other texture scales, to improve the adhesion of matrix proteins, such as fibronectin and vitronectin, and to stimulate cellular migration and proliferation, important steps in the process of osseointegration, i.e. the formation of bone around the implant [6], [7]. Functionalized and modified nanostructured devices for biomedical applications have become increasingly investigated in the hybrid science field named nanobiotechnology [8]. The metallic, ceramic, polymeric and composite nanomaterial properties may be integrated with biomolecules to promote combined and synergistic effects from the hybrid systems, such as biomolecule-nanoparticles [8], [9]. The hybrid nanomaterials combining inorganic and organic or even bioactive components into a single material are promising for using in biomedicine, for example, the organic-inorganic hybrid hydrogel, polymers and the magnetic Janus particles that have physical properties on their two or more dissimilar faces for drug delivery [10], [11], [12]. Inorganic/organic hybrid materials can be obtained by atomic layer deposition to promote the modification of polymer surfaces [13].
Drugs may be delivered with release in a target site of the organism, even at the cellular level for delivery of genes into cells using nanocarriers, such as inorganic nanoparticles and quantum dots, for example, carbon dots developed with glucose and polyethyleneimine [14], [15], [16]. Specific nanomaterials to use as vehicles depending of the characteristics of the therapeutic biomolecule; for that the delivery and expression of optogene used in the optogenetics may to stimulate or inhibit the neural activity should be taken into account the optical, electrical, thermal properties and proper bio-functionalization of nanomaterials [17]. Upconversion nanoparticles, luminescent materials that can absorb near-infrared light and emit UV–visible light, covalently conjugated with a photosensitizer has been a highly specific and targeted treatment option in the photodynamic therapy studies [18]. Gold nanostructures can be synthesized with size controlled using reduction of copper; the properties of nanostructures depend on their morphology and are widely used in biomedicine, such as drug delivery and biosensing systems [19], [20]. Proteins and peptides from the eggshells participate of the nucleation of calcium carbonate crystals and play an important role in the eggshell biomineralization; such biomolecules can be used in the future with TiO2 nanostructured for manufacturing of implants [21], [22].
TiO2 nanotubes (TNTs) are tubular and self-organized nanostructures that have attracted considerable attention in implant manufacturing because of their mechanical stability, low cost of preparation and better biocompatibility compared to TiO2 film [23], [24], [25]. TNTs are able to form well-defined nanostructured platforms with favorable transport pathways, good adhesion to the substrate and high surface area, i.e., having a large number of atoms on their surface, available to interact with many biomolecules and allowing their use as an electrode in the manufacture of biosensors [26]. TNTs can be obtained in large quantities by various synthesis techniques, such as the sol-gel method, hydrothermal treatment and electrochemically by anodization [27]. Anodic oxidation (or anodization) is a simple and versatile technique that synthesizes TNTs with controlled structure and morphology, being aligned perpendicularly and easily to the Ti substrate [28], [29].
Therapeutic failure in the use of implants may occur due to insufficient bone formation in the tissue surrounding the biomaterial, as ineffective bone fixation may lead to bacterial infection [30]. Research has been carried out with the aim of improving the functionality of these implants made of TNTs, for example, by the immobilization of biomolecules on the surface of these nanotubular matrices. The administration of growth factors such as bone morphogenetic protein 2 (BMP2) in TNT implants may improve their osteoinductive capacity [31], [32]. TNTs can be used as matrices for the immobilization of proteins and enzymes for use in biosensors, such as the enzyme glucose oxidase (GOx) on the surface of TNTs for the preparation of an enzymatic biosensor capable of detecting glucose [33].
The objective of this review is to address some techniques used for the immobilization of biomolecules in TNTs, since its synthesis, to make the applicability of these functionalized TNTs in biomedicine more efficiently, either as implants or as biosensors.
2. Immobilization of biomolecules in TNTs
The growth of TNTs by electrochemical anodization occurs in aqueous electrolytes with fluoride ions and organic electrolytes such as glycerol or ethylene glycol. This method is based on an oxidation-reduction reaction that occurs in an electrochemical cell in which titanium is used as the anode and at the cathode is an inert material such as platinum [29], [34]. An electrical potential from a power source is applied in the process to promote an electrical field and thus the diffusion of oxygen ions present in the electrolyte to form an oxide layer on the surface of the anode [35].
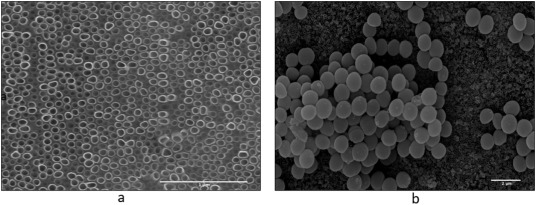
Fig. 1 shows the scanning electron microscope (SEM) image of TNTs at a magnification of 100,000 × (Fig. 1a), showing an ordered layer of the nanotubes obtained by the anodization process. Fig. 1b is at 7000 × and bacterial Staphylococcus aureus cells can be seen forming clusters on the surface of the TNTs.
 Fig. 1. SEM top view images of TNTs at different orders of magnification: (a) 100,000 × and (b) 7000 × with evidence of S. aureus colonies.
Fig. 1. SEM top view images of TNTs at different orders of magnification: (a) 100,000 × and (b) 7000 × with evidence of S. aureus colonies.Fig. 2 presents a schematic showing the formation of TNTs and their functionalization with biomolecules. The reaction that occurs on the anode describes the growth of oxide on the surface of Ti (Fig. 2a), in which the oxidized species of the metal react with the O2 − ions, provided by the water molecules, to form the TiO2 layer (Fig. 2b). The fluoride ions, present in the electrolyte, have the ability to form [TiF6]2 − complexes, which are soluble in water and promote a chemical attack, that is, the dissolution of the TiO2 formed. As soon as the oxide layer is obtained, there is decay of the current applied in the anodization and then the nanopores begin to grow on the surface of the metal (Fig. 2c). The equilibrium state is reached when the growth rate of the nanopores at the oxide-metal interface is the same as the rate of dissolution of the oxide, allowing continuous growth of the nanotubes (Fig. 2d) on the Ti surface [36]. Depending on the application, the TNTs can undergo a heat treatment to convert their amorphous structure into nanocrystalline structures such as anatase and rutile [34].
 Fig. 2. Synthesis of TNTs and their functionalization with biomolecules.
Fig. 2. Synthesis of TNTs and their functionalization with biomolecules.The reaction that leads to the synthesis of TNTs in Ti (a) begins with the formation of the TiO2 layer on the metal (b); then this oxide undergoes dissolution by fluoride ions that leads to the appearance of nanopores (c); these nanopores become deeper and deeper until they form an orderly and compact layer of TNTs (d). Nanotubes can still have their functionality enhanced by the immobilization of biomolecules by loading and/or coating them (e).
The biomolecules can be immobilized in the nanotubular matrices by coating the surface of the TNTs and/or by loading them (Fig. 2e). The adsorption of biomolecules in TNTs can occur by physical methods such as hydrophobic interactions, hydrogen bonds and electrostatic interactions, or by chemical methods such as the covalent attachment with formation of ether, amide and thioether linkages, for example.
The sol-gel coating methods have been classified into two types: dip coating and spin coating [37]. These techniques are being applied to promote the coating of TNTs with biomolecules, such as the coating of these nanotubular matrices with chitosan biopolymer [38], [39]. Spin coating is based on the application of a solution on a rotating substrate with subsequent ejection and evaporation of the solvent. Dip coating consists of immersing a substrate in a solution followed by gravitational drainage and evaporation of the solvent. Both methods allow the formation of a homogeneous film on the surface of the substrate [40].
Spin coating and dip coating can also be used for Layer-by-Layer (LbL) assembly, which is a technique capable of forming Polyelectrolyte Multilayers (PEMs) by adsorption of oppositely charged polyelectrolytes attracting by electrostatic interaction, on the surface of a given substrate [41]. TNTs could be coated by LbL using the polysaccharides chitosan and sodium hyaluronate, with positive and negative charges, respectively [42].
Spin coating is a fast method to obtain films homogenous with thickness easily changed mainly by changing spin speed, or the viscosity of the solution to be deposited [40], [43]. A limiting factor to the spin coating technique is the substrate size because large substrates cannot be spun at a sufficiently high rate in order for formation thin film [43]. Besides, dip coating does not need sophisticated apparatus characterizing a low cost solution deposition; a thin film of solution onto a plate, cylinder, or irregular shaped substrate, that is, without defined geometry, is a distinguishing feature of this technique [44], [45], [46]. There are those who consider one drawback the fact that in the dip coating process occurs the film formation in both sides of the substrate [45]. However, this factor may be favorable for the immobilization of biomolecules on the TNTs, since in certain anodization conditions the TNTs formation occurs in both sides of the Ti foil, so, unlike spin coating that promotes the immobilization on a single side, the dip coating may to allow the functionalization in the two sides of the anodized Ti foil, that is, more functionalized biomolecules on the TNTs may be obtained.
Biomolecules are also covalently immobilized on TNTs. BMP2 can functionalize TNTs through a covalent bond of this protein to the catechol and quinine groups derived from dopamine polymerization [31]. In another approach, a TNT/polypyrrole hybrid matrix was prepared for the covalent immobilization of GOx, where the chemical bond  CH
CH N
N was formed between the enzyme and polypyrrole through the glutaraldehyde cross-linker [47].
was formed between the enzyme and polypyrrole through the glutaraldehyde cross-linker [47].
TNTs can be loaded with bioactive molecules to promote localized release of them into a specific body compartment. Lyophilization has been a technique used to fill TNTs, for example, with connective tissue growth factor (CNN2) and the antimicrobial peptide cyproterin B (CecB) [42], [48]. Lyophilization or freeze drying is a process that removes a solvent, usually water, from a product frozen by sublimation (primary drying) and desorption (secondary drying) obtaining biomolecules that can be labile with purity and high stability [49], [50].
3. Functionalized TNTs with biomolecules
In order to increase the biocompatibility of TNTs used in implants, these matrices have been functionalized with cytocompatible biomolecules (Fig. 3) that may be able to stimulate the migration of cells to the implant site, increase cell adhesion, promote osteogenic differentiation of mesenchymal stem cells or even stimulate the proliferation of osteoblastic cells when the biomolecule is endowed with mitogenic activity. Another approach used in studies is the use of bactericidal or bacteriostatic biomolecules or those that only help prevent the adherence of planktonic (free-floating) bacterial cells on the surfaces of the TNTs in order to consequently avoid the formation of biofilm that can culminate with a peri-implant infection and implant loss.
 Fig. 3. Use in implants of TNTs functionalized with biomolecules improving cell adhesion for better osseointegration and preventing bacterial adhesion as a way to avoid possible infection.
Fig. 3. Use in implants of TNTs functionalized with biomolecules improving cell adhesion for better osseointegration and preventing bacterial adhesion as a way to avoid possible infection.The immobilized biomolecules may be able to render the surface of TNTs attractive for cell adhesion, such as mesenchymal stromal cells (MSCs) and mature osteoblasts. Another artifice that can be exploited of some biomolecules is the possibility of them preventing bacterial adherence, thus avoiding the formation of biofilm in these functionalized nanotubular matrices.
Table 1 summarizes some biomolecules that functionalize TNTs and their main applications. Different peptides and proteins were immobilized in the TNTs mainly aiming at the osseointegration process in comparison to the nonfunctionalized nanotube matrices. However, enzymes were also immobilized in these matrices and were successful for the detection of a specific analyte for use in bioenergetics. Table 1 shows the use of chitosan for the functionalization of TNTs. This bioactive polymer, besides being biocompatible and able to be used as a polyelectrolyte for surface coating, also has antimicrobial property which can act in synergism with the functioning of drugs that are loaded in TNTs [51]. The flavonoids quercetin and icariin were used as alternative, natural compounds capable of improving the biocompatibility of biomaterials (Table 1).
Table 1. Biomolecules and their applications after functionalizing TNTs.
| Biomolecules | Applications | References |
|---|---|---|
| Antimicrobial peptides | Possess antimicrobial activity for use in localized drug delivery | [52], [53] |
| Arg-Gly-Asp peptide | Promote initial attachment and proliferation of human mesenchyme stem cells (MSCs) and improve adhesion of rat bone marrow stromal cells (BMSCs) from rat and osteogenic gene expression | [54], [55] |
| Gly-Arg-Gly-Asp-Ser peptide | Stimulate cell spreading and proliferation of the osteoblast-like cell | [56] |
| Lys-Arg-Ser-Arg peptide | Increase preosteoblast adhesion and spreading on TNTs | [57] |
| Epidermal growth factor | Promotes rat MSCs proliferation and prevents cellular apoptosis induced by TNTs with a diameter of 100 nm | [58] |
| Bone morphogenetic protein-2 | Osteoinductive action, reduces inflammatory responses, and promotes enhanced bone remodeling in vivo | [31], [32], [58], [59], [60], [61] |
| Gelatin | Stabilizes gold nanoparticles improving osteoblast adhesion and propagation; gelatin is used mainly as coating to control drug release profile | [60], [62], [63], [64] |
| Hemoglobin | Detection of hydrogen peroxide | [65] |
| Glucose oxidase | Detection of glucose | [26], [66], [67], [68] |
| Urate oxidase | Detection of uric acid | [26] |
| Trehalose | Together with BMP2 on TNTs have osteogenic potential on BMSCs and anti-inflammatory properties | [32] |
| Chitosan | Controls the release of drugs, has antimicrobial action, good osteoconductivity, and manufacturing nanoparticles | [38], [39], [42], [60], [63], [64], [69], [70], [71], [72] |
| Hyaluronic acid/hyaluronate | Fabrication of bacteria triggering antibacterial and manufacturing nanoparticles | [42], [72] |
| Palmitoyl-oleoyl phosphatidyl-choline | Used as a barrier for controlling and sustaining release of drug | [53] |
| Quercetin | Loads TNTs and its release into the environment as an alternative for the treatment of post-operative infection, inflammation and quick healing with better osseointegration | [73] |
| Icariin | Enhances bioactivity of osteoblasts | [63] |
| Small interfering RNA (siRNA) targeting tumor necrosis factor alpha (TNF-α) | Suppresses inflammation and improves osteogenesis | [72] |
4. Mechanisms through which the functionalizing biomolecules of TNTs improve the use of biomaterials
The definition of biomaterials developed by the National Institutes of Health Consensus of 1982 is still used, which considers biomaterials as any substance or combination of substances, of natural or synthetic origin, that can be used for any period of time, as a whole or a part of a system in order to treat, increase or replace any tissue, organ or body function [74]. Biomaterials can be classified as metals, crystalline elements when solids, characterized by their opacity, ductility, conductivity and unique brightness; ceramics, hard, brittle materials, resistant to heat and corrosion, generally made by combining metal elements with oxygen or carbon; and polymers, high molecular weight compounds, derived from addition or condensation of many smaller molecules with elimination of water, alcohol or the like [75].
In relation to the biological reaction of the tissue to the biomaterials, these can be classified into three distinct categories: biotolerant, such as stainless steel and polymethyl-methacrylate, are substances that become surrounded by fibrous connective tissue after implantation and, although they promote the release of substances in non-toxic concentrations, they are not necessarily rejected; bioinert, such as alumina and zirconia, which allow bone apposition on its surface despite being susceptible to encapsulation in fibers; and bioactive, such as hydroxyapatite and bioglass, which are able to promote the formation of new bone on the surface, and interdiffusion of ions and chemical bonds with tissue [3], [76], [77]. Bioactive biomaterials can further be classified into osteoconductors, which are capable of binding to hard tissue, for example tricalcium phosphate; and osteoproductive biomaterials, such as Ti and niobium, which are those that bind spontaneously to the cells of the bone tissue and stimulate the growth of a new bone on its surface [76].
The main factors needed to achieve direct bone fixation of implants used in the areas of dentistry and orthopedics include surface properties and implant design, the quality of the host bone, the preparation of the surgical site, loading conditions and the prevention of initial and chronic infections [78]. Orthopedic implants include temporary ones such as plaques and screws, and permanent ones that are used to replace the hip, knee, spinal column and finger, for example [79]. The two main types of dental implants are subperiosteal, inserted into the top of the bone that lies below the periosteum over the bone cortex, and the endosteal, inserted into the cortical/basal bone usually in the maxilla or mandible and typically has a screw format to mimic the root of the tooth [79], [80], [81].
Fig. 4 shows an endosteal implant, with biomolecule-functionalized TNTs, inserted into the bone and replacing a dental root. In the conventional process, after the placement of these implants a period, generally three to six months, is expected for osseointegration and to be able to place a crown prosthesis. However, there is also the immediate loading of these implants with the prosthesis to shorten the time of the complete treatment and guarantee aesthetics [82], [83]. The interactions that may occur between the functionalizing biomolecules of the TNTs with the cells in the peri-implant microenvironment are also elucidated in Fig. 4 as mechanisms necessary to accelerate osseointegration.
 Fig. 4. Endosteal implantation of TNTs functionalized with biomolecules and their interaction with cells to accelerate osseointegration.
Fig. 4. Endosteal implantation of TNTs functionalized with biomolecules and their interaction with cells to accelerate osseointegration.After implant insertion, immobilized biomolecules in TNTs can enhance osseointegration through their binding to cell surface receptors culminating in the following: differentiation of mesenchymal stromal cells into osteogenic lineage (a); stimulation of osteoblast proliferation (b); and inhibition of the production of proinflammatory cytokines by leukocytes, such as macrophages (c).
4.1. Cellular response in bone formation
The first biological component to come into contact with the implant after its insertion is blood, resulting in a series of biological processes on the surface of the material [84]. Some extracellular matrix proteins that are adsorbed on the surface of the implant contain the Arg-Gly-Asp tripeptide that is capable of interacting with adhesion proteins (integrins) present in the cell membrane, culminating in cell adhesion [85], [86]. The peptide Lys-Arg-Ser-Arg linker of heparan sulfate and a constituent of transmembrane proteoglycans, has also been used for the adhesion of osteoblasts [87].
Early interactions of blood cells and fibrin on the surface of implants influence platelet activation and clot formation [88]. Platelets contain in their granules growth factors, such as vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF), which contribute to the recruitment of osteogenic cells [89]. Therefore, the fibrin matrix acts as a scaffold (osteoconduction) for the migration of mesenchymal cells and eventual differentiation (osteoinduction) of these cells in the osteoblastic lineage [84], [90]. Fig. 4a illustrates these osteoconduction processes with the consequent osteogenic differentiation, since the functionalized biomolecules in the TNTs can interact with cell surface receptors of the MSCs resulting in cascades of intracellular signaling and activation of transcription factors that culminates with osteoblastogenesis [91]. It is known that some transcription factors, such as runt-related transcription factor 2 (Runx2) and osterix (Osx, also known as Sp7), are fundamental for the differentiation of MSCs in osteoblasts, since these factors regulate the expression of genes related to osteoblasts including that of osteocalcin [91], [92].
Bone morphogenetic proteins (BMPs) are a group of proteins that can play a vital role in cell stimuli, such as differentiation, proliferation and inhibition of growth of various cell types, depending on the cellular microenvironment and other regulatory factors [93]. There are approximately 20 known BMPs, but BMP2 and BMP4 act as the main triggers for osteogenic differentiation [94]. BMP2 has been widely used to functionalize TNTs (see Table 1), including a recombinant human form, to make osseointegration faster.
Stem cells differentiate into osteoprogenitors with limited self-renewal capacity, after osteoinduction; they become pre-osteoblasts with limited proliferation, until they mature into mature osteoblasts that synthesize the osteoid which is the non-mineralized, organic component of the bone matrix. The osteoid is then mineralized to form the trabecular bone that eventually restructures into lamellar bone in direct contact with the surface of the implant [90], [95]. The optimal implant surface nanostructure for osteoblast proliferation is yet to be established [96].
Cells may proliferate or remain quiescent in response to the cellular environment; while the progression of the G1 phase to the S phase of interphase is considered the point of no return, since in the absence of stress such as DNA damage, the cell is committed to complete the cell cycle and divide [97]. The stimulus of biomolecules for the proliferation of osteoblasts is illustrated in Fig. 4b; the p38 mitogen-activated protein kinase (MAPK), extracellular, signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) can be activated, resulting in the stimulation of osteoblast proliferation. BMPs, for example, are capable of activating these three pathways [98].
Biomaterials capable of modulating the response of osteoblasts and osteoprogenitor cells may be crucial for the mechanical fixation of implants. Biomolecules may be options for making these biomaterials able to do such modulation, as in icariin immobilized in TNTs, which has been found to promote the proliferation of osteoblasts by regulating the expression of genes related to osteogenesis [63], [95].
4.2. Anti-inflammatory action
Surgical injury caused by the insertion of an implant may result in an inflammation that typically occurs in the absence of microorganisms and is called sterile inflammation. The monocytes and neutrophils that circulate in the capillaries surrounding the implant are attracted to and activated in the peri-implant space due to cytokines released by the platelets; such activated leukocytes produce proinflammatory cytokines and chemokines such as interleukin 1 (IL-1) and tumor necrosis factor (TNF), which can induce bone resorption by osteoclasts [84], [99].
Surfaces of TNTs with a diameter of about 78 and 80 nm have been able to promote in vitro adhesion and proliferation of macrophages by reducing the expression of the mRNA of proinflammatory cytokines, even in a lipopolysaccharide (LPS)-induced inflammation of Escherichia coli, used to mimic a stimulus of inflammation caused by bacterial infection [100], [101]. Matching the characteristics of nanotopography with bioactive molecules capable of attenuating the inflammatory response may increase the chances of success in post-implant rehabilitation. Expression of BMP2 protein can be stimulated by proinflammatory cytokines [102]. TNTs functionalized with BMP2 and the carbohydrate trehalose, in addition to enhancing osseointegration, also promoted inhibition of IL-1 and TNF-α production following stimulation with LPS [32]. Fig. 4c demonstrates the recognition and binding of biomolecules to their specific receptors on the surface of cells involved in the inflammatory response, such as macrophages, which can trigger signal transduction and lead to inhibition of proinflammatory cytokine production. For example, activated transcription factor Nrf2 (NF-E2-related factor-2) inhibits the transcription of genes expressing IL-6 and IL-1β in macrophages [103].
It is known that quercetin has an anti-inflammatory action by the rat-paw edema test induced by carrageenan and also by the ability to reduce the production of proinflammatory cytokines by mast cells [104], [105]. Icariin reduced the expression and secretion of IL-1, IL-6 and TNF-α in a model of osteolysis in rat calvaria induced by Ti particles [106]. Therefore, both flavonoids may have an additional, anti-inflammatory effect in their use when immobilized on TNTs in addition to their action on peri-implant bone formation.
4.3. Drug delivery
Osteoblasts can compete with bacteria for adherence to implant surfaces. When bacteria win this competition, they soon secrete extracellular polymeric substances (EPS), forming microcolonies until they develop into a mature biofilm. This leads to an infection that is one of the most recurrent causes of implant loss [107], [108].
Usually peri-implant infections are treated by the administration of systemic drugs, but these are distributed throughout the body rather than to specific sites of interest which entail a series of complications and limitations such as poor biodistribution and low selectivity [109]. Localized drug delivery systems have been the most promising methods to promote a controlled and persistent release of drugs from TNTs to treat specific sites not only for infections but also inflammation and even cancer [110], [111], [112], [113], [114].
In order that the therapeutic agents used to load the TNTs may have extended release, they have been encapsulated into micelles and/or the nanotubular matrices have been coated by biopolymers so that the TNTs do not elute rapidly. Fig. 5 illustrates how such micelles and coatings are being manufactured by biomolecules such as d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) and chitosan, respectively [39], [112], [113], [114], [115].
 Fig. 5. Drug release from TNTs can be extended by encapsulating them in micelles, coating the TNTs with biopolymers or using the two mechanisms at the same time.
Fig. 5. Drug release from TNTs can be extended by encapsulating them in micelles, coating the TNTs with biopolymers or using the two mechanisms at the same time.TPGS is a natural derivative of vitamin E conjugated with polyethylene glycol with amphiphilic and nonionic properties; its voluminous structure and large surface area makes it an excellent emulsifier and solubilizer of hydrophobic drugs. One such example is TPGS micelles encapsulating indomethacin and itraconazole to load TNTs [114], [116], [117]. The antibacterial property of chitosan occurs due to electrostatic interaction between its NH3+ groups and the phosphoryl groups of the phospholipid components of the membrane of bacterial cells, leading to damage in the latter [118]. Therefore, chitosan may converge with the action of antibiotics in preventing bacterial adhesion on the surfaces of TNTs, in addition to prolonging the release of these drugs and favoring better adherence of osteoblasts as mentioned in Table 1.