1. Introduction
Pharmaceuticals are defined as therapeutic drugs used to prevent or treat humans and animal diseases or infections while personal care products (PCPs) are used to improve the quality of human life. In recent years, the increasing levels of these PPCPs in various components of the aquatic environment (water, sediment, and biota) have been increasing causing detrimental effects to aquatic organisms [1]. PPCPs are compounds having one or more ionizable or non-ionizable groups with a molecular weight of less than 500 Da. The European Union (EU) and United States Environmental protection agency (USEPA) categorize various PPCPs such as diclofenac, Ibuprofen, carbamazepine, iopamidol, clofibric acid, triclosan, phthalates and bisphenol A as priority pollutants since they pose a serious threat to both flora and fauna in receiving water bodies [2].
The physicochemical nature of PPCPs makes them recalcitrant to conventional water treatment processes resulting in a prevalence of PPCPs in all water bodies. PPCPs in water are either persistent or pseudo-persistent as they are continuously discharged into the environment. They pose a greater risk to biota (toxicity) than other organic contaminants such as pesticides, polychlorinated biphenyls, perfluoroalkyl substances, dyes, etc., The levels of these compounds are replenished (due to indiscriminate discharge) even though they may be acted upon by natural environmental processes such as biodegradation, photodegradation, and sorption onto sediments/soil. PPCPs and their metabolites have been categorized into lowly, moderately, and highly persistent compounds based on their dissipation time (DT50) in water/sediment [3]. Pharmaceuticals such as paracetamol, Ibuprofen, 2-hydroxyibuprofen and CBZ-diols are classed as lowly persistent compounds since their DT50 is 3–7 days while oxazepem, Iopromide, and Ivermectin were moderately persistent as their DT50 is 15–54 days. Certain pharmaceuticals such as carbamazepine are rated as highly persistent as their DT50 is 119–328 days [3].
PPCPs tend to bioaccumulate in a non-target aquatic organism, especially in fishes. The exposure of goldfish (carauratus) to waterborne gemfibrozil for over 14 days results in a bioaccumulation factor of 113 [4]. The antiepileptic drug, carbamazepine displays a bioaccumulation factor of 2.2 in algae, Pseudokirchneriella subcapitata [5]. The presence of 43 pharmaceuticals in mussel tissues from different classes of Mollusca, has been reported [6]. A bioaccumulation factor ranging from 0.66 to 3.2 for metformin and sertraline has been observed. In Texas- Wastewater treatment plants (WWTP), the presence of two antimicrobial agents namely, triclocarban (TCC), and triclosan (TCS) along with a metabolite methyl-triclosan (M-TCS) was detected in algal samples with bioaccumulation factor ranging from 700 to 1500 for M-TCS; 900–2100 for TCS and 1600–2700 for TCC respectively [7].
The role of PPCPs in the human system is to maximize biological activity at low doses by targeting metabolic, enzymatic, or cell-signaling processes. However, these pharmaceuticals, may be pharmacologically active even in non-target organisms as their mode of action is similar for all biological species. A mode of action for an anti-depressant, Fluoxetine, which targets the serotonin (5-H) signaling pathway has been proposed [8]. Any alteration in 5-HT pathway signaling has adverse effects on reproduction, metabolism, and locomotion in mussels. Apart from affecting signal transmission, these materials may interact with the endocrine system to produce undesired effects by disruption of homeostasis. Cooperative toxic effects has been observed for the combination of an antiepileptic drug, carbamazepine and a lipid-lowering agent, clofibric acid, towards Daphina Magna [9] compared to the sum of the impacts for the single compounds at the same concentrations.
The major concern for the presence of PPCPs in the environment is the development of antibiotic-resistant strains in natural bacterial populations. Extensive use of antibiotics in human medicine and animal husbandry is the major cause of the increase in antibiotic -resistant bacteria which pose a serious threat to the treatment of various infectious diseases such as COVID-19, and SARS caused by pathogenic microorganisms. Natural bacterial strains in WWTP have developed resistance against six commonly used antibiotics such as ciprofloxacin, tetracycline, ampicillin, trimethoprim, erythromycin, and sulphamethoxazole. The toxicity of PPCPs may vary depending on the type of exposed organism, the duration of exposure and contaminant concentrationlevels, as well as the pH, and dissolved oxygen concentration [10] in the supporting medium. Many pharmaceutical contaminants enter the environment after human consumption. As a consequence, metabolite concentrations may play a more significant role than the parent compounds in inducing toxic effects. Some metabolites are more toxic than the parent compound, For instance, the presence of N-acetyl sulfapyridine has been found to be more toxic than the parent sulfapryidine towards algae [11]. In addition, acidic pharmaceuticals like fluoroquinolones and tetracyclines may exhibit different toxicological responses depending on the pH levels in the receiving environment. The sources, fate, and behavior of PPCPs in an aquatic environments and ways to control the levels of PPCPs using VLP methods has been described.
2. Sources, fate, behavior of PPCPs
2.1. Sources of PPCPs
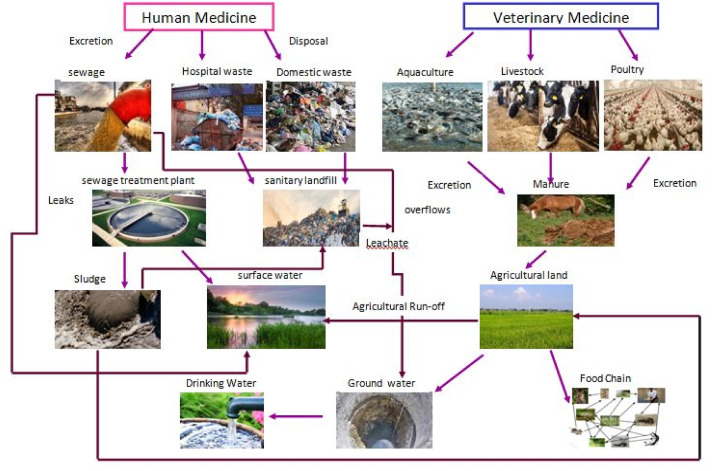
The sources of PPCPs in various spheres of the environment from different routes are presented in Fig. 1. The major sources are sewage treatment plants(STPs), WWTP, and sanitary landfill leaching. PPCPs are not completely removed in conventional treatment plants. Detectable amounts with concentrations ranging from ng/L to μg/L [12] may be present in reclaimed surface water. The presence of PPCPs in the freshwater environment mainly arises through STP where the excretion from humans (consuming therapeutic drugs) is released into the sewage system or septic tank without any primary treatment. Another major source of PPCPs is the effluent from the pharmaceutical industry (manufacturer), which directly enters into STPs containing either treated or untreated PPCPs and their metabolites. After the sewage treatment, the wastewater is reused for irrigation and the sludge (biosolids) is applied directly as a fertilizer to the agricultural land. PPCPs can reach freshwater through run-off as a non-point source from agricultural land treated with the digested sludge. It can also reach groundwater through leaching from the soil posing a serious danger to the availability of potable groundwater [13]. Veterinary medicines which are released into the environment through animal wastes in both solid and liquid form are sprayed on agricultural land as fertilizer. These medicine along with their metabolite pollute the soil and thereby enter the food chain [14]. Personal care products like Triclosan which are discharged into the sewer enter the environmental cycle through washing sinks, bathing, swimming, shower waste, etc.,
 Fig. 1. Source and Transport of PPCPs in the environment.
Fig. 1. Source and Transport of PPCPs in the environment.2.2. Transport of PPCPs
As the PPCPs are released into the environment depending on the physicochemical properties of PPCPs, they are distributed in the aqueous environment, soil/sediment, and in the food chain. PPCPs are transported into the groundwater due to leaching from the biosolids which are mostly applied onto agricultural fields as a fertilizer. A part of the PPCPs was assimilated by the crops as nutrients which pose a potential health hazard to humans as it enters through dietary intake. Runoff from biosolids containing PPCPs either from landfills or from agricultural land to the nearby waterways contributes a serious threat to aquatic life and public health. Sorption of PPCPs onto the sediments may influence the concentration of PPCPs in the aqueous media thereby reducing their bioavailability and toxicity to aquatic life [15]. At the same time, there is a possibility of continuous release of these chemicals from the sediments to overlying water resulting in their persistence or pseudo persistent nature of PPCPs.
2.3. Fate of PPCPs
In an natural aqueous environment, PPCPs undergo transformation through biodegradation, photodegradation, and other abiotic processes like hydrolysis, adsorption, absorption, and ion-exchange reactions which reduces the concentrations of PPCPs in the environment with ultimate mineralization of these compounds [16]. However, these compounds are converted into newer compounds with lesser molecular weight (degraded products or metabolites) which in some cases are more toxic to aquatic life than the parent compound.
The extent of photodegradation in aqueous environments depends on the intensity of solar light, light penetration, organic matter, latitude, seasonality, salinity, dissolved solids, the concentration of PPCPs, etc., It has been observed that the carbamazepine undergoes photodegradation in artificial estuarine water to acridine (the metabolite formed) which are more toxic, mutagenic and carcinogenic in nature [17] while certain PPCPs like Ibuprofen, Metronidazole, Acetaminophen also undergo photolysis to harmless products suggesting a major route for their removal from the surface waters [18]. On the other hand, PPCPs like Tetracycline used in animal husbandry cannot be photodegraded due to sorption onto the sediment [19]. It has been reported that many PPCPs undergo microbial mediated degradation in STP/WWTP and in the environment resulting in the formation of transformation products. Microorganisms utilize PPCPs as substrates of carbon or energy sources to bring their degradation up to a certain threshold concentration, beyond a certain limit (PPCPs) become toxic to microorganisms exhibiting an inhibitory effect [20].
3. Global prevalence of PPCPs
3.1. America and European Union
The presence of 25 pharmaceuticals like dextropropoxyphene, erythromycin, sulphamethoxazole, tetracyclin, and theophylline up to 1 μg/L has been detected in the River Lee, UK [21]. Investigations on STP effluents and random river samples in Germany has detected the presence of antibiotic residues like erythromycin, roxithromycin, and sulfamethoxazole up to concentrations of 6 μg/L [22]. Even in the lower reaches of River Tyne, Tees, Mersey, Thames PPCPs like clotrimazole, dextropropoxyphene, diclofenac, mefenamic acid, and tamoxifen are identified at a concentration of measurable levels. Acidic pharmaceuticals like ibuprofen, naproxen, ketoprofen, and diclofenac in Danube river water and sediment in Budapest, Hungary has been observed [23]. The surface waters and treated waters of Louisiana, USA, and Ontario, Canada also revealed the presence of PPCPs like sulfamethoxazole, atrazine, carbamazepine, dilantin, and diclofenac with concentrations ranging from 22 to 107 ng/L. The presence of clofibric acid (25–65 ng/L); caffeine (3–27 ng/L); ibuprofen (1–34 ng/L); naproxen (1–135 ng/L), triclosan (9–26 ng/L), bisphenol A (1–147 ng/L) and 17β-estradiol were also detected in Mississippi, New Orleans, USA [24]. In Cape Cod, Massachusetts, USA, frequently used pharmaceuticals like sulfamethoxazole, carbamazepine, and phenylotin were found at concentrations of 113 ng/L, 72 ng/L, and 66 ng/L in the ground water [25]. Even in San Francisco Bay, California the presence of triclocarban in sediment, valsartan in surface water, and N, N-diethyl meta-toluamide (DEET) in mussels at concentrations of 33 ng/g; 92 ng/L and 14 ng/g respectively were reported [2].
3.2. Asia, Australia, and Africa
In continents like Asia, high concentrations of ciprofloxacin up to 31 mg/L and cetirizine up to 1.2 mg/L were detected in WWTP in Patancheru, Hyderabad, India. The presence of antiepileptic, antimicrobial, and preservatives has been identified in surface waters and sediments of Kaveri, Tamiraparani, and Vellar Rivers Tamil Nadu, India [26]. Several pharmaceutical residues such as cimetidine, caffeine, acetaminophen, and sulfamethoxazole were found in the surface water of River Han, Korea. The presence of pharmaceutical residues of ibuprofen, carbamazepine, naproxen, ketoprofen, diclofenac, and clofibric acid has been detected in tapwater, groundwater, WWTPs, river water (Fu-Hsing River) in China [27]. Compounds like DEET, mefenamic acid, fluoxetine, ibuprofen, and carbamazepine with concentrations ranging between 2 and 80.8 ng/L has been identified in Dongting Lake, China [28]. In Northern China, Tianjin the surface waters and sediments contains six estrogens includes, diethylstilbestrol (DES), estrone (E1), estriol, β-estradiol, 17α-ethynylestradiol. In a national survey on trace organic contaminants in Australian Rivers, the common pharmaceuticals like salicylic acid, paracetamol, carbamazepine, and caffeine were reported with maximum concentrations ranging from 1500 to 7150 ng/L [29]. The detection of pharmaceuticals in the estuarine-receiving environment around Auckland, New Zealand with acetaminophen and naproxen in the concentration of 7.7 ng/g and 5.5 ng/g respectively. In the Africa subcontinent, Tanzania [30], antimalarial drugs like artemether and lumefantrine along with amoxicillin were detected at concentrations in surface waters ranging from 3 to 32 μg/L. In Nairobi River, Kenya [31] up to 10 classes of pharmaceutically active ingredients for antibiotics, antimalarial and antiviral drugs were detected up to 10–30 μg/L. The sediment samples collected from Msunduzi River, KwaZulu-Natal, South Africa indicates the presence of aspirin, diclofenac, ketoprofen, and ibuprofen in the concentrations between 212 and 427 ng/g [32]. The presence of carbamazepine and trimethoprim has been observed in the sediment samples of Buffalo and Sundays River Estuary, South Africa [33]
It is therefore apparent from the prevalence data that PPCPs exist in almost every surface water, sediments, WWTPs, and STP around the globe. It has been reported that PPCPs has been distributed in 260 lakes in 44 countries. In Asia continent, PPCPs are present in 115 lakes and are concentrated only in 3 countries such as China, India and Sri Lanka. In Europe, PPCPs are present in 95 lakes and the majority is found in Germany, Sweden. PPCPs are found in many lakes in Africa, Antarctica, Oceania and South America which requires much more attention since the WWTP are not efficient in handling these wastes. The removal efficiency of PPCPs in waste stabilization ponds (commonly used as biological treatment in African countries) varies with the nature of PPCPs with negligible removal in case of carbamazepine to 100% removal of paracetamol [34].
4. Analytical methods for PPCPs
Indiscriminate use of medicines and house hold chemicals release huge amounts of PPCPs and their metabolites into the environment. These compounds are ubiquitously present in all waters (surface, ground and wastewaters) at concentration ranging from ng/L to μg/L [35]. The extraction and detection of PPCPs at low concentrations includes high-performance liquid chromatography tandem mass spectrometry (HPLC/MS), triple quadrupole mass spectrometry (TQ-MS), Ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC/MS), gas chromatography/mass spectrometry (GC/MS), liquid chromatography-electrospray tandem mass spectrometry (LC-ES/MS). Table 1 summarizes the different analytical methods employed in detecting PPCPs. In all these methods, sample preparation forms a central part of analysis and the quantification results largely depends on the procedure for sample collection and storage of the samples. The choice of the chromatographic method employed for detecting and analyzing PPCPs depends on the analyte polarity. As PPCPs are either polar or moderately polar these compounds are analyzed by liquid chromatography (LC-MS) methods. Gas chromatographic methods (GC/MS) are more amenable to non-polar analytes. Among various chromatographic methods, UHPLC has emerged as a powerful tool to detect antibiotics, antiretroviral in the effluents a 1 μg/L.
Table 1. Detection of PPCPs in surface waters and wastewater treatment plants by different analytical methods.
| Occurrence | PPCP | Instrument | Concentration (μg/L) | Reference |
|---|---|---|---|---|
| Surface water-South Africa | Ibuprofen | HPLC-MS | 19.2 | [36] |
| Surface water-South Africa |
Bisphenol A, Methyl paraben, Ethyl paraben, o-phenyl phenol |
UHPLC-MS | 0.0064–0.071 | [37] |
| Surface water- Jordan | Acetaminophen | LC-MS/MS | 36.7 | [38] |
| Hai River, China | Sulfadiazine | SPE-HPLC/MS | 0.027 | [39] |
| WWTP, India | Ibuprofen | GC-MS | 210 | [40] |
5. Treatment methods for PPCPs
As seen in the above section, the persistent and refractory nature of PPCPs towards conventional WWTPs find their way into the receiving water bodies along with the discharged effluent of the WWTPs. The concentration of PPCPs in the aquatic environment ranges from ng/L to mg/L posing a serious threat to the biota by inducing acute and chronic toxicity with the proliferation of antibiotic-resistant genes. Conventional treatment processes, such as adsorption, coagulation, and biological treatment were found to be ineffective in the removal of PPCPs. Among several treatment methodologies, the visible light-mediated advanced oxidation process using photocatalyst has gained considerable attention due to its ability to completely mineralize PPCPs to CO2and water irrespective of the nature of the organic contaminants.
5.1. Basics of photocatalysis
Photocatalysis is one of the promising technology used to mineralize organic recalcitrant contaminants like PPCPs by using UV/visible light/solar light and a photocatalyst like TiO2, ZnO, CdS. The mineralization/degradation of contaminants takes place by adsorption of the PPCPs present in the aqueous solution onto the surface of the photocatalyst with the absorption of light whose photon energy is greater than or equal to the bandgap (Eg) of photocatalyst(TiO2-3.2 eV; ZnO-3.37eV) for the generation of electron-hole pairs (e−-h+) pairs. The separation of these e−-h+ pairs on the surface of the photocatalyst in the valence band and conduction band results in the generation of reactive oxygen species (ROS). The adsorbed PPCPs undergo oxidation in the presence of ROS resulting in complete mineralization of CO2and H2O [[41], [42], [43]] as shown in Fig. 2. Depending on the nature of the photocatalyst, photocatalysis is of two types 1) single catalyst process, where only one photocatalyst, usually a TiO2 or ZnO is used for the treatment process 2) dual catalyst, where two different photocatalyst used forms a heterojunctionat the interfaces of the photocatalyst for the reaction to take place.
 Fig. 2. Schematic diagram representing the photochemical process for the degradation of PPCPs.
Fig. 2. Schematic diagram representing the photochemical process for the degradation of PPCPs.Photocatalysis of organic contaminants using a single photocatalyst is not gaining importance due to its inherent drawbacks [44]: 1) limited absorption of the light spectrum, For eg., TiO2 absorbs only UV light which contributes to a mere 2–3% of the solar spectrum 2) Recombination of electron-hole pairs is high resulting in the dissipation of energy as represented in Fig. 3 rather than photocatalysis process. To enhance the photocatalysis process, heterojunction type photocatalysts have been developed. The heterojunction is a contact interface formed by the hybridization of two semiconductors/metals with a dissimilar bands and electronic structures. The two semi-conductor materials (SA and SB) work in a coordinated fashion which minimizes the recombination losses. The advantages of heterojunction surfaces compared to single catalyst photocatalysts are [42]: 1) On combining the visible light responsive semiconductor or metal with a photocatalyst of either TiO2 or ZnO, a heterojunction is formed thereby the absorption of light can be extended to higher wavelength region in the visible region of the spectrum. 2) Enhanced separation of e−- h+ pairs: In heterojunction structures, the charge carriers structures migrate due to the interaction of semiconductor A and B resulting in the separation of charge carriers. 3) Enhanced photocatalysis: The presence of heterojunctions, capable of absorption in the visible light generates charge carriers on the surface of SA (TiO2/ZnO) as well as other components, SB [45]. Hence, more charge carriers are available for the generation of ROS, responsible for enhanced photo mineralization of the contaminants.
 Fig. 3. Schematic diagram representing the a) Single catalyst Photochemical process b) Recombination losses c) Dual catalyst.
Fig. 3. Schematic diagram representing the a) Single catalyst Photochemical process b) Recombination losses c) Dual catalyst. Fig. 4. Schematic diagram representing visible light mediated photocatalyst.
Fig. 4. Schematic diagram representing visible light mediated photocatalyst.5.2. Heterojunction Band gap engineering
Depending on the electronic structures and bandgap engineering the heterojunctions formed from semiconductor A (SA) and semiconductor B (SB) are of three types:
-
1)
Type 1- Straddling Gap: In these heterojunctions, the edge of the conduction band (CB) and the valence band (VB) of SA are higher and lower than that of CB and VB of SB respectively as shown in Fig. 4. The e− and h+ generated on SA migrate to the CB and VB of SB respectively [46]. The inherent nature of the photocatalyst doesn't effectively separate the charge carriers due to the accumulation of the carriers on the same semiconductor, SB.
-
2)
Type II - Staggered Gap: In these heterojunctions, the edges of the CB and the VB of SA are lower than that of CB and VB of SB respectively. Hence, the e− migrates to the CB of SA and h+ to the VB of SB resulting in enhanced spatial charge separation [44] thereby promoting higher photocatalytic efficiency.
-
3)
Type III- Broken Gap: In these heterojunctions, the edges of the CB and VB of SA are lower than that of CB and VB of SB respectively like Type II heterojunction except the staggered gap is so wide thereby e−-h+ migration and separation between SA and SB does not occur.
Among these heterojunctions, Type –II heterojunction has been found to be the most effective in photodegradation of organic contaminants like PPCPs as presented in Table 2, due to its spatial separation of e−-h+ pairs. Most of the PPCPs reported in Table 2 for the photocatalytic degradation reactions belong to the antibiotics class with other groups of pharmaceuticals like β-blockers, antiepileptic drugs, cytosolic drugs, and personal care products like sunscreens, toothpaste are reported rarely. There are reports that degradation using visible light-mediated photocatalyst with Type II heterojunctions was found to degrade only 55.83% of 5 mg/L of carbamazepine [58]. Hence, more efficient photocatalysts are needed to increase the degradation efficiency of the non-biodegradable PPCPs.
Table 2. Type II Heterojunction photocatalyst for the degradation of PPCPs.