1. Introduction
Tuberculosis (TB), caused by infections with Mycobacterium tuberculosis (Mtb), is an infectious disease of enormous public health impact. TB is an airborne disease initiated by the inhalation of infected droplets that travel the upper respiratory tract and bronchi, being deposited in the lower airways. Here, Mtb is thought to be recognized and phagocytosed by macrophages, specifically alveolar macrophages. In the majority of cases, this initial interaction between Mtb and immune cells results in the development of a host protective response capable of eliminating the invading pathogen. In some cases however, the immune response does not eliminate the infection thereby resulting in a spectrum of disease with different phenotypes and clinical manifestations [1]. It is estimated that approximately one-third of the world population has contacted with Mtb and part of this population is likely latently infected [2]. Although these individuals do not show signs or symptoms of TB, as the bacteria proliferation is under immune control, they have a 10% increased risk of progressing into active TB during their life time being therefore important reservoirs for transmission [3].
The current anti-TB therapy protocols comprise of a six-month combination course of rifampicin (R), isoniazid (I), pyrazinamide (Z) and ethambutol (E), which are first-line anti-TB drugs. All four drugs are taken during the first two months of the treatment following a period of four months where only rifampicin and isoniazid are taken. Although the success rate of this regimen has been estimated to be over 85% [2], the full length of treatment is crucial for the effective and complete eradication of the pathogen. However, the incorrect use of anti-TB drugs or the use of ineffective drug formulations such as single drug regimen, poor quality medicines or inappropriate storage conditions and premature treatment interruption due to the long duration of the therapeutic regimen and the associated toxic side effects, lead to drug resistance [2].
Treatment for drug resistant strains, which include rifampicin-resistant TB (RR-TB), multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) is longer (up to 2 years) and requires more expensive and more toxic drugs with treatment success rates lower than 54% [2].
Therefore, in addition to classical drug discovery strategies, new approaches are urgently needed to get a faster, more efficient and less harmful treatment. In this regard, the development of novel therapies aiming at pulmonary delivery and drug targeting to the site of infection could be a promising solution to allow a sustainable and controlled release of medicines with therapeutic action, while decreasing the dosage and frequency of conventional chemotherapy and minimizing side effects. Moreover, these approaches may lead to a higher efficiency of the treatment and to a higher patient compliance, minimizing the risk of therapy failure and the development of drug resistant strains.
2. Delivery of anti-tuberculosis drugs to the lungs
2.1. Advantages of the pulmonary route
Despite the natural barriers to prevent invasion of unwanted airborne particles or living entities, such as airway geometry, humidity, mucociliary clearance and resident populations of macrophages, the lungs are constantly challenged with infectious agents, including Mtb, which make these air-filled organs an attractive target for drug delivery strategies as it provides direct access to the infection site. Delivery systems for TB treatment through the pulmonary route present advantages when compared to the conventional oral and injectable routes. These include drug delivery directly to the infected area, increasing local drug concentration which will impact in bacterial burden and reduce the systemic dosage of the drug [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. Additionally, inhalable systems avoid unwanted side effects often caused by drug metabolism in the gastrointestinal tract before the drug reaches the systemic circulation [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. Unlike the injectable route, inhalation is a pain free and self-administrable delivery means favoring patient convenience and compliance to the treatment.
2.2. Particulate carriers as inhalable delivery systems
Pure drug formulations are typically burst released in the lungs and rapidly undergo unspecific distribution [5,[14], [15], [16], [17], [18]]. In order to achieve a more efficient solution, therapeutic agents can be formulated into particulate carrier systems such as microparticles, nanoparticles, liposomes, micelles or dendrimers. Such systems allow protection of drugs from direct contact with the lung tissue, avoiding early degradation, preclude rapid clearance from the body and assist the control and sustain release of drugs over long periods of time. Particulate carriers also reduce drug toxicity, circumvent undesirable physicochemical properties of the drugs (e.g. low water solubility) and improve drug up-take by macrophages [19].
In recent years the advantages of inhalable particulate carrier systems have been allied to the benefits of dry powder formulations to be delivered by dry powder inhalers (DPIs) for an improved drug delivery system. Dry powder formulations have improved stability as a result of its dry form, do not require refrigeration and allow longer storage periods. Formulations are often combined with pharmaceutically suitable excipients such as lactose, leucine, mannitol or trehalose to improve processing and aerodynamic properties [20,21]. DPIs are propellant free, portable, easy to use and cost-effective devices. Moreover, DPIs are activated by the inspiration effort of patients, allowing a rapid and higher dose administration and a more efficient pulmonary drug deposition [22,23]. Thus, DPIs are currently considered the most convenient and suitable alternative for inhaling anti-TB drugs [24].
2.3. Design and features of inhalable particulate systems
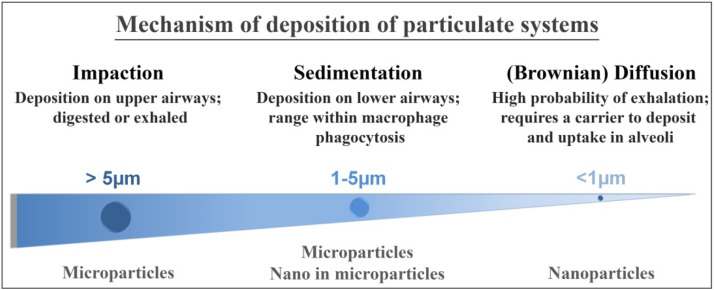
Medicinal particles are designed and developed so their deposition in the respiratory tract can be predicted rather precisely (Fig. 1). The behavior of inhaled particles and their deposition depend of several parameters including particle dimensions, density, shape, composition, concentration, surface properties such as particle charge and the breathing pattern (air flow) of the individual [13,25].
 Fig. 1. Schematic representation of the particle deposition within the upper or lower airways considering their dimensions and the type of carrier systems used for their delivery.
Fig. 1. Schematic representation of the particle deposition within the upper or lower airways considering their dimensions and the type of carrier systems used for their delivery.The aerodynamic diameter is an important parameter affecting the pulmonary trajectory and depends on both particle dimension and density. Particles with an aerodynamic diameter in the range 1–5 μm are deposited in the lower airways, which is a desirable location for anti-TB drugs [13,[26], [27], [28]]. On the other hand, particles larger than 5 μm undergo deposition in the upper regions, such as the nasopharynx and they are consequently swallowed, while particles smaller than 1 μm are likely to be exhaled or retained in the alveoli if transported by a carrier system [13,26,29]. After reaching the lower airways, particulate systems are likely to be either phagocytized by alveolar macrophages, adhere to the lung tissue or enter the blood circulation via lung vascular tree. Another potential destination is the entrapment of these particles within the granulomas which are compact and organized aggregates of immune cells where Mtb persists [30,31].
2.4. Types of microparticulate dry powder systems
Liposomes, micelles and polymeric particles have been developed as inhalable dry powder particulate systems aiming at anti-TB drug delivery strategies. Liposomes and micelles studies were reviewed elsewhere [32,33]. Polymeric particles offer great opportunities in target drug delivery and controlled and sustained release of drugs. The possibility of surface modification of polymeric particles can give rise to systems with a great diversity of physicochemical properties and, if desired, favor a more efficient targeting approach [34]. Additionally, the production of polymeric particles is considerably more cost-effective than lipidic systems.
Polymeric particles include microparticles and nano-in-microparticles and these can further be divided into nanocomposites (also referred as nano-embedded microparticles) and porous nanoaggregate particles (PNAPs) (Fig. 2). Nano-in-microparticle systems have been developed to utilize nanoparticulate systems, often exhaled from the lungs, into successful inhalable drug carrier systems (Fig. 1). In nanocomposites, drug loaded nanoparticles are incorporated in a micronized sugar matrix (e.g. lactose, mannitol, leucine, maltodextrin) to form micro-scaled particles of 1–5 μm. These nanocomposites can then be decomposed into nanoparticles in the lower airways, since the sugar moiety is soluble in the lung lining fluid. On the other hand, PNAPs consist of spherical nanoaggregates at the micron-size level that also re-disperse into the elementary nanoparticles in the lung lining fluid.
 Fig. 2. Developed inhalable dry powder systems for anti-TB drug delivery based on polymeric particles.
Fig. 2. Developed inhalable dry powder systems for anti-TB drug delivery based on polymeric particles.A considerable focus has been given to synthetic polymers poly(lactide-co-glycolide) (PLGA) and polylactide (PLA) due to their biocompatible and biodegradable properties, non-toxicity [[35], [36], [37]] and to the ability to encapsulate hydrophobic anti-TB drugs. Synthetic polymers also benefit from high purity products and batch-to-batch uniformity. However, more recently, the trend for dry powder production has been shifted towards the use of natural polymers such as chitosan, gelatin or guar gum due to their abundance and availability in nature, cost effectiveness and the fact that these raw materials require a minimal use of organic solvents to be processed. Also, combinations of polymers have been tested to merge properties of the polymers involved in order to obtain more efficient delivery systems.
2.5. Techniques used to develop polymeric microparticulate dry powders
A brief explanation on the most common production techniques to prepare inhalable polymeric drug delivery microparticulate and nano-in-microparticulate systems is described in Table 1. The techniques presented in this table have shown to produce carrier systems with suitable aerodynamic diameters using a wide range of synthetic and natural based polymers.
Table 1. Summary of the techniques most often used in the preparation of polymeric microparticulate and nano-in-microparticulate systems for anti-TB pulmonary drug delivery.
| Technique | Brief explanation of working principle | Advantages of the process | Disadvantages of the process | Ref. |
|---|---|---|---|---|
| Emulsion/solvent evaporation | An organic solvent is used to dissolve the drug and polymer followed by the addition of an aqueous solution containing a dispersion stabilizer. The emulsion is sonicated followed by solvent evaporation. | Preserves the physicochemical characteristics of the polymer |
Relatively wide size distribution of microparticles Use of organic solvents Difficult to scale up Two step process |
[38,47] |
| Spray-drying (SD) | Transforms a feed in the fluid state into a dried particulate form by spraying into a hot drying medium. |
Simple, fast, one-step, continuous and reproducible production process No final drying step High versatility Easy to scale up Higher drug incorporation when compared with other techniques |
Higher polydispersity when compared with other techniques Expensive equipment and operation conditions |
[38,48] |
| Supercritical anti-solvent | The drug and polymer are dissolved in an organic solution and the supercritical fluid (e.g. SCO2) acts as an anti-solvent promoting the precipitation of the microparticles. | Single-step process |
Use of organic solvents difficult to scale up Expensive Labour intensive |
[49] |
| Anti-solvent Precipitation/SD | Drop-wise addition of an anti-solvent (e.g. ethanol, diethyl ether)/drug solution to a polymer solution under rapid mixing. The drug loaded nanoparticles are next spray dried with excipients to form nanocomposites. | Low cost |
Two-step process Use of organic solvents Difficult to scale-up |
[50] |
| Ionotropic gelation/SD | Drop-wise addition of cross-linking agent (e.g. TPP, CaCl2) into polymer solution containing drug and under stirring. The drug loaded micro- or nano- particles are next spray dried with or without excipients to form microparticles or nanocomposites. | Avoids the use of toxic reagents used in the chemical cross-linking |
Two step process Difficult to scale-up |
[51,52] |
Legend: SCO2: supercritical carbon dioxide; SD: Spray-drying; TPP: tripolyphospate.
Emulsion followed by solvent evaporation is commonly used to produce microparticles, in particular PLGA microparticles, however these are produced with a relatively wide size distribution [38]. Makino et al. have used this technique with a Shirasu porous glass (SPG) membrane to formulate microparticles with lower size dispersion [39,40]. In order to overcome the low yield of this process, a premix membrane emulsification method was used to prepare monodispersed PLGA microparticles [41,42]. Alternatively to SPG membrane emulsification, an adjuvant strategy based on glass beads facilitated stirring, improved the homogeneity of PLGA microparticles produced by the emulsion/solvent evaporation method [43]. Although emulsion/solvent evaporation is often used to produce anti-TB drug loaded microparticles, it is labor intensive and not practicable for large production, which can limit translational strategies into therapies.
Inhalable microparticles and nano-in-microparticles were successfully obtained using the spray-drying (SD) technique [38,[44], [45], [46]], the most used technique for production of inhalable dry powders for anti-TB delivery. SD is a simple, fast and versatile technique that can be used with different spray nozzles. For instance, rifampicin dihydrate microcrystals were coated with PLGA and/or PLA by SD with a three-fluid spray nozzle producing core-shell microcapsules with the appropriate aerodynamic properties to reach the alveoli and with controlled release properties not achieved by using only rifampicin dihydrate microcrystals [14,15]. Due to the existence of three independent channels, one for the core (drug), one for the shell (polymer) and the other for the atomizing gas, the process can be done in a single step.
Nanocomposites containing rifampicin-PLGA nanoparticles and mannitol have also been prepared in a one-step approach using a four-fluid nozzle spray drier [53]. In this technique two liquid and two gas passages allowed the drug and the carrier to be prepared in different solvents avoiding the limitations associated to using a common solvent as in the traditional two fluid spray-drier.
Spray-drying can also be combined with techniques such as anti-solvent precipitation and ionotropic gelation that are oriented to nanoparticle production, to fabricate nano-in-microparticulate systems. Inhalable guar gum nanoparticles were prepared using the precipitation technique with ethanol as anti-solvent [50]. Chitosan and alginate nanoparticles have been prepared by the ionotropic gelation, a method based on the complexation between oppositely charged species namely chitosan and tripolyphosphate anion or calcium cation and alginate [51,52,54]. The physical cross-linking by electrostatic interactions avoids toxic reagents used in the chemical cross-linking such as glutaraldeyde.
The supercritical technique has also been proposed for the production of inhalable polymeric microparticles. Patomchaiviwat et al. prepared rifampicin loaded PLA microparticles, in a size range suitable for lower airways deposition, using a supercritical anti-solvent process [49]. Supercritical anti-solvent is a quite recent and barely explored technique for anti-TB drug delivery. Although it is a single step process this technology is expensive and labor intensive and requires deeper investigation to assess its full potential for anti-TB drug delivery.
2.6. Carrier systems oriented for macrophage targeting
Phagocytosis of drug carrier systems by macrophages especially by infected macrophages is a very interesting approach leading to the internalization of these systems, and to an intracellular influx of the drug in the phagosome, where the bacilli reside, which could result in a more efficient anti-TB approach.
Among the physical properties, the size of the particulate carrier system is one of the most important characteristics affecting up-take via phagocytosis (Fig. 3) [55].
 Fig. 3. Physico-chemical properties of microparticulate systems undergoing phagocytosis by alveolar macrophages.
Fig. 3. Physico-chemical properties of microparticulate systems undergoing phagocytosis by alveolar macrophages.Phagocytosis ranged particles (1–6 μm) enter the macrophage and can potentially deliver larger amounts of anti-TB drugs directly to the infection than oral or injected drug doses [56]. Particle shape also plays an important role in the up-take process as the local shape determines the initial contact with macrophages and their phagocytic fate [57]. Using shape-switching particles it was shown that elliptical disks have potential to mitigate phagocytosis, however when they switched their shape into spheres they were internalized by macrophages [58]. In relation to surface charge an increase up-take was observed when PLGA microparticles were coated with cationic polymer polyethylenimine relative to uncoated ones [43]. Furthermore, harder and non-porous particles are more efficiently taken up than soft and porous particles and hydrophobic and insoluble particles are more easily opsonized and this fact increases the probability of recognition by alveolar macrophages [55]. The chemical composition of the particle is also a significant feature for macrophage response. PLGA microparticles prepared with different proportions of lactide and glycolide and with different PLGA molecular weights result in different interactions with alveolar macrophages [59]. The presence of mannose moiety in the structure of polymers such as guar gum and mannan results in higher up-take by macrophages [51,60,61].
The carrier surface can be functionalized to incorporate ligands that target macrophage surface receptors in a process that is known as active targeting. Mannosylated gelatin microparticles have been developed to target macrophage mannose membrane receptor [62]. Other ligands including carbohydrate binding receptors such as galactose, β-glucan, N-acetylglycosamine and folic acid have been explored for macrophage targeting aiming at a wide range of diseases [63]. However, their potential towards therapeutic TB strategies requires further investigation.
3. Emerging inhalable polymeric dry powders
The main outcomes on recent works on dry powder microparticles and nano-in-microparticles for anti-TB drug delivery envisioning the improvement of inhalable administration of therapeutic drugs are compiled in Table 2, Table 3, respectively.
Table 2. Inhalable polymeric microparticulate dry powders for TB treatment.
| Polymer(s) | Drug(s) | Excipient(s) | Fabrication method | MMAD (μm) | FPF (%) | Drug loading (%) | Loading efficiency (%) | Release profile | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Synthetic polymers | |||||||||
| PLGA | R | – | Emulsion/SE; SDa | – | – | Emulsion/SE: 20; SD, 30 | Emulsion/SE: 44; SD: 100 | Burst release in the first 24 h followed by slow release phase. Higher release percentages were observed for SD microparticles than for emulsion/SE microparticles. Higher release percentages at pH 7.4 than at pH 5.2 | [38] |
| R | – | Emulsion/SE | 4.5 | 52 | 34.2 ± 4.0 | 68.5 ± 7.2 | Gradual and almost complete drug release over 6 days | [47] | |
| R | – | Membrane emulsion/SEb | – | – | <17 | 57.5–90.9 | Drug release profile dependent on PLGA molecular weight and monomer ratio and pH of release medium (77–104% release at pH 7.4 and 13–25% release at pH 4.0, in 60 days) | [39] | |
| R | Trehalose | Membrane emulsion/SEb | – | – | 4 | 40 | The release ratio increased in the presence of pulmonary surfactants and was greater at pH 7.4 than 4.0 | [29] | |
| R | – | Premix membrane emulsion/SE | 2.63 | 54 | 5.2 ± 0.6 | 20.8 ± 2.4 | Linear drug release profile, with 40% R released over 4.5 days | [42] | |
| Rc | – | Premix membrane emulsion/SE | 3.43–4.93 | 46.7–69.9 | 4.9–16.5 | 34.6–66.9 | Initial burst release followed by slow release phase. 80% R release from 12 h to 4 days | [41] | |
| R | – | Emulsion/SE with glass beads | – | – | 2 | 50–70 | About 80% of drug released within 7 days. Maximal release rate at pH 7.4 on the first day and then drug release rate decreased until day 15 | [43] | |
| R | Leucine | Emulsion/SD | <4.7 | 43.4 ± 5.7 | 15.46 ± 0.01 | 88.9 ± 1.4 | After 4 h, 69.7% release at pH 7.4 and 62.9% in pH 5.0 | [64] | |
| R | – | SD | – | – | 10 | 100 | The release ratio was not affected by pulmonary surfactants but was dependent on pH of release medium (higher release at pH 7.4 than 4.0) | [48] | |
| R | – | SD | 4.67–5.11 | 22.9–34.2 | – | – | Higher polymer contents led to slower release rates (100% to 20% in 6 h) | [65] | |
| Rd | – | SDe | 3.5–4.2 | 32.7–43.5 | 50 | – | Initial burst release (about 60% in 1 h) followed by sustained release | [15] | |
| Rpt | – | Emulsion/SE; SDa | 2.4–3.0 | 41–57 | emulsion/SE <1; SD, 9 and 22 | Emulsion/SE <10; SD, 95–100 | Initial burst release followed by sustained release (up to 50% release in 7 days) | [46] | |
| Cm | – | Emulsion/SEf | – | – | 1–20 | – | – | [66] | |
| Cmg | – | SD | – | – | 11.55 ± 1.56 | 90.74 ± 12.27 | – | [44] | |
| PLA | R | – | SD | 2.22–2.86 | 55.2–68.4 | – | – | Higher polymer contents led to slower release rates (88% to 42% in 6 h) | [65] |
| Rd | – | SDe | 3.6–4.5 | 26.4–44.5 | 50 | – | Initial burst release (about 50% in 1 h) followed by sustained release; 32% less release of R at pH 5.2 than 7.4 | [15] | |
| R | – | SAS | – | – | 3.3–66.4 | 33.4–91.7 | 70% and 80% PLA microparticles give sustained release without initial burst | [49] | |
| Ih | – | HIP/PCAi | 1–3 | – | 30 | > 100 | Initial burst followed by a period of slower release. Higher drug loading caused higher release ratios. Similar release patterns at pH 7.4 and 5.0. | [67] | |
| Cmj | – | SD | 3.46 | – | 19.8 | – | – | [68] | |
| Ofxk | – | SD | 2.5 | – | 30 | – | Ofx-Pd-PLA microparticles show very low release at pH 7.4 compared to Ofx microparticles; release increased slightly at lower pH | [69] | |
| R, I | – | Emulsion/SE | – | – | R, 11.0; I, 4.3 | R, 45.2; I, 49.6 | – | [4] | |
| R, I | – | SD | 3.57 | 79.0 ± 8.4 | R, 37.5; I, 12.5 | >90 | – | [45] | |
| Rbt, I | – | SD | 3.57 | 78.9 ± 8.4 | Rbt, 25; I, 25 | – | I releases faster than Rbt regardless the pH (7.4 or 5.2) and about 70% of the drugs were released in 10 days. I releases faster at pH 7.4 than at pH 5.2 and on the other hand RFB releases faster at pH 5.2 than at 7.4 | [5] | |
| PCL | I | – | Emulsion/SDl | 3.0 | 51.83 ± 1.00 | – | 64.83 | Release of I from I-PCL microparticles was slower and more sustained than from I alone microparticles. Faster drug release at pH 4.5 than 7.4 for I-PCL microparticles | [16,70] |
| HPMC | R | Lactose | SD | 3.41–3.73 | 52–60 | – | 65–70 | – | [71] |
| Eto | Lactose | SD | 3.01–3.33 | 55–63 | – | 70–75 | – | [72] | |
| Natural polymers | |||||||||
| Chitosan | R | Lactose, leucine | SD | 1.85–3.62 | 29.65–47.29 | 10.09–32.77 | 90.81–98.31 | 68–84% drug release in 6 h | [73] |
| R | Lactose | SD | 2.68–2.98 | 55–73 | – | 93–99 | – | [71] | |
| Rm | Lactosen | Ionotropic gelation/SD | ~5 | 21.46 | – | 45–60 | 90% drug release in 12 h | [74] | |
| Rbtm | Lactosen | Ionotropic gelation/SD | ~5 | 29.97 | – | 70–89 | 50% drug release in 24 h | [74] | |
| I | Lactose, leucine | SD | 2.71–3.85 | 55–67 | – | 88–108 | 90% drug release in 1 h | [75] | |
| I | – | SD or ionotropic gelation/SDo | – | – |
non-crosslinked: 24.2–44.5; cross-linked: 38.3 ± 0.1 |
Non-crosslinked: 89.0–96.8; Cross-linked: 114.9 ± 0.2 |
<50% drug release after 6 h for cross-linked microparticles; non-cross-linked microparticles showed a faster release | [76] | |
| Ofx | Lactosep | Emulsion | – | – | 27.41 ± 0.74 | – | – | [60] | |
| Eto | Lactose | SD | 2.28–2.58 | 58–76 | – | 93–95 | – | [72] | |
| Gelatin | Iq | – | Emulsion/SE | – | – | 18.38 ± 1.08 | 55.9 ± 2.4 | 80% release in 24 h. Release not influenced by pH | [62] |
| HA | Ofx | Lactose | SD | – | 43 | – | 50.0 ± 2.5 | – | [6] |
| LBG | I | – | SD | 1.30–1.83 | – | 8.8 ± 0.1 | 88.8 ± 1.5 | 86% drug release of I in 20 min | [61] |
| Rbt | – | SD | 0.89–1.78 | – | 1.8–10.3 | 86–100 | 37% release of RFB in 20 min | [61] | |
| Rbt, I | – | SD | 6 | 38 | Rbt, 4.4 ± 0.1; I, 8.2 ± 0.3 |
Rbt, 102.1 ± 1.1; I, 94.9 ± 3.3 |
73% release for RFB and 84% for I after 1 h | [77] | |
| Combination of polymers | |||||||||
| Chitosan/ethyl cellulose | Rbt | – | Emulsion/SD | – | – | 4.61–6.11 | 59.40–78.65 | Initial burst release followed by sustained release phase; release rates of cross-linked microparticles were slower than non-cross-linked (7.89% to 15.57% release in 24 h) | [78] |
| Lysine/alginater | I | – | SD | – | – | 44.73–70.16 | – | Sustained release of I for up to 48 h. Release not influenced by pH | [79] |
| PLGA/Gelatins | R | Mannitol | Emulsion/SE | 3.45 | 49.0 ± 6.2 | 25.67 ± 0.73 | 16.75 ± 0.49 | 29% of R release after 4 days and sustained release for up to 14 days | [80] |
| Cm | Mannitol | Emulsion/SE | 3.45 | 49.0 ± 6.2 | 10.81 ± 0.33 | 7.2 ± 0.22 | 40% of Cm release after 4 days and sustained release for up to 14 days | [80] | |
| PAS | Mannitol | Emulsion/SE | 3.45 | 49.0 ± 6.2 | 18.45 ± 0.45 | 12.3 ± 0.3 | 40% of PAS release after 4 days and sustained release for up to 14 days | [80] | |
Legend: ALG: alginate; Cm: capreomycin; CHI: chitosan; EC: ethyl Cellulose; Eto: ethionamide; FPF: fine particle fraction (fraction of the total inhaled dose that reaches the stages corresponding to the cut-off diameter of 5 μm); HA: hyaluronic acid; HIP/PCA: hydrophobic ion-pairing/compressed anti-solvent process; HPMC: hydroxy propyl methyl cellulose; I: isoniazid; LS: lysine; LBG: Locust Bean Gum; MMAD: mass median aerodynamic diameter; Ofx: ofloxacin; PAS: para-aminosalicylic acid; PBS: phosphate-buffered saline; PCL: poly-caprolactone; PLA: polylactide; PLGA: poly(lactide-co-glycolide); Rbt: rifabutin; Rpt: rifapentine; R: rifampicin; SAS: supercritical anti-solvent process; SE: solvent evaporation; SD: spray-drying; SLF: simulated lung fluid.
- a
-
Microparticles were prepared using two different techniques: emulsion/solvent evaporation and spray-drying.
- b
-
Emulsion/solvent evaporation technique with a SPG membrane.
- c
-
A rifampicin-(2-hydroxypropyl)-β-cyclodextrin complex was used.
- d
-
Rifampicin dehydrate microcrystals were coated with PLGA or PLA.
- e
-
Spray-drier with a three fluid spray nozzle.
- f
-
A double-emulsion/solvent evaporation technique was used.
- g
-
Hydrophobic ion pairing with oleate.
- h
-
Ionizable prodrug of isoniazid.
- i
-
Hydrophobic ion-pairing/compressed antisolvent process.
- j
-
A capreomycin‑palladium complex was used.
- k
-
A ofloxacin‑palladium complex was used.
- l
-
The microparticles were obtained by double emulsion followed by spray-drying.
- m
-
Drug-loaded chitosan/tripolyphosphate-microparticles.
- n
-
Drug-loaded microparticles were mixed with lactose
- o
-
Non-cross-linked microparticles were prepared by SD and cross-linked microparticles were prepared by ionic gelation with TPP followed by SD.
- p
-
Lactose did not increased the aerosolization efficiency.
- q
-
Isoniazid loaded-mannosylated gelatin.
- r
-
Mannosylated lysine-co‑sodium alginate conjugate.
- s
-
PLGA microparticles were coated with gelatin.
Table 3. Inhalable polymeric nano-in-microparticulate dry powders for TB treatment.
| Polymer(s) | Drug | Excipient(s) | Fabrication method | MMAD (μm) | FPF (%) | Drug loading (%) | Loading efficiency (%) | Release profile | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Synthetic polymers | |||||||||
| PLGA | R | Lactose, trehalosea | Emulsion/SE/SDb | 1.5–2 | 15–20 | 4.0 | – | – | [20,21] |
| R | Mannitolc | SDd | – | 35 | – | 104.0 ± 2.8 | – | [53] | |
| R | Leucinee | Emulsion/SE/SDf | – | PNAP40, 35.5 ± 2.1; PNAP80, 44.7 ± 2.3g | PNAP40, 5.2 ± 0.1; PNAP80, 10.0 ± 0.1g | – | Burst release of drug (80%) in 1 h and remainder released over beyond 8 h | [28] | |
| HPMC | R | Mannitol and leucinee | Anti-solvent precipitation/SDh | 3.82 | – | – | 69.1 ± 1.8 | 59% of R was released in 24 h | [98] |
| I | Mannitol and leucinee | Anti-solvent Precipitation/SDh | 2.74 | – | – | 69.2 ± 2.1 | 76% of I was released in 24 h | [98] | |
| Natural based polymers | |||||||||
| Alginate | R | Mannitol and leucine | Ionotropic gelation/SDi | 1.58 ± 0.09 | – | – | 56.2 ± 3.9 | Initial burst (30–40% release up to 4 h) followed by sustained release phase (90% up to 60 h) | [52] |
| I | Mannitol and leucine | Ionotropic gelation/SDi | 1.23 ± 0.07 | – | – | 53.3 ± 4.3 | Initial burst (30–40% release up to 4 h) followed by sustained release phase (90% up to 60 h) | [52] | |
| Chitosan | R | Mannitol and leucinec | Ionotropic gelation/SDj | 1.17 ± 0.02 | – | 42.5 ± 4.9 | 70.8 ± 6.6 | Initial burst (80% release in 24 h) followed by sustained release | [51] |
| R | Lactosec | Ionotropic gelation/physical mixingk | 3.3 ± 0.2 | 33.3 ± 0.9 | 12.7 ± 0.1 | 72.0 ± 0.1 | 90% drug release in 24 h | [99] | |
| I | Lactose, mannitol and maltodextrin, w/ or w/o leucinel | Ionotropic gelation/SDj | – | 7.05–45.00 | 1.92–6.00 | 9.30–17.00 | Burst release in 4 h (40–50%) followed by sustained release for 6 days | [54] | |
| I | Mannitol and leucinec | Ionotropic gelation/SDj | 1.21 ± 0.02 | – | 40.2 ± 5.1 | 68.8 ± 7.0 | Initial burst (80% release in 24 h) followed by sustained release | [51] | |
| E | – | SD/physical mixingm | 2.3–2.7 | 32–42 | 99.2–107.2 | – | – | [100,101] | |
| Guar gum | R | Mannitol and leucinee | Precipitation/SD | 3.11 | – | – | 50.4 ± 2.8 | 60% drug release in 24 h and sustained release up to 48 h | [50] |
| R | Mannitol and leucinec | Precipitation/SD | 1.57 ± 0.01 | – | 31.1 ± 4.2 | 52.4 ± 5.0 | 70% drug release in 24 h followed by sustained release | [51] | |
| I | Mannitol and leucinee | Precipitation/SD | 3.53 | – | – | 51.3 ± 2.2 | 70% drug release in 24 h and sustained release up to 48 h | [50] | |
| I | Mannitol and leucinec | Precipitation/SD | 1.43 ± 0.01 | – | 37.2 ± 5.3 | 63.4 ± 4.0 | 70% drug release in 24 h followed by sustained release | [51] | |
| Mannan | R | Mannitol and leucinec | SD | 1.92 ± 0.02 | – | 23.3 ± 6.3 | 45.4 ± 5.0 | 80% drug release in 24 h followed by sustained release in PBS 7.4 | [51] |
| I | Mannitol and leucinec | SD | 1.76 ± 0.03 | – | 41.3 ± 4.2 | 58.4 ± 6.1 | 85% drug release in 24 h followed by sustained release | [51] | |
| Combination of polymers | |||||||||
| Chitosan/guar gum | R | Mannitol and leucinec | Ionotropic gelation/SDn | 1.58 ± 0.03 | – | 35.2 ± 2.4 | 62.4 ± 5.0 | 75% drug release in 24 h followed by sustained release | [51] |
| I | Mannitol and leucinec | Ionotropic gelation/SDn | 1.64 ± 0.02 | – | 26.1 ± 3.2 | 60.8 ± 5.1 | 65% drug release in 24 h followed by sustained release | [51] | |
Legend: E: ethambutol; FPF: fine particle fraction (fraction of the total inhaled dose that reaches the stages corresponding to the cut-off diameter of 5 μm); HPMC: hydroxy propyl methyl cellulose; I: isoniazid; MMAD: mass median aerodynamic diameter; PLGA: poly(lactide-co-glycolide); PNAPs: porous nanoaggregate particles; PNAP40: PNAPs containing 40% nanoparticles; PNAP80: PNAPs containing 80% nanoparticles; R: rifampicin; SD: spray-drying; SE: solvent evaporation.