1. Introduction
Due to their exceptional mechanical qualities, metal based-materials (i.e., metal oxide and composite) are widely used in industry and construction. Metal oxide is a type of metal-based material that is frequently used in industrial process systems. Strontium derivatives (i.e., SrwCaxFeyOz), barium titatinium oxide derivatives (i.e., BaTiOx), nickel ferum oxide (i.e., NiFexOy), and ytterium barium oxide (i.e., YBaxCuyOz) have received a lot of attention in recent years. These compounds are ferromagnetic, paramagnetic, and resistant to heat. As a result, these compounds are frequently used as metal alloys to enhance the performance and resistance of basic materials like carbon steel and its derivatives. Slimani et al. [1] looked at how different ratios of the ferrimagnetic Sr0·92Ca0·04Fe12O19 and ferroelectric BaTiO3 (abbreviated BTO) phases affected the structure, microstructure, optical, electrical, dielectric, and magnetic properties in composites. Due to the ferrimagnetic component's stable magnetic properties, high anisotropy, enhanced magnetization, and significant coercivity, it was chosen for surface coating and mixture for industrial construction. In addition, they group also created BaTiO3/(WO3)x ceramics (where x = 0, 0.5, 1, 2, and 5% wt) using a solid state reaction [2]. The increase in grain size and the absence of impurities were all effective in improving dielectric properties. A high WO3 content results in high dielectrics with low tangent loss, which is ideal for radio frequency and microwave applications.
Almessiere et al. [3] examined the impact of dysprosium (Dy) ions on the structural, microstructural, and magnetic characteristics of nickel nanospinelferrite, NiFe2O4. The cubic phase of Ni nanoferrite had formed. Due to the influence of spin disorder in the surface regions of NiDyxFe2-xO4(0.01 ≤ x ≤ 0.10) NPs, the calculated squareness ratios are discovered to be much less than 0.5. By increasing the remanence Mr, coercivity Hc, and magnetic moment nB values, the Dy3+ ion substitution makes nickel nanoferrite samples more magnetically hard. M(H) curves of magnetization versus field show superparamagnetic nature at room temperature and ferrimagnetic nature at low temperatures. Furthermore, YBa2Cu3Oy-based material also has high superconductivity for use in material construction. Hamrita et al. [4] investigated how ball milling affects the superconducting characteristics of polycrystalline YBa2Cu3Oy compounds. The samples were created by taking into account a thermal cycle with two stages of 950 °C sintering, each followed by an intermediate crushing step. Ball milling has been shown to be a successful method for producing tiny particles with a size of about 15 nm that are gathered into coral-like agglomerates and embedded in the matrix. The intra-grain critical current density of the material under a magnetic field is improved by such a decorated finer structure. Slimani et al. [5] investigated the electrical conductivity fluctuation of YBa2Cu3O7d and Y3Ba5Cu8O18x polycrystalline samples that had nano-sized ferrite cobalt particles added to them. According to SEM observations, the yttrium-based superconductor (YBCO) matrix's CoFe2O4 content increases with a corresponding decrease in grain size. Therefore, the grain size of YBa2Cu3Oy has an important effect in increasing the critical current density itself. However, all of these metal oxide materials still have disadvantages when in contact with a corrosion agent.
The destruction of a metal's surface as a result of environmental interaction is known as corrosion. The metal becomes a significant problem because it results in significant financial loss as a result of exposure to a gas and a fluid. When outed to corrosive environments like high humidity, pH variation, and temperature, especially carbon steel typically exhibits poor corrosion resistance[6]. Various methods to control corrosion, such as the use of chemicals as corrosion inhibitors, surface coatings, cathodic and anodic protection, modifying destructive climate, etc., to combat the risky and hazardous effects of corrosion [7]. Between many all of these methods, using corrosion inhibitors during the industrial pickling process is one of the most crucial ones. In order to create coating materials with exceptional magnetic, electrochemical, and mechanical properties, electrodeposition is one of the most significant and promising techniques [8]. When revealed to wear and corrosion circumstances, the components' lifetime can be extended by accumulating a layer of nanocomposite material [9].
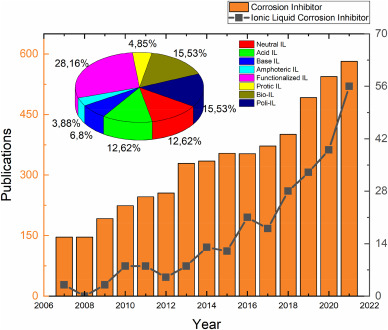
Corrosion inhibitors are compounds that are introduced into the working fluid in small amounts and absorbed into the metal surface chemically, mechanically, or both to prevent further metal dissolution and corrosion [[10], [11], [12], [13]]. The use of corrosion inhibitors has the distinct benefit of not interfering with ongoing activities. Corrosion inhibitors are used in a variety of industries, including petroleum refining, oil and gas exploration and production, water treatment, heavy manufacturing, chemical manufacture, and product additives [14]. In terms of efficiency, Roberge [14] claims that corrosion inhibitors produce 95% inhibition at a concentration of 0.008%-wt. And 90% inhibition at a concentration of 0.004%-wt. The availability, pricing, and toxicity of the product are further factors to consider [14]. According to science, the most essential characteristics for considering inhibitors are electron density, molecular structure, hydrophilicity, hydrophobicity, and dispersibility [10,15,16]. Due to their high electron density and basicity, corrosion inhibitors are mostly organic molecules containing heteroatoms such as nitrogen, sulfur, oxygen, and phosphorus [17]. The number of publications related to corrosion inhibitor and ionic liquid corrosion inhibitor indexed by Scopus is presented in Fig. 1.
 Fig. 1. The number of publications related to corrosion inhibitor and corrosion inhibitor indexed by Scopus. Queries (TITLE (“corrosion inhibitor”) and TITLE-ABS-KEY (“ionic liquid” “corrosion inhibitor”); Accessed on December 23rd, 2021.
Fig. 1. The number of publications related to corrosion inhibitor and corrosion inhibitor indexed by Scopus. Queries (TITLE (“corrosion inhibitor”) and TITLE-ABS-KEY (“ionic liquid” “corrosion inhibitor”); Accessed on December 23rd, 2021.This paper discusses recent research about ionic liquid (IL) as corrosion inhibitors, present chemical structures, name of IL, optimum environment of the experiment, electrode, and maximum efficiency, corrosion hindrance mechanism and performance, adsorption site, isothermal adsorption equation, reaction mechanisms, reactions of mechanism after certain hours (presented in supplement chapter), and velocity impact on corrosion inhibitors. This paper also discusses the characterization and performance of IL corrosion inhibitors, which contain thermodynamical parameters related to measuring IL performance and some tools to observe the performance of IL; recent issues related to IL as corrosion inhibitors; the actual greenness of IL; a brief view of the economic perspective of IL; and IL application to hindrance corrosion as an inhibitor. The final section will summarize everything and provide an opinion on the future of IL as a corrosion inhibitor, including prospects and challenges. Previous review on IL as corrosion inhibitors as Gurjar et al. (2021) [18] specifically discuss analytical techniques of IL, Kobzar et al. (2021) [19] summarizing corrosion inhibitors performance parameters and discuss polymer coating protection, Deyab et al. (2020) [20] discuss IL for oil-based industry, Verma et al. (2021) [9] discuss several approaches for IL performance as corrosion inhibitor, Ardakani et al. (2021) [21] uniquely highlighting imidazolium and azoles based IL. The main difference of this review is a brief discussion about IL greenness and economics before being applied as industrial corrosion inhibitors.
2. ILs as corrosion inhibitor
Hajipour and Rafiee have made a general classification of IL-based on their similarity in terms of conductivity, solubility, viscosity, basicity or acidity, and water miscibility [22]. Based on Hajipour and Rafiee categorization, the types ILs as corrosion inhibitors can be summarized as follow.
2.1. Neutral ILs
Because neutral IL has weak electrostatic interactions with cations, it has a low viscosity and a low melting temperature [[23], [24], [25], [26]]. Neutral IL is known for its ease of handling and lower moisture sensitivity, which leads to good stability in water [27]. The neutral IL corrosion inhibitor structure and adsorption mechanism are tabulated in Table 1. The most general kinds of neutral IL used are 1-butyl-3-methylimidazolium hexafluorophosphate and tetrafluoroborate. These neutral ILs are frequently employed in radical polymerization processes [28]. Even so, the stability of neutral IL is still imperfect since 1-butyl-3-methylimidazolium hexafluorophosphate in water or heated at a higher temperature liberates traces of hydrofluoric acid [29], an extremely corrosive acid, and chloroaluminate based IL for up to 200 °C starts to generate AlCl3 vapor and higher than 250 °C starts to produce HCl vapor [30]. The research on the effect of degradation on corrosion inhibitor performance is still in its infancy. Schoetz et al. [31] compared the electrochemical conductivity of Lewis acid, neutral, and basic binary IL of 1-ethyl-3-methyl-imidazolium chloride with that of aluminum chloride. The conductivity of neutral chloroaluminate IL is the highest (21 mS cm−1 at 21.9 °C) compared to acid (14 mS cm−1 at 22.2 °C) and basic (2.07 mS cm−1 at 21.4 °C). This could stimulate inhibition mechanisms that are more powerful than acidic and basic-based IL [15,32].
Table 1. Neutral IL corrosion inhibitors.
| Chemical Structure | Name | Optimum Environment | Eff. | Adsorption Mechanism | Ref |
|---|---|---|---|---|---|
|
|
1-methyl-3-methylimidazolium thiocyanate | 75 ppm on 0.5 M HCl and 100 ppm on 0.5 M H2SO4 API 5L X52 steel | 83.9% for H2SO4and 74.4% for HCl | Langmuir isotherm, mixed adsorption, N+became center adsorption | [33] |
 |
1-vinyl-3-dodecylimidazolium hexafluorophosphate | 100 ppm or 0.192 mM at carbon steel SAE 1018 in 1.0 M H2SO4 solution | 97% | Langmuir isotherm, mixed adsorption influenced by alkyl chain length which is bound to N+ | [34] |
 |
1-vinyl-3-butylimidazolium hexafluorophosphate | 100 ppm at carbon steel SAE 1018 at 1 M H2SO4 | 85% | Langmuir isotherm, mixed adsorption influenced by alkyl chain length which is bound to N+ | [34] |
  |
1-vinyl-3-octylimidazolium hexafluorophosphate | 100 ppm at carbon steel SAE 1018 at 1 M H2SO4 | 88% | Langmuir isotherm, mixed adsorption influenced by alkyl chain length which is bound to N+ | [34] |
  |
1-vinyl-3-octadecylimidazolium hexafluorophosphate | 100 ppm at carbon steel SAE 1018 at 1 M H2SO4 | 95% | Langmuir isotherm, mixed adsorption influenced by alkyl chain length which is bound to N+ | [34] |
 |
1-vinyl-3-undodecylimidazolium hexafluorophosphate | 100 ppm at carbon steel SAE 1018 at 1 M H2SO4 | 91% | Langmuir isotherm, mixed adsorption influenced by alkyl chain length which is bound to N+ | [34] |
|
|
oleyl ammonium tosylate | 150 ppm or 0.341 mM at carbon steel at 1 M HCl | 97.7% | Langmuir isotherm, mixed adsorption, displace water from the metal surface, mainly influenced by blocking geometry | [35] |
 |
1-butyl-3- methylimidazolium hexafluorophosphate | 0.1%w at mild steel at 2 M HCl | 86% | Langmuir isotherm. Chain length size and shape influence adsorption. Anion becomes an intermediate bridge of the cation core and metal surface. High dipole cation facilitate adsorption | [36] |
 |
1-ethyl-3-methyl-imidazolium hexafluorophosphate | 5 mM at mild steel alloy at 1 M HCl | 82.95% | Langmuir isotherm. The high dipole moment of cation facilitates adsorption to the high dipole moment of the metal surface | [37] |
 |
1-methyl-3-pentyl-imidazolium hexafluorophosphate | 5 mM at mild steel alloy at 1 M HCl | 87.60% | Langmuir isotherm. The high dipole moment of cation facilitates adsorption to the high dipole moment of the metal surface | [37] |
  |
1-methyl-3-octylimidazolium hexafluorophosphate | 5 mM at mild steel alloy at 1 M HCl | 94.62% | Langmuir isotherm. The high dipole moment of cation facilitates adsorption to the high dipole moment of the metal surface | [37] |
  |
1-methyl-1-octyl-pyrrolidinium hexafluorophosphate | 5 mM at mild steel alloy at 1 M HCl | 92.23% | Langmuir isotherm. The high dipole moment of cation facilitates adsorption to the high dipole moment of the metal surface | [37] |
 |
1-methyl-1-octyl-pyrrolidinium thiocyanate | 5 mM at mild steel alloy at 1 M HCl | 93.94% | Langmuir isotherm. The high dipole moment of cation facilitates adsorption to the high dipole moment of the metal surface | [37] |
2.2. Acid ILs
Acid ILs are classified according to the type of acid function they link into the molecule [38]. The acid IL corrosion inhibitor structure and adsorption mechanism are tabulated in Table 2. The acidity of Brønsted acid ILs was related to their ionizable protons, whereas Lewis acid ILs acidity was related to their electron deficiency. In one molecule, this function group could exist as more than one, or a combination of both kinds [[39], [40], [41]]. Lewis acid ILs based on chloroaluminate exhibit good conductivity and low viscosity but lack moisture stability. Zn, Sn, and Fe are used as alternatives for moisture-insensitive IL. Brønsted IL is made from a stoichiometric reaction between Brønsted acid and Brønsted base. PolyILs (PILs) also consist of an acidic group [42]. To simplify and differentiate it with polyionic groups, we considered adding acidic PIL to PIL corrosion inhibitors.
Table 2. Acid IL corrosion inhibitors.
| Chemical Structure | Name | Optimum Environment | Eff. | Adsorption Mechanism | Ref |
|---|---|---|---|---|---|
 |
1,1′-(1,4-phenylenebis (methylene)bis(3-(carboxymethyl)-1H-imidazole-3-ium) dihydrogen phosphate |
75 ppm on 0,5 M HCl and 100 ppm on 0,5 M H2SO4 API 5L X52 steel | 91.1% | Langmuir isotherm. Mixed adsorption. Blocking geometry delivers the main inhibition. Physical adsorption as initiator. Anion became the first bridge for cation to permanently adsorbed to the metal surface. | [43] |
 |
1,1′-(1,4-phenylenebis (methylene)) bis(3-(carboxymethyl) -1H-imidazole -3-ium) chloride |
100 ppm or 0.192 mM at carbon steel SAE 1018 in 0.5 M HCl solution | 94.8% | Langmuir isotherm. Mixed adsorption. Carboxylate especially O atom unshared electron pairs donated into vacant d-orbital Fe. Two imidazolium tends to parallel arrangement on the metal surface. | [44] |
|
|
1-(2-carboxylic acid) ethyl-3-methyl imidazolium hydrogen sulfate |
150 ppm or 0.341 mM at carbon steel at 1 M HCl | 75.8% | Langmuir isotherm. Mixed adsorption. N and O become adsorption centers. Replacing water at the metal surface. | [45] |
|
|
1-(3-carboxylic acid) propyl-3-methyl imidazolium hydrogen sulfate |
100 ppm at carbon steel SAE 1018 at 1 M H2SO4 | 89.5% | Langmuir isotherm. Mixed adsorption. N and O become adsorption centers. Replacing water at the metal surface. | [45] |
|
|
1-(4-sulfonic acid) propyl-3-methyl imidazolium hydrogen sulfate |
100 ppm at carbon steel SAE 1018 at 1 M H2SO4 | 93.0% | Langmuir isotherm. Mixed adsorption. N and O become adsorption centers. Replacing water at the metal surface. | [45] |
|
|
1-(4-sulfobutyl)-3-methylimidazolium hydrogen sulfate | 100 ppm at carbon steel SAE 1018 at 1 M H2SO4 | 80% | Langmuir isotherm. Mixed adsorption. Negative Metal-SO4-electrostatically interacted to cation core. | [46] |
|
|
1-(4-sulfobutyl)-3-methylimidazolium tetrafluoroborate | 0.1%w at mild steel at 2 M HCl | 90% | Langmuir isotherm. Mixed adsorption. Negative Metal-SO4-electrostatically interacted to cation core. | [46] |
 |
1-(4-sulfonic acid) butyl-3-ethyl imidazolium hydrogen sulfate |
15 mM at 0.5 M HCl at carbon steel | 80.8% | Langmuir isotherm. Mixed adsorption. Alkyl caused compact protective will sweep water. | [47] |
 |
1-(4-sulfonic acid) butyl-3-decyl imidazolium hydrogen sulfate |
15 mM at 0.5 M HCl at carbon steel | 97.7% | Langmuir isotherm. Mixed adsorption. Alkyl caused compact protective will sweep water. | [47] |
|
|
1-butyl-3-methylimidazolium hydrogen sulfate | 10 mM at 1 M HCl at mild steel | 93.6% | Langmuir isotherm. Mixed adsorption. | [48] |
|
|
1-butyl-3-methylimidazoliumhydrogen sulfate | 10 mM at 0.5 M H2SO4at copper | 88.8% |
Langmuir isotherm. Adsorption occurs at –C N- groups at cation. Electron donating group at cation increase electron density –C N- groups at cation. Electron donating group at cation increase electron density –C N-. N-. |
[48] |
|
|
1-hexyl-3-methylimidazoliumhydrogen sulfate | 10 mM at 0.5 M H2SO4at copper | 90.3% |
Langmuir isotherm. Adsorption occur at –C N- groups at cation. Electron donating group at cation increase electron density –C N- groups at cation. Electron donating group at cation increase electron density –C N-. N-. |
[48] |
|
|
1-octyl-3-methylimidazoliumhydrogen sulfate | 10 mM at 0.5 M H2SO4at copper | 95.5% |
Langmuir isotherm. Adsorption occurs at –C N- groups at cation. Electron donating group at cation increase electron density –C N- groups at cation. Electron donating group at cation increase electron density –C N-. N-. |
[48] |
2.3. Base ILs
Lactate, formate, acetate, other carboxylates, and dicyanamide are the anion that counts as base ILs [23,49]. Lewis base IL with 1,4-diazabicyclo-[2.2.2.] octane cation and bis(trifluoromethanesulfonyl) amide anion exhibits electrochemical stability and a wide electrochemical window for voltages greater than 4 V [23]. It is easier to achieve basic properties and avoid thermal instability by incorporating basic groups on cations rather than anion [49]. The ability of tertiary amine nitrogen to interact with metal is another quality associated with basic cation. The base IL corrosion inhibitor structure and adsorption mechanism are presented in Table 3.
Table 3. Base IL corrosion inhibitors.
| Chemical Structure | Name | Optimum Environment | Eff. | Adsorption Mechanism | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
1-hydroxyethyl-3-methyl-imidazolium bromide | 10 mM at carbon steel at 1 M HCl | 87.87% | Langmuir isotherm. Mixed adsorption. | [50] | ||||
 |
1-butyl-3- benzyl- imidazolium acetate | 100 ppm at mild steel at 0.5 and 1 M HCl | 39% at 0.5 M HCl and 57% at 1 M HCl | Temkin isotherm. Mixed adsorption through the imidazole ring. | [51] | ||||
|
|
1,3-dibencilimidazolio acetate | 75 ppm API 5LX52 steel in 1 M HCl and H2SO4 | 14% in H2SO4and 85% in HCl | Langmuir isotherm. Mixed adsorption. | [52] | ||||
|
|
2-hydroxyethyl-trimethylammonium- acetate | 1,8 mM at 1 M HCl at mild steel | 96.59% | Temkin isotherm. Blockage geometry is instrumental to adsorption. | [53] | ||||
 |
1-methyl-1-octyl-pyrrolidinium dicyanamide | 5 mM at mild steel alloy at 1 M HCl | 95.74% | Langmuir isotherm. Mixed adsorption. | [37] | ||||
|
|
1,3-dibencilimidazolium dodecanoate | API 5LX52 steel in 1 M HCl and H2SO4 | 31% for 50 ppm in H2SO4and 88% for 100 ppm in HCl | Langmuir isotherm. Mixed adsorption. | [52] | ||||
|
|
1-aminoethyl-3-methylimidazolium bromide | 10 mM at Mild steel in 1000 mg/L HCl and 200 mg/L H2S | 94.16% | Langmuir isotherm. Mixed adsorption. | [50] | ||||
2.4. Amphoteric ILs
Amphoteric ILs such as hydrogen sulfate and dihydrogen phosphate are examples of small numbers of anions [23]. The dihydrogen phosphate offers hydrogen donor and acceptor sites. Finding biocompatible anion and cation for corrosion inhibitors is an exciting area for future research [49]. The amphoteric IL corrosion inhibitor structure and adsorption mechanism are shown in Table 4as follows.
Table 4. Amphoteric IL corrosion inhibitors.
| Chemical Structure | Name | Optimum Environment | Eff. | Adsorption Mechanism | Ref |
|---|---|---|---|---|---|
|
|
1-propyl-3-methylimidazolium bis(trifluoromethyl-sulfonyl) imide | 500 ppm at mild steel at 1 M HCl | 66.3% | Langmuir isotherm. Mixed adsorption. | [54] |
 |
1-butyl-3-methylimidazolium bis(trifluoromethyl-sulfonyl) imide | 500 ppm at mild steel at 1 M HCl | 68.9% | Langmuir isotherm. Mixed adsorption. | [54] |
 |
1-hexyl-3-methylimidazolium bis(trifluoromethyl-sulfonyl) imide | 500 ppm at mild steel at 1 M HCl | 75.7% | Langmuir isotherm. Mixed adsorption. | [54] |
 |
1-propyl-2,3- methylimidazolium bis(trifluoromethyl-sulfonyl) | 500 ppm at mild steel at 1 M HCl | 86.7% | Langmuir isotherm. Mixed adsorption. | [54] |
2.5. Functionalized ILs
The quaternization method for 1-alkyl imidazole and functionalized alkyl halides, as well as deprotonation of imidazole by NaH/KH followed by the addition of two equivalents of functionalized alkyl halides or heating a mixture of 1-trimethylsilyimidazole, are the most common procedures for the preparation of functionalized IL cations [50]. Besides imidazolium, pyridinium, pyridazinium, 1,2,4-triazolium, triazine, and phosphazene are also extensively researched [55]. Liu et al. [56] studied the density, dynamic viscosity, and electrical conductivity of two hydrophobic functionalized ILs. At 298.15 K, electrical conductivity follows the order of [HEMMor][NTf2] < [EimCH2CONHBu][NTf2] < [McmMI][NTf2] < [MCNMIM][NTf2] <[EOHMIM][NTf2] [57] van der Waals forces, hydrogen bond, and molecular size all have an effect on conductivity for the same anion as before [56]. The functionalized IL corrosion inhibitor structure and adsorption mechanism are shown in Table 5 as follows.
Table 5. Functionalized IL corrosion inhibitors.
| Chemical Structure | Name | Optimum Environment | Eff. | Adsorption Mechanism | Ref |
|---|---|---|---|---|---|
 |
3-(4-fluorobenzyl)-1-methyl-1H-imidazole-3-ium bromide | 0.01 M at mild steel at 0.5 M H2SO4 | 99.48% | Langmuir isotherm. Pi-electron from imidazolium or heteroatoms offer electrons to vacant d-orbital Fe. | [58] |
 |
1-aminoethyl-3-methyl-imidazolium bromide | 10 mM at carbon steel at 1 M HCl | 94.16% | Langmuir isotherm. | [50] |
 |
3,3′-(1,4-phenylenebis(methylene))bis(1-octyl-1H-imidazole-3-ium)bromide | 100 mg/L at stainless steel 0.5 M H2SO4 | 86.8% | Langmuir isotherm. | [6] |
 |
3,3′-(1,4-phenylenebis(methylene))bis(1-decyl-1H-imidazole-3-ium)bromide | 100 mg/L at stainless steel 0.5 M H2SO4 | 95.9% | Langmuir isotherm. | [6] |
 |
3,3′-(1,4-phenylenebis(methylene))bis(1-dodecyl-1H-imidazole-3-ium)bromide | 100 mg/L at stainless steel 0.5 M H2SO4 | 72.8% | Langmuir isotherm. | [6] |
 |
3-(4-chlorobenzoylmethyl)-1-methylbenzimidazoliumbromide | 0,68 mM at carbon steel 1 M HCl | 97.9% | Langmuir isotherm. | [59] |
 |
octahydroxyethylamino pentaertheritoltetrapropanoate p-toluene sulfonate | 10 ppm at carbon steel at 1 M HCl | 95.0% | Langmuir isotherm. Heteroatoms offer free pair electron to vacant d orbital of Fe. Geometrical blocking occurs to retarded corrosion. | [60] |
 |
1-(3-ethoxy-3-oxopropyl)-2,3,3-trimethyl-3H-indolium bromide | 0.6 mM at carbon steel at 0.5 M HCl | 84% | Langmuir isotherm. | [61] |
 |
1-(2-ethoxy-2-oxoethyl)-2,3,3-trimethyl-3H-indolium bromide | 0.6 mM at carbon steel at 0.5 M HCl | 75% | Langmuir isotherm. | [61] |
 |
N-octyl-2-(4-hydroxybut-2-ynyl) pyridinium bromide | 40 μM at X70 steel at 5 M HCl at 308 K | 97% | Langmuir isotherm. Mixed adsorption. | [62] |
 |
N-dodecyl-2-(4-hydroxybut-2-ynyl) pyridinium bromide | 40 μM at X70 steel at 5 M HCl at 308 K | 97.1% | Langmuir isotherm. Mixed adsorption. | [62] |
 |
N-hexadecyl-2-(4-hydroxybut-2-ynyl) pyridinium bromide | 40 μM at X70 steel at 5 M HCl at 308 K | 97.3% | Langmuir isotherm. Mixed adsorption. | [62] |
 |
1-2-(4-chloro phenyl) 2-oxoethyl)-4, N–N-dimethyl) picolinium bromide | 1 mM at C38 steel at 1 M HCl | 87% | Langmuir isotherm. | [63] |
 |
benzotriazole 2-naphtalenesulphonic acid | 1,5%wt at mild steel at 5%wt of HNO3 | 99.97% | Langmuir isotherm. Mixed adsorption. | [64] |
 |
benzotriazole benzenesulfonic acid | 1,5%wt at mild steel at 5%wt of HNO3 | 98.29% | Langmuir isotherm. Mixed adsorption. | [64] |
 |
3-((4-amino-2-methylpyrimidin-5-yl) methyl)-5-(2-hydroxyethyl)-4-methylthiazol-3-ium chloride | 40 ppm at carbon steel at 1 M HCl | 91.4% | Langmuir isotherm. Mixed adsorption. | [65] |
 |
3,3′-Diethylthiadicarbocyanine iodide | 4 mM at copper at 0.1 M HCl | 96.5% | Langmuir isotherm. Mixed adsorption. | [66] |
 |
1-(2-(4-chlorophenyl)-2-oxoethyl) pyridazinium bromide | 1 mM at steel at 1 M HCl | 91% | Langmuir isotherm. Mixed adsorption. | [67] |
 |
1-(2-(4-nitrophenyl)-2-oxoethyl) pyridazinium bromide | 1 mM at carbon steel at 1 M HCl | 85% | Langmuir isotherm. Mixed adsorption. | [67] |
 |
(2-amino benzyl) triphenyl phosphonium bromide | 10 mM at mild steel at 0.5 M HCl | 99% | Langmuir isotherm. Mixed adsorption. | [68] |
 |
(1-napthylmethyl)-triphenyl phosphonium chloride | 1 mM at mild steel at 0.5 M HCl | 99.72% | Langmuir isotherm. Mixed adsorption. | [68] |
 |
(4-Methoxybenzyl)-triphenyl phosphonium bromide | 1 mM at mild steel at 0.5 M HCl | 97.76% | Langmuir isotherm. Mixed adsorption. | [69] |
2.6. Protic ILs
To differentiate from Brønsted acid IL, protic ILs in this review is restricted to acidic hydrogens that reside in the cationic group [39]. The protic IL corrosion inhibitor structure and adsorption mechanism are shown in Table 6. Initial prediction of protic IL ideal ionicity by calculating ΔpKa = pKa, base precursor – pKa, acid precursor. ΔpKa larger than 8–10 has ideal ionicity [70]. Another general method used to measure ionicity is the Walden plot [71]. The Walden plot consists of log equivalent conductivity versus log fluidity. Smith et al. [72]. Investigate the H-bond network and the solvophobic effect on the rheological properties of five protic ILs, including ethyl ammonium nitrate, propylammonium nitrate, ethanol ammonium nitrate, ethyl ammonium formate, and dimethylammonium formate. Primary ammonium ILs act as Newtonian fluids at low stress but shear thin at high stress. The viscosity is influenced by the spongelike nanostructure and H-bond strength. In the non-polar phase, a sterically hindered tail group on the cation side reduces viscosity.
Table 6. Protic IL corrosion inhibitors.
| Chemical Structure | Name | Optimum Environment | Eff. | Adsorption Mechanism | Ref |
|---|---|---|---|---|---|
  |
benzotriazole 2-naphtalenesulphonic acid | 1.5%wt at 5%wt of HNO3 at the brass | 98% | Langmuir isotherm. Mixed adsorption. | [64] |
 |
1-butyl-3-methylimidazolium tetrafluoroborate | 0.38 mM at carbon steel at saturated Ca(OH)2 solution with 3.5%wt NaCl | 89% | Langmuir isotherm. Mixed adsorption. | [73] |
  |
1-decyl-3-methylimidazolium tetrafluoroborate | 500 ppm at carbon steel at 1 M HCl | 98.08% | Langmuir isotherm. Mixed adsorption. | [74] |
 |
bis(2-hydroxyethyl) ammonium stearate | AISI 316L Steel disk | Langmuir isotherm. Mixed adsorption. | [75] | |
 |
di-bis[2-hydroxyethyl] ammonium succinate | AISI 316L Steel disk | Langmuir isotherm. Mixed adsorption. | [75] |
2.7. Bio ILs
Certain halide-contained ILs have certain drawbacks such as toxicology, ecology, and economic issues, and one of the alternative approaches is to use biodegradable and non-toxic materials, which leads to another kind of IL: bio-ILs. This characteristic is potentially upgraded to support a recent concern about green corrosion inhibitors. The bio IL corrosion inhibitor structure and adsorption mechanism are summarized in Table 7. The presence of anisotropichydrogen bonds in bio ILs may have a significant impact on their properties [76]. Fukaya et al. [77] suggest the intra-molecular hydrogen bonds in an anion would lead to the delocalization of negative charge and weaken the electrostatic interaction with the cation and lead to lower melting temperature and viscosity. Muhammad et al. [78] investigated the physical properties and cytotoxicity of choline-based ILs, while Restolho et al. [79] investigated surface tension.
Table 7. Bio IL corrosion inhibitors.
| Chemical Structure | Name | Optimum Environment | Eff. | Adsorption Mechanism | Ref |
|---|---|---|---|---|---|
 |
2-hydroxyethyl-trimethyl ammonium chloride |
1.8 mM at 1 M HCl at mild steel | 96.59% | Langmuir isotherm. Mixed adsorption. | [53] |
 |
2-hydroxyethyl-trimethyl ammonium iodide |
1.8 mM at 1 M HCl at mild steel | 96.59% | Langmuir isotherm. Mixed adsorption. | [53] |
  |
L-phenyl alanine methyl ester saccharinate | 100 ppm at 1 M HCl at mild steel | 79.9% | Langmuir isotherm. Mixed adsorption. | [80] |
  |
l-Leucine methyl ester saccharinate | 100 ppm at 1 M HCl at mild steel | 77.78% | Langmuir isotherm. Mixed adsorption. | [80] |
  |
l-Alanine methyl ester saccharinate | 100 ppm at 1 M HCl at mild steel | 69.87% | Langmuir isotherm. Mixed adsorption. | [80] |
  |
tetra-n-butyl ammonium l-methioninate | 1.59 mM at mild steel at 1 M HCl | 95.1% | Langmuir isotherm. Mixed adsorption. | [81] |
 |
glycine propyl ester lauryl sulfate |
100 ppm at mild steel at 1 M HCl | 93.3% | Langmuir isotherm. Mixed adsorption. | [82] |
  |
glutamic acid propyl ester lauryl sulfate |
50 ppm at mild steel at 1 M HCl | 93.42% | Langmuir isotherm. Mixed adsorption. | [75] |
 |
proline nitrate | 300 ppm at mild steel at 1 M HCl | 91.78% | Langmuir isotherm. Mixed adsorption. | [83] |
 |
2-(3-(carboxy methyl)-1H-imidazole-3-ium-1-yl) acetate | 0.55 mM at mild steel at 1 M HCl | 90.0% | Langmuir isotherm. Mixed adsorption. | [84] |
 |
2-(3-(1-carboxy ethyl)-1H–imidazole-3-ium-1-yl) propanoate | 0.55 mM at mild steel at 1 M HCl | 93.47% | Langmuir isotherm. Mixed adsorption. | [84] |
 |
2-(3-(1-carboxy-2-phenylethyl)-1H-imidazole-3-ium -1-yl)-3-phenylpropanoate |
0.55 mM at mild steel at 1 M HCl | 96.08% | Langmuir isotherm. Mixed adsorption. | [84] |
  |
1,1,3,3-tetramethyl guanidine L histidine |
Blended with PEG 200 2%wt. | Reduce 0.0007 g weight loss | Langmuir isotherm. Mixed adsorption. | [85] |
  |
1,1,3,3-tetramethyl guanidine L glutamic acid |
Blended with PEG 200 2%wt. | Reduce 0.0003 g weight loss | Langmuir isotherm. Mixed adsorption. | [85] |
  |
1,1,3,3-tetramethyl guanidine L aspartic acid |
Blended with PEG 200 2%wt. | Reduce 0.0001 g weight loss | Langmuir isotherm. Mixed adsorption. | [85] |
  |
tetra butyl phosphonium tryptophan |
2%wt of the water in steel | Corrosion current density reduced from 0.911 μA/cm2to 0.016 μA/cm2 | Langmuir isotherm. Mixed adsorption. | [86] |
2.8. Poly ILs
An IL linked to a compound's monomer is called a polyIL (PIL). The tunability of the IL ion pair combination or the polymer backbone might change the PIL's nature. At the solid-liquid interface, polymeric compound adsorption activity is known to display a lateral diffusion phenomenon, which can also involve Brownian motion, in which polymeric IL is transported from one active metal surface to another, or even to bulk solutions [15,[87], [88], [89], [90]]. To investigate lateral diffusion, single-molecule tracking is employed [[90], [91], [92]]. PILs are prepared by mechanisms other than traditional copolymerizationtechniques, such as free radical polymerization, step-growth polymerization, atom transfer radical polymerization, reversible addition-fragmentation chain transfer, cobalt-mediated radical, nitroxide-mediated, and ring-opening metathesis polymerization, can expand their structural toolbox [93,94]. The PIL corrosion inhibitor structure and adsorption mechanism are shown in Table 8. PIL conductivity becomes the primary influencer of delocalized ions' conductivity. Low charge results in low mobility of PIL and, as a result, low conductivity, and vice versa. The presence of counter ions has a strong impact on PIL behavior in fluid, when PIL is combined with nonpolar ions, its solubility improves. Nonetheless, high temperatures cause depolymerization, scission, or thermal deterioration of the PIL backbone. Some polymeric compounds, such as Arabic Gum, chitosan, polyethylene glycol, polyvinyl alcohol, polyacrylamide, polyanthranilic acid, polyvinyl pyrrolidine, carboxymethyl cellulose, octyl phenol ethoxylate, aniline, piperazine, formaldehyde, vinylpiridine-based polymer, and others, have previously been used as corrosion inhibitor compounds [90].
Table 8. PIL corrosion inhibitors.
| Chemical Structure | Name | Optimum Environment | Eff. | Adsorption Mechanism | Ref |
|---|---|---|---|---|---|
 |
poly[3-butyl-1-vinylimidazolium]bromide | 400 ppm at 1 M HCl mild steel | 96.4% | Langmuir isotherm. Mixed adsorption. | [94] |
 |
2-hydroxyethyl-trimethyl Ammonium iodide |
200 ppm at 1 M HCl C1020 steel | 89% | Langmuir isotherm. Mixed adsorption. | [95] |
 |
poly [1,1’-(butane-1,4-di-yl)-diimidazole-3,3′-diphenyl-diium bromide] | 0.2 g/L at 0.5 M H2SO4at pure copper | 98.2% for PDIBMB | Langmuir isotherm. Mixed adsorption. | [96] |
 |
poly[2-acrylamido-2-methylpropane sulfonic acid] | 250 ppm at 1 M HCl at the steel | 85.0% | Langmuir isotherm. Mixed adsorption. | [97] |
 |
poly[2-acrylamido-2-methylpropane sulfonic acid diethyl ethanolamine] | 25 ppm at 1 M HCl at the steel | 95.8% | Langmuir isotherm. Mixed adsorption. | [97] |
 |
poly[2-acrylamido-2-methylpropane sulfonic acid-triethanolamine] | 250 ppm at 1 M HCl at X-65 Steel | 91.4% | Langmuir isotherm. Mixed adsorption. | [98] |
 |
Poly[2-acrylamido-2-methylpropane sulfonic acid-triethylamine] | 250 ppm at 1 M HCl at X-65 Steel | 83.7% | Langmuir isotherm. Mixed adsorption. | [98] |
 |
Poly[2-acrylamido-2-methylpropane sulfonic acid-trimethylamine] | 250 ppm at 1 M HCl at X-65 Steel | 80% | Langmuir isotherm. Mixed adsorption. | [98] |
 |
chitosan-p-toluene sulfonate lauric | 250 ppm at 1 M HCl at X-65 steel | 98.6% | Langmuir isotherm. Mixed adsorption. | [99] |
 |
chitosan-p-toluene sulfonate myristic | 250 ppm at 1 M HCl at X-65 steel | 95.6% | Langmuir isotherm. Mixed adsorption. | [99] |
 |
chitosan-p-toluene sulfonate palmitic | 250 ppm at 1 M HCl at X-65 steel | 93% | Langmuir isotherm. Mixed adsorption. | [99] |
 |
chitosan-p-toluene sulfonate stearic | 250 ppm at 1 M HCl at X-65 steel | 88.4% | Langmuir isotherm. Mixed adsorption. | [99] |
3. Characterization and performance of IL-based corrosion inhibitors
The potentiodynamic study was used to calculate efficiency and illustrate the general phenomenology of IL. The difference in corrosion potential with IL minus without IL smaller than 85 mV is a mixed-type inhibitor. On the other hand, if the potential difference is negative and under −85 mV, the IL could be cathodic type, and if the potential difference is greater than 85 mV, the IL could be an anodic type [100]. If the value is significantly large, then the IL could be involved in the electrocatalytic effect process [94,101]. Most IL from literature studies is shown as a mixed inhibitor. The mixed-type phenomenon is hampering the cathodic and anodic corrosion processes. Goyal et al. [69] potentiodynamic polarization curve of (1-napthyl-methyl)-triphenyl phosphonium chloride as an inhibitor for carbon steel immersed in 0.5 M H2SO4for concentration with sequences of 10−5 M, 10−4 M, 10−3 M, 10−2 M, and 0.5 M, which is shown in Fig. 2.
 Fig. 2. The top is the potentiodynamic polarization curve of a carbon steel surface immersed in 0.5 M H2SO4 under the (1-napthylmethyl)-triphenyl phosphonium chloride (4-NpMe-TPB) influence for concentrations of 10−5, 10−4, 10−3, 10−2, and 0.5 M at various temperatures of (a) 298 K, (b) 308 K, (c) 318 K, (d) 328 K, reprinted with permission from Ref. [69]; The bottom is potentiodynamic polarization curves for mild steel in 1 M HCl solution at 303 ± 1 K in the absence and presence of different concentrations. Potentiodynamic polarization curves of mild steel in 1 M HCl without and with various concentrations of (A) [EMIM][BF4], (B) [BDMIM][BF4], and (C) [C10MIM][BF4], reprinted with permission from ref. [100].
Fig. 2. The top is the potentiodynamic polarization curve of a carbon steel surface immersed in 0.5 M H2SO4 under the (1-napthylmethyl)-triphenyl phosphonium chloride (4-NpMe-TPB) influence for concentrations of 10−5, 10−4, 10−3, 10−2, and 0.5 M at various temperatures of (a) 298 K, (b) 308 K, (c) 318 K, (d) 328 K, reprinted with permission from Ref. [69]; The bottom is potentiodynamic polarization curves for mild steel in 1 M HCl solution at 303 ± 1 K in the absence and presence of different concentrations. Potentiodynamic polarization curves of mild steel in 1 M HCl without and with various concentrations of (A) [EMIM][BF4], (B) [BDMIM][BF4], and (C) [C10MIM][BF4], reprinted with permission from ref. [100].The difference between uninhibited and inhibited corrosion potentials from highest to lowest at (a) 298 K is 14 mV–2 mV, (b) 308 K is 39 mV–13 mV, (c) 318 K is 52 mV–4 mV, and (d) 328 K is 14 mV–3 mV. 1-NpMe-TPC works as a mixed-type IL corrosion inhibitor that inhibits both mild steel acidic dissolution and H+ cathodic. This no inclination to anodic type suggests that IL is also a relatively safe inhibitor if present in scant amounts since there will be no intensification of localized corrosion rate. Other ILs results also showed similar results as in the bottom study by Sassikumar et al. [74] (A) [EMIM][BF4], (B) [BDMIM][BF4], and (C) [C10MIM][BF4] at concentrations of 50 ppm, 100 ppm, 300 ppm, and 500 ppm, for [EMIM][BF4] the highest and lowest differences in Ecorr are 15 mV (100 ppm) and 2 mV (500 ppm), while the highest and lowest differences in Ecorr for [BDMIM][BF4] are 73 mV (50 ppm) and 8 mV (500 ppm) and Ecorr for [C10MIM][BF4] are 73 mV (50 ppm) and 8 mV (500 ppm). The potentiodynamic gradient also reveals information about other events that occur.
The fact that the IL adsorption mechanism has a significant effect on cathodic branch slope, resulting in a decrease in cathodic branch current density, suggests that the IL adsorption mechanism removes hydrogen evolution. Fig. 2(a), (b), Fig. 2(c), and Fig. 2(d) depict this mechanism shift. The blank solution is represented by the light blue curve. With the naked eye, we can see that the concentration increases as the curve becomes more slanted, implying that hydrogen evolution is considerably slowed [102]. The lack of a substantial variation in slope between the anodic branch and the blank solution shows that the metal dissolving process is unchanged. Fig. 2 indicates that there are no differences in slopes for any concentrations when compared to the blank solution. The declivous curve appears exclusively in the top picture, implying that passivation occurs, and we expect that 4-NpMe-TPB IL increases passivation rather than only adsorption to protect the metal surface.
ΔGoads is a strong indication of the adsorption of IL; more negative means more strength and vice versa. It is also a preliminary determination of how adsorption works. ΔGoads smaller than −20 kJ/mol indicate the charged moiety of the inhibitor and the charged metal surface interact with each other, supporting physisorption work dominantly. ΔGoads larger than −40 kJ/mol charge share or electron transfer from the bulk electron density compound moiety for making covalent bonds with the metal surface or chemisorption[33,103]. The fraction of the IL-occupied first monolayer on the surface to available metal surface sites is known as the surface coverage degree, which is denoted, θ and the value lies between 0 and 1. Surface coverage degree 0 means no sites occupied by IL, and surface coverage degree 1 means all sites available are occupied by first monolayer IL. Kads is an adsorption equilibrium constant. Kads were obtained by using the most suitable adsorption equation. With those two values, ΔGoads could be calculated by eq. (1) R is the universal gas constantwith a value of 8.314 JK−1mol−1, 55.5 is the standard condition of water molarity in water, and T is the absolute temperature in Kelvin.(1)
Another thermodynamic variable is adsorption enthalpy, ΔHoads, which can be calculated using eq. (2) and adsorption entropy, ΔSoads, which can be calculated using eq. (3) [51,68,104] Adsorption enthalpy can be used instead of adsorption Gibbs to distinguish between physisorption and chemisorption. Physisorption is defined as enthalpy less than −40 kJ/mol, whereas chemisorption is defined as enthalpy more than −100 kJ/mol. The value in the middle represents a hybrid pathway, with physisorption or chemisorption depending on how close the two values are [16,105,106].