1. Introduction
The durability of rehabilitative treatments depends on the availability of materials capable of minimizing the risk of mechanical failure, especially in applications involving the treatment of large defects subject to high loads or where it is necessary to reduce the implant dimensions [1]. Progress in this area has achieved improvements in treatment performance and longevity; nevertheless, failures still occur [2]. In order to overcome these failures, dental implant materials should present high fatigue strength, low elastic modulus, high strength [3], and good corrosion resistance and biocompatibility [4].
Commercially pure titanium (cpTi) is the material of choice for the manufacture of dental implants [5]. However, its use is limited in areas subjected to high wear and tensile and fatigue strength [2], [6], [7]. Because Ti is a relatively soft material [8], fatigue may occur, particularly when it is used in small-diameter implants, which must fulfill high requirements for mechanical stability to avoid overload and implant fracture [9]. In addition, high-elasticity modulus and difficulty in improving its mechanical properties without any reduction in biocompatibility have been considered characteristics that limit the use of cpTi as a material of dental implants [4]. The use of Ti alloy made by grinding Ti with other metals is an alternative option to obtain better mechanical properties [8].
Several elements may be combined with Ti, resulting in alloys with distinct properties and patterns closer to the ideal for use as dental implants. Ti-6Al-4V alloy is widely used owing to its excellent mechanical performance [2]. On the other hand, this alloy showed negative effects on cell viability by the release of Al and V [10], with a consequent adverse influence on implant biocompatibility [11]. Indeed, Al has been linked to significant neurotoxic effects, especially when considering reports of its association with Alzheimer's disease, bone fragility [12], and potential causes of local inflammation [13]. These reports have discouraged the use of Ti-6Al-4V and stimulated the development of alloys free of toxic elements that are inert in the oral environment.
To extend their clinical application, experimental alloys must exhibit satisfactory mechanical properties, with sufficient strength and stability in a corrosive environment, besides being biocompatible and safe for in vivo use [14], [15]. Ti alloys have proven to be of great interest for biomedical applications due to their excellent strength and superior biocompatibility [3]associated with properties such as high tensile strength, good corrosion resistance [16], and elastic modulus comparable to that of bone tissue [17], [18]. These outstanding properties have pointed to Ti alloys as viable options to be used as an alternative to cpTi in the manufacture of dental implants, and in many cases, for use as the first choice in treatment [19].
Although several alloys are designed for biomedical applications, many studies are inconclusive concerning the possibility of using these new materials as substitutes to cpTi. In addition, few studies have tested experimental alloys in vivo to consolidate their use. In this article, we provide a summary of several relevant aspects of Ti alloys for use as dental implants. Existing information about the mechanical, chemical, electrochemical, and biological properties of the main alloys developed over the past few years is deeply reviewed to provide scientific evidence in favor of using Ti-based alloys as alternative of cpTi with its alloys in the clinical scenario.
2. Classification of Ti alloys
Ti can take on two different crystal forms in a temperature-dependent manner. The α phase has a hexagonal closed-packed (HCP) structure and is stable from room temperature to 882 °C. The β phase has a body center cubic (BCC) structure and is stable at temperatures higher than those mentioned above [8], [19], [20]. Ti also presents metastable phases, such as the hexagonal martensite α′ and orthorhombic α″ phases [21]. The transition temperature between the α and β phases can be changed by combining elements with Ti, which consequently modifies its microstructure. Besides the constitution of the alloy, the processing approach and heat treatment conditions affect the material's microstructure [22].
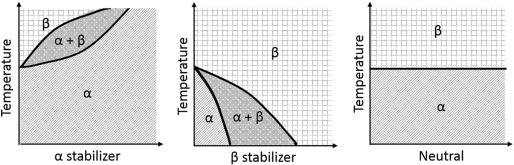
The microstructure of Ti alloys is defined according to the type and concentration of the alloying elements, as well the crystalline phases present at room temperature [20], [23]. Elements that may constitute Ti alloys are classified into three categories: α-stabilizers (Al, O, N, C) tend to stabilize the α phase by increasing the transition temperature; β-stabilizers (Mo, V, Fe, Cr, Ni, Co, Nb) depress the transition temperature by stabilizing the β phase; and elements such as Zr and Sn exhibit no effect on the stability of any phase, being considered neutral elements [11], [19]. Table 1 summarizes this information for better understanding. To understand such mechanism, the phase diagram of Ti as a function of stabilizers constituents is shown in Fig. 1. The effect of elements addition at the transition temperature between α and β phases can be clearly seen.
Table 1. Main components of Ti alloys and their influence on the transition temperature and Ti matrix.
| Element | Influence on the transition temperature | Main effect on Ti matrix | |
|---|---|---|---|
| α-stabilizer | Al, O, N, C | Increase | Hardening |
| β-stabilizer | Mo, V, Fe, Cr, Ni, Co, Nb | Decrease | Grain Refiners |
| Neutral | Zr and Sn | No significant effect | Hardening |
 Fig. 1. Phase diagram of Ti according to the stabilizers constituents.
Fig. 1. Phase diagram of Ti according to the stabilizers constituents.Depending on the proportion of each phase, Ti can be further classified as near α, α, α + β, near β, and β phases [21]. The near-α alloys contain approximately 1–2% of β-stabilizers and approximately 5–10% of β phases; alloys that present in their constitution higher amounts of β- stabilizers, resulting in 10–30% of β phases in the microstructure, are classified as α + β alloys; the near β and β alloys have higher amounts of β-stabilizers and predominantly β phase in their microstructures [19]. Fig. 2 shows the relationship between the concentration of stabilizer elements incorporated into the Ti and its microstructural phases.
 Fig. 2. Relationship between the concentration of stabilizer elements incorporated into titanium and its microstructural phases.
Fig. 2. Relationship between the concentration of stabilizer elements incorporated into titanium and its microstructural phases.It is known that microstructure has a great influence on the physical and chemical properties of the material [22] and is widely affected by the volume fraction, morphology, distribution, and size of the α phase precipitates within the matrix [24]. Knowing the elements that influence the microstructure of Ti and understanding how this relationship occurs has driven many researchers to incorporate elements to pure Ti to produce implants with greater performance than those made of cpTi.
The addition of V and Al to Ti forming Ti-6Al-4V, for example, was considered to provide a biphasic structure (α + β) because of the stabilizing effects of α and β. The alloys that exhibit α + β structure are characterized by higher strength, higher ductility and higher low-cycle fatigue [19]. Furthermore, alloys containing Al provide a high rate of solid solution hardening to the Ti matrix [14], with Ti-6Al-4V being the most common Ti alloy used for biomedical applications where high strength is required [25].
Similar to Al, Zr and Bi have been used to induce a solid solution hardening effect [7], [26], [27]. When Zr is cast only with Ti, it can form α alloys of various proportions, which usually increase the mechanical strength (such as tensile strength, hardness and flexural strength) and improve the corrosion potentialand wear resistance of Ti [21]. In contrast, β alloys have in their constitution β-stabilizers acting as grain refiners [3]. Among them, Nb, Mo and Ta have received emphasis for forming β alloys characterized by a combination of improved mechanical properties and excellent biocompatibility [7], [22]. They also present low elastic modulus [22], [28], superior corrosion resistance [22], good plasticity, high yield strength [28], hardenability, fracture toughness, and reasonable ductility [24]. These characteristics have made them the most promising alloys for the manufacture of implants [20], [22]. The relationship among the chemical elements, microstructure, and alloy properties is better discussed in the following topics.
3. Thermomechanical process of alloys
Good mass proportion of the elements involved and a melting method associated with an appropriate sequence of heat treatments is necessary to obtain alloys with enhanced mechanical, physical, and chemical characteristics [26]. An appropriate thermomechanical process depends on the time, temperature, strain rates, cooling rates, and the alloy's chemical composition [29]. There are elements that are unlikely to produce alloys by conventional grinding and melting processes [30]. Additionally, some microstructural phases are not stabilized at room temperature if there are no changes in the thermomechanical process.
Thermomechanical processing affects the nature and ratio of microstructural phases [19] and modifies the size, shape, and amount of microstructural constituents [22]. Cold-rolled alloys showed increased dislocation density, microstrain, and size changes of the grains and sub-grains, which vary depending on the percentage reduction in alloy and annealing time. Also, it directly influences the strength, elongation, and elastic modulus of the alloy [31], [32]. Annealed materials generally exhibit better ductility than castings or those worked cold; however, they show decreases in tensile strength [4].
A previous study [22] evaluated the effect of the cooling process (furnace cooling (FC), air cooling (AC), or water quenching (WQ)) on the microstructure and mechanical properties of a Ti-Nb-Zr-V alloy. Air-cooled samples had a relative grain refinement with the presence of α microstructure distributed in a β matrix, ensuring higher values of hardness of the alloy. The cooling time is crucial to the presence of the α phase (i.e., water quenching is a fast process that is insufficient for the precipitation of this phase in the microstructure).
Sintering is another step that can be modified to obtain improved properties of alloys. Ideally, it should retain the developed microstructure while preventing or minimizing undesirable grain growth [33]. It has been reported that the fracture strength of Ti-Nb-Ag alloy fabricated by a process that involves the combination of amperage and pressure (Spark Plasma Sintering - SPS) was nearly three times higher than that of a vacuum furnace-sintered sample, and promoted a dense structure without any pores [34]. SPS proved to be a promising approach to producing grain refinement. It has been further observed that increasing the sintering temperature leads to pore reduction and grain size decrease, which has a positive effect on raising the hardness of the alloy [33].
The process that obtains fine structures with smaller grain size has been shown to be of greater interest to increase the mechanical strength and toughness of the alloy; in addition, the microstructure and the general properties obtained are more isotropic [35]. The possibility of modifying the thermomechanical process during the development of Ti alloys is a wide field of study, with the possibility of changing the properties of the alloys using simple techniques. However, there is a certain difficulty in standardizing the procedures that are effective for all alloys.
4. Mechanical properties
Mechanical properties and biocompatibility are the primary considerations in the design of a new alloy [3]. Dental implants require good mechanical properties because they are exposed to loads and fatigue cycles in function [36]. To improve the mechanical properties of Ti and its alloys, mechanisms such as solid solution strengthening by interstitial and substitutional atoms, grain refinement, precipitation, work hardening, and dispersion strengthening, including lamellar and dispersed phases, can be used [4].
Biomaterials are expected to exhibit a combination of high strength and low elastic modulus [29]. However, these characteristics seem to be interexclusive for solid materials, particularly for metals and alloys [18]. Elements such as Mo, Nb, Ta, and Zr are generally used to increase strength and reduce the elastic modulus of the Ti alloy [7]. The search for a balance between strength and elastic modulus is important to improve the performance of implants compared with those made from cpTi. Therefore, it is necessary to understand the properties of the biomaterials and to predict their behavior when fixed to the bone [37]. Table 2 shows the mechanical properties of Ti alloys developed for biomedical applications. Conclusions from the analysis in Table 2 must be drawn with caution, as it is a summary of several studies in which the methodologies were not standardized.
Table 2. Mechanical properties of Ti alloys used for biomedical applications.
| Alloy type | Alloy | Thermomechanical process | Ultimate tensile strength (MPa) | Yield strength (MPa) | Elastic modulus (GPa) | Elongation (%) | Hardness (HV) | Ref. |
|---|---|---|---|---|---|---|---|---|
| α | CpTi | Conventional | 465 | 320 | 110 | 36 | 220 | [25] |
| Tia-5Zr | Conventional or heat treated | – | – | ≈ 86 | – | ≈ 246–357.40 | [21], [38] | |
| Ti-10Zr | Conventional or heat treated | – | – | ≈ 95 | – | ≈ 257–384.00 | [21], [38] | |
| Ti-15Zr | Conventional or heat treated | – | – | ≈ 113 | – | ≈ 250–412.0 | [21], [38] | |
| Ti-2Bi | Conventional | ≈ 360 | ≈ 310 | 102.0 | ≈ 25 | ≈ 210 | [7] | |
| Ti-5Bi | Conventional | ≈ 370 | ≈ 310 | 100.1 | ≈ 20 | ≈ 225 | [7] | |
| Ti-10Bi | Conventional | ≈ 520 | ≈ 425 | ≈ 97.9 | ≈ 15 | ≈ 300 | [7] | |
| Ti-20Bi | Conventional | ≈ 585 | ≈ 535 | 94.3 | ≈ 3 | ≈ 365 | [7] | |
| Ti-1Ag | Conventional | – | ≈ 985–1105 | – | – | ≈ 380–435 | [39] | |
| Ti-3Ag | Conventional | – | ≈ 975–1045 | – | – | ≈ 360–370 | [39] | |
| Ti-5Ag | Conventional | – | ≈ 960–1040 | – | – | ≈ 350–370 | [39] | |
| Ti-47Zr-7.3Al | Conventional or hot rolled | 1144–1564 | 992–1447 | 106–117 | 3.08–10.08 | – | [23] | |
| Ti-10Zr-15Si | Conventional | – | 1231 | 150 | 2.7 | – | [40] | |
| α + β | Ti–35Nb | Hot rolled and water quenched | 561 | 160 | 73.9 | 59 | – | [29] |
| Ti-8Fe | Conventional | ≈ 1560 | ≈ 130 | ≈ 567 | [41] | |||
| Ti-6Al-4V | Conventional | 1000 | 910 | 114 | 18 | 400 | [25] | |
| Ti-6Al-7Nb | Annealed | 1000–1100 | – | – | 10–15 | – | [10] | |
| Ti-13Nb-131Zr | ST† and MPCR¶ | 896–1027 | 840–935 | – | 7,8–14 | – | [42] | |
| Ti-35Nb-5Zr | Heat treated | 486 | – | – | – | 240 | [26] | |
| Ti–35Nb-2.5Sn | Hot rolled and water quenched | 566 | 139 | 65.8 | 55 | – | [29] | |
| Ti–7Ta–5Fe | Conventional | – | 1250 | 110 | – | 430 | [43] | |
| Ti-8Fe-5Ta | Conventional | ≈ 1045 | – | ≈ 120 | – | ≈ 387 | [41] | |
| Ti-8Fe-8Ta | Conventional | ≈ 1130 | – | ≈ 107 | – | ≈ 380 | [41] | |
| Ti-9Fe-2Ta | Conventional | ≈ 1075 | – | ≈ 110 | – | ≈ 388 | [41] | |
| Ti-9Fe-5Ta | Conventional | ≈ 1075 | – | ≈ 103 | – | ≈ 370 | [41] | |
| Ti-9Fe-9Ta | Conventional | ≈ 1010 | – | ≈ 99 | – | ≈ 350 | [41] | |
| Ti-10Fe-10Ta | Conventional | ≈ 1005 | – | ≈ 92 | – | ≈ 342 | [41] | |
| Ti-2.0Al-12.8Sn-18.2Cr | Cooling in different ways | 1005–1075 | 45.1–72.8 | – | – | – | [3] | |
| Ti-6.9Al-4.1Zr-7.0Mo-9.9Sn-10.1Cr | Cooling in different ways | 1065–1113 | 46.2–72.8 | – | – | – | [3] | |
| Ti-15Zr-4Nb-4Ta-0.2Pd-0.1O-0.005N | Annealed or ST and aged | 714–919 | – | 94–99 | 18–28 | 243–289 | [10] | |
| Ti-15Zr-4Nb-4Ta-0.2Pd-0.2O-0.005N | Annealed or ST and aged | 881–1026 | – | 97–100 | 14–27 | 301–317 | [10] | |
| Ti-15Sn-4Nb-2Ta-0.2Pd-0.05O-0.005N | Annealed or ST and aged | 860–1109 | – | 89–103 | 10–21 | 292–351 | [10] | |
| Ti-15Sn-4Nb-2Ta-0.2Pd-0.2O-0.005N | Annealed or ST and aged | 966–1189 | – | 86–98 | 14–20 | 336–361 | [10] | |
| β | Ti-30Nb | Conventional | – | – | 93 | – | 248 | [44] |
| Ti-38Nb | Cold rolled and Annealed | 1020 | 850 | 56 | – | – | [17] | |
| Ti-40Nb | Conventional | – | 544 | 62 | 28 | – | [40] | |
| Ti-45Nb | Conventional or heat treated | 480–527 | 460–438 | 64–65 | 21 | 185–233 | [25], [45] | |
| Ti-26.88Fe | Conventional | 2627 | 2028 | – | 7.5 | – | [46] | |
| Ti-15Mo | Conventional | 1150 | 1070 | 100 | 17 | 360 | [25] | |
| Ti-12Mo-3Nb | ST and water quenched | – | 450 | 105 | 41 | – | [47] | |
| Ti-30Nb-2Sn | Conventional | – | – | 89 | – | 246 | [44] | |
| Ti-30Nb-4Sn | Conventional | – | – | 86 | – | 235 | [44] | |
| Ti-33Nb-4Sn | Cold rolled and annealed | ≈ 853 | ≈ 763 | ≈ 36 | – | – | [18] | |
| Ti-35Nb-5Sn | Hot rolled and water quenched | 563 | 130 | 68.2 | 36 | – | [29] | |
| Ti-35Nb-7.5Sn | Hot rolled and water quenched | 478 | 152 | 86.4 | 38 | – | [29] | |
| Ti-13Nb-13Zr | Conventional | 680 | 515 | 80 | 28 | 265 | [25] | |
| Ti-35Nb-10Zr | Heat treated | 546 | – | – | – | 185 | [26] | |
| Ti-41.1Nb-7.1Zr | Heat treated and water quenched | 490 | 490 | 65 | 16 | 182 | [48] | |
| Ti-26.88Fe-2Ta | Conventional | 2560 | 2300 | – | 1.0 | – | [46] | |
| Ti-26.88Fe-4Ta | Conventional | 2531 | 2215 | 175 | 5.0 | – | [46] | |
| Ti-12Nb-5Fe | Cold crucible levitation melting | – | 740 | 90 | – | 293 | [43] | |
| Ti-10Ta-4Fe | Cold crucible levitation melting | – | 1360 | 121 | – | 410 | [43] | |
| Ti-20.6Nb-13.6Zr-0.5V | ST and cooling by different ways | 741–785 | 520–572 | 77–81 | 15–19 | 260–280 | [22] | |
| Ti-35.3Nb-7.1Zr-5.1Ta | Heat treated and water quenched | 550 | 550 | 63 | 21 | 173 | [48] | |
| Ti-23.72Nb-4.83Zr-1.74Ta | Spark Plasma Sintering | 2793 | 1143 | 35 | – | – | [49] | |
| Ti-10Zr-15-Nb-15Si | Conventional | – | 1185 | 120 | 3 | – | [40] | |
| Ti-31Nb-6Zr-5Mo | Hot rolled and heat treated | 672–747 | 610–712 | 44–48 | 20.6–26.7 | – | [50] | |
| Ti-13.6Nb-6.5Al- 6Cu-5.1Ni | Conventional | 1145 | 1050 | 82 | 3.7 | – | [51] | |
| Ti-14.1Nb-6.7Al-4Cu-3.4Ni | Conventional | 880 | 820 | 72 | 14 | – | [51] | |
| Ti-23.72Nb-4.83Zr-1.74Ta-2Si | Spark Plasma Sintering | 3263 | 1296 | 37 | – | – | [49] | |
| Ti-23.72Nb-4.83Zr-1.74Ta-5Si | Spark Plasma Sintering | 3267 | 1347 | 40 | – | – | [49] | |
| Ti-22.3Nb-4.6Zr1.6Ta-6Fe | Spark Plasma Sintering | 2650 | 2425 | 75 | – | – | [52] | |
| Ti-10Zr-31Cu-10Pd-4Sn | Spark Plasma Sintering | 2060 | – | 114 | – | – | [53] | |
| Ti-35Nb-4Sn-6Mo-9Zr | ST and water quenched | 834 | 802 | 65 | 11.0 | – | [31] | |
| Ti-35Nb-5Sn-3Mo − 3Zr | ST and water quenched | 690 | 668 | 70 | 18.0 | – | [31] | |
| Ti-35Nb-5Sn-6Mo-3Zr | ST and water quenched | 770 | 729 | 85 | 10.5 | – | [31] | |
| Ti-35Nb-3Sn-9Mo-9Zr | ST and water quenched | 833 | 781 | 70 | 10.2 | – | [31] | |
| Ti-35Nb-4Sn-3Mo-6Zr | ST and water quenched | 666 | 654 | 55 | 8.9 | – | [31] | |
| Ti-35.3Nb-7.3Zr-5.7Ta | Conventional or ST | ≈ 550 | ≈ 450 | ≈ 62–66 | ≈ 21 | – | [54] | |
| Ti-35.3Nb-7.3Zr-5.7Ta-1Si | Conventional or ST | ≈ 620 | ≈ 560 | ≈ 70–72 | ≈ 12 | – | [54] | |
| Ti-35.3Nb-7.3Zr-5.7Ta-2Fe | Conventional or ST | ≈ 720 | ≈ 590 | ≈ 79–81 | ≈ 22 | – | [54] | |
| Ti-13.6Nb-6Co-5.1Cu-6.5Al | Conventional | 1550 | 1110 | 92 | – | – | [55] | |
| Ti-13.6Nb-6Co-5.1Cu-6.5Al-0.5B | Conventional | 1570 | 1150 | 97 | – | – | [55] | |
| Ti-13.6Nb-6Co-5.1Cu-6.5Al-1B | Conventional | 1680 | 1200 | 100 | – | – | [55] | |
| Ti-35.3Nb-7.3Zr-5.7Ta-0.5Si + 1Fe | Conventional or ST | ≈ 685 | ≈ 630 | ≈ 75–78 | ≈ 14 | – | [54] | |
| Ti-35.3Nb-7.3Zr-5.7Ta-0.5Si + 2Fe | Conventional or ST | ≈ 840 | ≈ 720 | ≈ 82–84 | ≈ 15 | – | [54] | |
| Ti-35.3Nb-7.3Zr-5.7Ta-1Si + 1Fe | Conventional or ST | ≈ 610 | ≈ 650 | ≈ 79–84 | ≈ 7 | – | [54] | |
| Ti-2.1Al-3.9Zr-10.1Mo-4.9Nb-5.1Ta-8.0Sn-5.8Cr | Cooling in different ways | – | 722–767 | 41.7–100.9 | – | – | [3] |
†ST - Solution treated.
¶MPCR - multi-pass caliber-rolled.
- a
-
The values of Ti mass% are proportional to the other elements of the alloy.
4.1. Strength and ductility
The alloy strength should be high enough to bear the load to which the implant is subjected [3], including tension, compression, bending, and torsion [22]. Tensile strength and fracture toughness are essential properties of materials that are used as hard tissue substitutes. They are responsible for protecting the integrity of the implant and preventing plastic deformation during insertion, thus assuring stability between the implant and the prosthetic components [4]. On the other hand, ductility facilitates several manufacturing processes [29], which is important because implants have complex geometry. It is possible to enhance the strength properties and the plasticity of the alloy by controlling the microstructure and grain size of the material.
The tensile strength of Ti-Al-V is significantly higher than that of cpTi; this is due to the addition of Al and V, which generates changes in solid solution alloy with particle precipitation and transformation from the α to the α + β phase [4]. Similarly, the addition of Al to Ti-Nb and Si to Ti-Nb-Zr-Ta resulted in solid solution strengthening and grain refinement [54], [56]. Grain growth suppression promoted by the addition of Si was caused by underpinning of the grain boundaries by silicide intermetallic particles [54]. Likewise, grain refinement by adding Nb to Ti enhances the yield strength and tensile strength 1.5–1.6 times over those of pure Ti [45].
The incorporation of Ta to Ti-Nb-Zr is also shown to be responsible for increasing the ultimate tensile strength and elongation of the alloy, which features an elastic and perfectly plastic material [48]. In a similar alloy composition, the use of a high-pressure torsion process was effective at increasing the tensile strength of the alloy by increasing the density of dislocations and resulted in refinement of the microstructure [16]. On other hand, Ta was responsible for decreasing the alloy strength of Ti-Fe, even though the alloy exhibited high hardness and compressive yield [41].
The incorporation of elements into the alloy will not always result in improved mechanical properties. In the case of Ti-Nb-Sn, the presence of high concentrations of Sn incorporated into the β phase of the alloy tends to reduce both ductility and tensile strength of the alloy [29]. A study conducted by Datta et al. [3] showed that the presence of a larger amount of β-stabilizers might be able to reduce the resistance of the material. This was initially observed in a predictive model, and subsequently verified by manufacturing a Ti-Al-Zr-Mo-Nb-Ta-Sn-Cr alloy, which showed lower strength than Ti-6Al-4V, while two other alloys with lower amounts of β-stabilizers had higher resistance levels. Further studies are needed to consolidate a thermomechanical alloy process to achieve a β microstructure with smaller fractions of β-stabilizers.
The interstitial content of Ti and its alloys (N, O and C) also is directly related to the strength and ductility of the material [4], [51]. The increase in interstitial solutes is deleterious to cpTi toughness reducing the elongation values; nevertheless, the interstitial solutes increase the strength of the material [4]. Intermetallic phases generated by substitute elements, such as Fe, Ni and Cu, are responsible for the same behavior in alloys. This was noted in a study that evaluated two alloys with different concentrations of Cu-Ni, in which the decrease in the fraction of these elements improved the ductility of the alloy, and its increase raised the tensile strength, reaching values of 1050 MPa [51].
The manufacturing process also influences the alloy's characteristics. Cold rolling, aging treatment, annealing, and incorporation of small amounts of ceramic particles into the matrix promote high strength and good ductility of the Ti alloy [16], [22], [54]. This will ensure the material's biomechanical compatibility with the bone, which is extremely important for the long-term success of dental implants, mainly because they are subject to loads or constant cyclic stresses [19].
The tensile strength of Ti alloys developed by different process ranges from 360 to 3267 MPa (Table 2). As an overall trend, high values of ultimate tensile strength and yield strength were found for the α + β type alloys. It is clear that Zr, Ta, Si, Fe, Al, and Mo have a tendency to increase alloy strength. Elements such as Nb, Sn, and Bi have little influence on this property. The processing method also affects alloy strength, where highest values were found using SPS.
4.2. Fatigue performance
As described in subchapter 4.1, most of the studies investigated the mechanical behavior of Ti alloys using compressive tests rather than fatigue tests. The use of cyclic loading to evaluate the fatigue behavior of dental implant materials is required because it represents a more realistic scenario than pure monotonic loading [57]. Besides, the environment that implants are inserted can influence the fatigue performance by accelerating the initiation of a surface flaw and propagating it to a critical size, leading implant to fracture [58]. Nevertheless, there are few studies evaluating the fatigue behavior of Ti alloys in human-like medium simulating this condition.
The number of fatigue cycles to failure the Ti–24Nb–4Zr–7.6Sn in 0.9% NaCl solution was larger than that in air, mostly due to the cooling effect of this medium, which can suppress material softening originated from the temperature increasing during the fatigue test [59]. Additionally, the oxide filmformed in NaCl solution may improve the corrosion fatigue resistance of such alloy. Ti–24Nb–4Zr–7.6Sn exhibited a much higher fatigue resistance in the strain-controlled fatigue test than Ti–6Al–4V ELI [59].
A corrosion fatigue test with Ti alloys was carried out in Eagle's solution. The fatigue strength of Ti-Zr-Nb-Ta-Pd-O-N and Ti-Sn-Nb-Ta-Pd-O at 108 cycles was ≈ 600 MPa, while for Ti-Al-Nb-Ta was ≈ 700 MPa. Ti-Mo-Zr-Al alloy showed worse results than previous alloys at 107 cycles [10]. Ti-15Zr discs and dental implants showed a significantly better fatigue performance when compared to Ti-Grade IV. The fatigue endurance limit was 560 MPa for the alloy and 435 MPa for the cpTi [60]. Meanwhile, Ti–6Al–4V and cpTi exhibited higher fatigue strengths than those of Ti–7.5Mo and Ti–13Nb–13Zr. Nevertheless, Ti–7.5Mo had the best fatigue performance, especially when the strain-controlled fatigue resistance is taken into consideration [61].
Improved fatigue behavior can be achieved by combining the material properties, surface properties and design optimization of implants [60]. In this context, the deposition of hard thin coatings, the use of different heat treatments and mechanical processing operations play an important role in the fatigue performance of Ti alloy [58]. Cold rolling raises the fatigue resistance by the production of much finer microstructures [59]. Aging treatment was responsible to increase the fatigue limit of Ti–29Nb–13Ta–4.6Zr (≈ 320 to ≈ 700 MPa) [62] and the Ti–24Nb–4Zr–7.6Sn (≈ 250 to ≈ 425 MPa) [59] owing to the combination of strength and ductility, which improved the resistance against small fatigue crack initiation and propagation [62]. The surface porosity generated by the casting process may be the main cause of fatigue cracks in alloys [61]. Similarly, the roughness created by surface treatments (e.g. SLA) proved to have a negative effect in the materials fatigue behavior, leading to a higher susceptibility to crack initiation [60].
In general, further studies are warranted to assess the fatigue behavior of the Ti alloys under cyclic loading in human-like medium, such as artificial saliva and simulated body fluid. This is required because the combination of a corrosive environment and cyclic mechanical loading is more detrimental for materials used in dental implants than the sum of the individual effects [57]. In addition, thermomechanical processes and surface treatments need to be improved to avoid manufacturing defects that can be stress concentration sites, which may lead to failures initiation/propagation.
4.3. Hardness and elastic modulus
Hardness is defined as the resistance of the material to permanent deformation [26]. It should be low during implant manufacturing to ensure good machinability but at the same time, should present adequate stiffness to avoid shielding the bones from stress [4]. The elastic modulus is an important property for biomechanical interaction between the implant and the bone. Lower elastic modulus values improve stress distribution at the implant–bone interface and decrease bone atrophy [4]. Therefore, the elastic modulus should be as close as possible to the bone to avoid the problem of “stress shielding” [3], [37], which is associated with bone resorption around the implant [2], [50] and osteoporosis [50]. However, the elastic modulus should not be too low, because it would lead to micro-motion of the metallic implant, resulting in implant loosening, and even failure of the prosthesis [45].
While higher values of alloy hardness are related to the presence of an α phase, its presence can be undesirable for the elastic modulus. The addition of α-stabilizers increases hardness because they act as a substitutional solute, which decreases the atomic mobility of the material [21] or promotes the precipitation of the α phase when the alloy is aged at low temperature [19]. With regard to the elastic modulus, a predictive model concluded that its value should initially increase with rising β content, but will decrease dramatically at higher concentrations [3]. Lower concentrations of β-stabilizers usually generates α + β alloys, which present higher values of elastic modulus due to the presence of the α phase. The suppression of the α phase of an alloy microstructure by the increase of β-stabilizers may be responsible for lower hardness values [26], [29], and consequently, the decrease in elastic modulus.
The addition of Zr and Bi to Ti increased the values of hardness when compared with cpTi [7], [21], [38]; however, with the increase in Zr concentration, the hardness decreased slightly [21], [38]. Zr works differently for the elastic modulus. The addition of 5%–10% in mass of Zr initially decreased the values; however, concentrations higher than 15% increased the value of this property above that of cpTi [21]. The authors explained the variation by a change in the distance between atoms of the alloy components, which is caused by the higher atomic radius of Zr compared with Ti. This leads to a change in the binding force between atoms that determine the elastic modulus. The incorporation of Nb into a Ti-Zr alloy also showed an increase in hardness, which was raised even more by aging heat treatment, responsible for the formation of a biphasic α + β microstructure [14]. However, this treatment usually increases the elastic modulus to above 100 GPa [54].
The addition of Sn to Ti-30Nb alloy culminated in an alloy with higher amounts of β phase generated by the diffusion of Nb in the Ti matrix, which, besides reducing the elastic modulus, did not change the material's hardness [44]. A Ti-33Nb-4Sn alloy subjected to the cold-rolled and annealing process, resulted in one of the lowest values of elastic modulus in the literature (≈ 36 MPa), which was possible due to the formation of a β microstructure with low levels of β-stabilizers [18]. Most of these alloys with low elastic modulus have been obtained using d electron alloy theory [32], [50], [63], [64], which takes into consideration the covalent bond strength between Ti and alloying elements and the d-orbital energy, relating to the radius and electronegativities of the elements [64].
Although the cpTi and its alloys present a much lower elastic modulus than stainless steel and Co-Cr alloy [2], [16], their values are still very high when compared with those of human bone (10–30 GPa) [4], [65]. Regarding elastic modulus, β-type alloys have shown more promising results than cpTi and α and α + β alloys [37], [66]. This may be related to the fact that the β phase has a body-centered cubic structure, with the density of atoms in the lattice lower than the hexagonal closed-packed structure found in the α phase [16]. This ensures a higher plastic deformability of the alloy [50]. These different structures have distinctive distances between the atoms, which lead to variations in the atomic-bonding force and, consequently, changes in elastic modulus [21].
Alloy elastic modulus may vary extensively with thermomechanical processing[3], [16]. For example, water-quenched samples showed lower elastic modulus values [3], [22], which is in accordance with Table 2. This decrease in elastic modulus is related to the inhibition of transformation from β to α phase, as a result of rapid cooling that limits the growth of the α phase [22]. According to the literature, the elastic modulus of Ti alloys varies between 35 and 175 MPa, with emphasis on the β-type alloys, which have values closer to that of bone (Table 2). We highlight the Ti-23.72Nb-4.83Zr-1.74Ta(-Si) and Ti-36Nb-4Sn alloys, which presented lower elastic modulus values. Regarding hardness, cpTi has one of the lowest values, which increases considerably with the addition of the elements Zr, Mo, Fe, and Al. Ti-Fe(-Ta), Ti-Al-V, and Ti-Zr have the highest hardness values.
5. Surface properties
Surface characteristics, such as composition and topography, exhibit high influence on the success of implant treatment. Implants require a surface that promotes osteogenic differentiation and proper mineralization during the initial integration stage [67]. Surface characteristics of the implant material (e.g., surface roughness, surface energy, and substrate composition) directly or indirectly influence the proliferation and differentiation of bone cells and, consequently, cell adhesion to bone apatite [68], [69].
For dental implants, it is desirable that the alloy preserves the topographic micro-roughness while maintaining the hydrophilic surface [70]. Implant surface roughness fits into micrometer (0.11–100 μm) and nanometer (1–100 nm) scales [71], which also applies for surface morphology (Fig. 3). There are indications that micrometer and nanometer levels influence bone response [72], ensuring proper cell attachment, higher bone/implant contact, and greater resistance to torque removal [67], [73]. It is expected that nanometric proteins present in the biological environment can easily penetrate into a nanotopography, with positive effects on cellular responses [74].
 Fig. 3. Schematical representation of the micro and nanoscale of implants and their effect in topographic features. Representative micrographs of microscale surfaces can be seen for machined Ti–6Al–4V–ELI (M) and acid etched in HCl (Ac) samples. Nanoscale features can be observed on the acid etched combined with alkaline treatment (AcAk) surfaces, in which a homogenous sponge-like structure is noticed. The nano-scale topography revel a decrease in water contact angle and consequently improvement in surface energy and hydrophilicity. SEM micrographs were reprinted from Mater Sci Eng C, 51, Oliveira DP, Palmieri A, Carinci F, Bolfarini C, Gene expression of human osteoblasts cells on chemically treated surfaces of Ti – 6Al – 4V – ELI/Results, 250, Copyright (2015), with permission from Elsevier.
Fig. 3. Schematical representation of the micro and nanoscale of implants and their effect in topographic features. Representative micrographs of microscale surfaces can be seen for machined Ti–6Al–4V–ELI (M) and acid etched in HCl (Ac) samples. Nanoscale features can be observed on the acid etched combined with alkaline treatment (AcAk) surfaces, in which a homogenous sponge-like structure is noticed. The nano-scale topography revel a decrease in water contact angle and consequently improvement in surface energy and hydrophilicity. SEM micrographs were reprinted from Mater Sci Eng C, 51, Oliveira DP, Palmieri A, Carinci F, Bolfarini C, Gene expression of human osteoblasts cells on chemically treated surfaces of Ti – 6Al – 4V – ELI/Results, 250, Copyright (2015), with permission from Elsevier.Rough topographies were found to be associated with the differentiation of osteoblasts while a smooth surface was related with cell proliferation. It is believed that the contacts between cells and rough surface may induce autophagic process by mechanically stimulation, which could lead cells toward differentiation [75]. An implant surface with defined nanoscale features and rough surface results in enhanced secretion of osteogenic markers and specific proteins related with the osteoblast maturation (e.g. osteocalcin, osteopontin, bone sialoprotein, osterix, nictric oxide, transcription factor Runx2, alkaline phosphatase), in which can provide faster and more reliable bone-to-implant contact [74], [75], [76]. A surface that induces the maturation of cells is crucial to improve and to accelerate osseointegration.
A previous study produced a Ti-6Al-4V alloy with different levels of surface roughness [71]. The roughness values ranged from 0.25 to 0.87 μm and the adhesion and cell proliferation were sensitive to changes in topography, where increased roughness produced better results. Furthermore, a greater quantity of total protein adhesion was observed on a rough surface, with fibronectin adsorbing up to 10 times more than on the smooth surface. Still need better explanation concerning the role of small differences in surface roughness (Ra < 0.50 μm) and its influence on the cellular response and protein adsorption [69].
Besides, an improved wettability is important to achieve better cell proliferation and adhesion on surfaces of Ti alloy implants [77]. Chemical and physical properties of the surface can influence the wettability of the alloy. The surface is considered hydrophilic when the water contact angle is < 90°, whereas the angle above of 90° represents an hydrophobic surface [78]. Ti-45Nb was shown to have an enhanced hydrophilicity with a lower contact angle (81.75°) when compared with cpTi (96.46°) [45]. Nevertheless, the Ti-50Zr alloy presented the higher surface energy and the lowest surface roughness (≈ 37 mN/m, 0.17 μm) than cpTi (≈ 34 mN/m, 0.20 μm) and the Ti-50Nb alloy (≈ 32 mN/m, 0.46 μm) [69]. This properties associated with the substrate composition assured a better in vitro biological profile (cells adhesion and proliferation) for Ti-Zr alloy. The cpTi showed a slightly rougher topography than Ti–Ta–Nb–Zr, despite the alloy presented a more enlarged surface area. Both materials reveal similar water contact angles, which were characteristic of hydrophobic surfaces [1].
Surface modifications by various treatment methods have also shown excellent results. Treatment by mechanical friction achieved different grain sizes on the Ti-25Nb-3Mo-3Zr-2Sn alloy [79]. The nano-grained alloy presented the lowest surface roughness associated with better wettability (7.0 nm; 50.4°) than ultrafine-grained (7.4 nm; 64.1°) and coarse-grained alloy (7.1 nm; 67.8°). Nanoscale grains (30 nm) showed better cell responses and higher protein adsorption as compared with grain sizes of 90 μm and 180 nm [79]. Two acid-etched surfaces associated with alkaline treatment was responsible for changing the topography of Ti–6Al–4V–ELI from micro- to nano-scale with a surface micro-roughness (0.14–0.48 μm), improving the wettability (≈ 70° to ≈ 35°) and surface composition of the material [80]. Some of the surfaces obtained are shown in Fig. 2. It can be clearly seen a modification of morphology between the machined surface (M) and acid-etching (Ac) for those that were treated with an alkaline solution (AcAk), in which presented an effective osteoconductive behavior.
A previous study [74] achieved a nanotopography by electrochemical anodization in Ti-6Al-7Nb alloy. The contact angle was dramatically decreased from 61.4° (machined surface) to 14.8° (treated surface). On the other hand, a nanoporous surface (pores < 15 nm) created by the anodization of Ti-25Nb-25Zr alloy did not changed its roughness (121 nm) and contact angle (56°) when compared with the machined surface (123 nm; 59°) [81]. Despite that, the morphology alteration and surface composition was able to ensure the adsorption of albumin and fibronectin, as well as significantly improving cellular responses such as adhesion, migration, proliferation and mineralization of mesenchymal cells. Rough or porous surfaces present reinforced biomechanical properties due to the larger area of contact between recently formed bone and the implant surface, stabilizing the connection between the implant and the surrounding tissue through microscale mechanical interlocking [67].
A hydrothermal treatment using an alkaline Ca-containing solution combined with a simple post-heat treatment, seems to be an effective way to enhance cell viability and differentiation on Ti-13Nb-13Zr surfaces via increased surface hydrophilicity (contact angle = 21°), possibly by increasing the surface area at the nanoscale and the formation of anatase structure, without markedly altering surface morphology [82]. The presence of anatase phase proved to be important in increasing the surface energy and the surface reactivity [78].
It has already been observed that a cpTi surface that combines a submicron roughness with high hydrophilicity promotes cell differentiation and maturation with osteogenic capacity, besides ensuring greater cell adherence [83], [84]. Moreover, few studies have compared the roles of surface characteristics of different alloys in the material properties and tissue response. Most research studies the surface properties only when it is related to surface treatments. From this perspective, further studies should deeply investigate the influence of the composition of different alloy surfaces on wettability, roughness, and biological properties.
6. Electrochemical properties
Metals used in the manufacture of dental implants tend to corrode under physiological conditions and in some cases, present exaggerated reactivity to the body [2]. When exposed to cells and body fluids, implants can release metal ions and wear debris into the surrounding tissue [85], [86]. The degradation process of the material may result in adverse effects on the body (e.g. osteolysis, bone resorption, infections) and loosening and failure of the implant [85] if their surfaces are not modified to tolerate changes in the physiological environment [87].
Corrosion resistance is correlated with the device's service life and with the harmfulness of corrosion processes taking place in the living organism [36]. Ti shows superior electrochemical properties to those of other metals due to the formation of a very stable passive layer of TiO2 on its surface [2]. This layer provides protection against the corrosive action of physiological fluids and reduces the release of ions, which leads to better biocompatibility and cytotoxicity [88]. It is known that higher corrosion resistance is related to strong and stable passive films [47], [89].
The TiO2 film provides corrosion resistance as long as its integrity is maintained [90]. Often times, its integrity and stability can be weakened by metabolites produced by micro-organisms [91], changes in salivary pH [92], and mechanical wear of the implant [25], which can lead to accelerated corrosion and structural degradation with consequent implant failure [93].
One way of improving the corrosion resistance of Ti and its alloys is to create more resistant materials and films. Several surface treatments have been investigated with the aim of improving the protective properties of the oxide layer [88]. Additionally, many alloys are created to reduce the anodic activity or to accelerate the cathodic reactions of the material, influencing the corrosion rates through the addition of elements to Ti, such as Mo, Ta, Zr, and Nb (anodic alloy elements) and Pd, Pt, and Ru (cathodic alloy elements) [90].
Similar to Ti, Zr has high corrosion resistance in biological environments [70]. Ti-Zr alloys showed enhanced corrosion resistance compared with cpTi, with greater protection against oxidation due to solid solution strengthening of the alloy [38]. Ti-Nb-Zr and Ti-Nb-Ta-Zr-Fe alloys presented a better corrosion resistance than Ti-6Al-4V [27], [94]. The difference can be attributed to the addition of elements such as Nb, Ta, and Zr in the alloy and the microstructure of the oxides that are formed on the material surface [47], as well as the increase in thickness of the upper layer of the passive film [27]. The oxide filmformed on the surface (composed of TiO2, Nb2O5, NbO2, Ta2O5, and ZrO2) seems to be more stable and inert, improving the corrosion resistance of the biomaterial [92], [94]. Furthermore, the addition of Nb, Sn, and V in Ti-Nb, Ti-Nb-Zr(-V), Ti-Nb-Sn, and Ti-Al-V alloys has increased the corrosion potential of the material due to the stabilizing effect of the β phase. This phase is considered nobler than the α phase [20], [22], [45]. Another explanation is related to the high corrosion resistance of added noble metals that enhance the potential of the alloy to exhibit nobler values than the critical potential for passivation of Ti, which improves its corrosion resistance [45].
A previous study [25] evaluated the mechanical and corrosive properties of α, β, and α + β alloys. All alloys exhibited similar potentiodynamic behavior due the TiO2 layer, which was formed on the sample surface; however, there was a difference in the passivation region. The Ti-Al-V achieved faster passivation, while the Ti-Mo lost its protection first. Ti-Al-V presented the best tribocorrosion behavior, but the Ti-Nb-Zr alloy showed the best combination of mechanical and electrochemical properties.
Ti-Pd and Ti-Mo-Ni alloys showed superior corrosion resistance than cpTi, which has been justified by the acceleration of the cathodic reactions caused by the incorporation of Pd and Ni, while the addition of Mo inhibited the anodic process [90]. The ion release rate decreases with increasing amounts of Pd, Zr, Nb, and Ta, highlighting the Pd element [10]. The same was observed for Ti- × Ag [95].
Although the Ti-Al-V alloys have improved the electrochemical properties, the toxic effects of Al and V have been a concern [4]. It would be better to use alloys that are free of toxic elements or to resort to surface treatments to improve corrosion resistance and to reduce the release of ions from the alloy. Some methods used to create a stronger and thicker oxide layer are heat treatment [67], plasma treatments [96], [97], [98], magnetron sputtering deposition [91], and galvanostatic anodization [73]. The results are promising, as some of these treatments produce thicker films that act as a barrier against the diffusion of ions, with the treated materials having noble behavior, improved stability, and excellent corrosion resistance [73], [96].
7. Biological properties
Implants should present not only improved mechanical properties but also be compatible with living tissues. To be considered successful, a fixed bond between the implant and the bone tissue is necessary [1]. Furthermore, implants must present unfavorable bacterial colonization and promote or, at least, not negatively interfere in bone regeneration and osseointegration [67].
7.1. Biocompatibility
The biocompatibility of Ti and its alloys is directly related to the forming capacity of stable and dense oxide films (i.e. TiO2) on the implant surface [99], as the interface between the implant and the biological environment involves the oxide layer, instead of Ti itself [100]. The biocompatibility of alloys is related to the body's response to its elements. Therefore, it is imperative that the alloy components are non-toxic and nearly inert to surrounding tissues.
Metallic particles of Ti, Zr, Sn, Nb, and Ta did not affect the relative growth rate of human cells and showed only a small amount of ion release to the environment. However, Al and V reduced cell viability in a concentration-dependent manner [10]. Other elements, such as Ag, Co, Cr, Cu, and In, are considered moderately cytotoxic, but when associated with Ti in a Ti alloy, their cytotoxicity is reduced [101].
A binary Ti-Zr alloy induced a lower inflammatory response and enhanced cell attachment when compared with cpTi, demonstrating its excellent cytocompatibility [10], [11], [102]. Further experimental alloys (Ti-5Zr, Ti-5Ta, and Ti-5Ta-5Zr) were considered biocompatible, as no influence on cell morphology and proliferation was noted [15]. The relative growth rates of cells in the presence of Ti-Zr and Ti-Sn alloys were slightly higher than those of cells exposed to cpTi and Ti-Al-V; and when a wear test was implemented, the alloy containing Al and V was the only one that showed a dramatic decrease in the cell growth rate [10]. Elements such as Pd and Pt did not influence the cell viability, with the Ti-5Pd alloy presenting better results than cpTi [101].
An alloy consisting of Ti, Ta, Nb, and Zr showed similar cytocompatibility to cpTi, but a lower inflammatory response, and became well osseointegrated [1]. Ti-Ta-Nb-Zr(-Si)(-Fe) also displayed superior biocompatibility to that of the widely-used Ti-6Al-4V alloy. In addition, the osteoblasts growing on Ti-Nb-Zr-Ta alloys spiked with Si and/or Fe produced more collagen I, which contains binding sites for calcium and phosphate ions that serve as nucleation sites for the formation of hydroxyapatite, and thus for bone matrix mineralization [54].
In general, Ti alloys have shown similar or better biocompatibility than cpTi. The biocompatibility and cytotoxicity of an alloy is directly linked to the elements composing it, which should not exceed the limits allowed to obtain a good tissue response. In some cases, the alloy may lose biocompatibility because of the increased ion release into the environment. Therefore, it is important to balance element incorporation into the Ti alloy to attain adequate biocompatibility while maintaining improved mechanical properties [21].
7.2. Osseointegration
The osseointegration ability and success of implants are directly related to the optimization of implant surface chemistry and topography to promote cell adhesion, differentiation, and proliferation. A previous literature review concluded that cpTi and Ti-Al-V have similar osseointegration and biomechanical anchorage [103]. However, cpTi and some alloys, such as Ti-15Mo-1Bi, Ti-15Zr, and Ti-24Nb-4Zr-7.9Sn, have shown better results in animal studies when compared with Ti-Al-V (Table 3). An overview about these studies and others that compare some alloys with the cpTi implants is demonstrated in Table 3.
Table 3. Overview of the Ti alloys used in animal model studies.
| Material | Animal model | Parameter | Follow up | Result | Ref. | |
|---|---|---|---|---|---|---|
| Experimental | Control | |||||
| Ti-10.1Ta-1.7Nb-1.6Zr | cpTi |
Male Sprague–Dawley rats; 38 Screw-shaped implants in each group. |
Removal Torque Evaluation (RT); Bone-implant contact (BIC) and bone area ratio (BA); Gene expression. |
4 weeks |
No significant difference was observed between the Ti and the Ti–Ta–Nb–Zr for RT; There was a higher BA (41.2%) for the cpTi compared with the Ti–Ta–Nb–Zr implants (p = 0.012); No significant difference was found in the BIC percentage; The gene expression of the implant adherent cells revealed an approximately 3-, 6- and 2-fold lower expression of bone formation, bone remodeling and the proinflammatory genes at the Ti–Ta–Nb–Zr implants compared with the Ti (p < 0.05). |
[1] |
| Ti-Zr, SLActive® Straumann Roxolid® |
Ti, SLActive® Straumann |
Mini pigs 18 RT implants in each group; |
RT evaluation; BA and BIC ratios |
4 weeks |
Ti-Zr implants revealed a higher mean value for peak RT than Ti implants (p = 0.013); There was more BA within the chambers of the Ti-Zr implants than within the chambers of the Ti implants (p = 0.023); No difference was observed for the BIC measurements (p = 0.96). |
[8] |
| Ti-45Nb | cpTi |
Beagle tibia implantation model; 12 cylindrical implants in each group; Rabbits. |
Bone tissue volume to total tissue volume (BV/TV); BIC and BA; Bone integration; Oral mucosa irritation. |
12 weeks |
Similar peri-implant bone volume/tissue volume was maintained in both groups; Ti–Nb alloy possessed comparable osseointegration effect to pure Ti; The bone contact and bone area of the Ti–Nb alloy increased rapidly up to 12 weeks, with no significant difference from that of Ti; Neither group showed irritation or trauma to the mucous membranes of mouth. |
[45] |
| Ti-35Nb-2Ta-3Zr | Ti-6Al-4V |
Male white rabbits; 48 implants in each group. |
Pull-out force and new BA; Surface bone apposition ratio (BAR) |
Up to 12 weeks | There were no significant differences in pull-out force, BAR, or BA between groups at any time. | [104] |
| Ti-10Cu | cpTi |
White rabbits; 96 implants in each group. |
BIC; Mineral addition rate (MAR); Mean optical density (MOD) in BMP-2 and TGF-b1 expression. |
Up to 12 weeks |
No difference in bone density, BIC, or MAR was found between groups at any time point; At weeks 1 and 4, the MOD value for BMP-2 was significantly higher in the Ti–10Cu group than in cp-Ti group; No difference could be found in TGF-b1 expression between groups at any time point. |
[105] |
| Ti-Zr, SLActive® Straumann Roxolid® |
Ti, SLA® Straumann |
Female Bama minipigs (Younger and aged groups); 13 screw-type implants in each group. |
Implant survival and success rate; RT evaluation. |
8 weeks |
The survival rate of Ti-Zr implants (85.7%) was equal to that of Ti implants in the aged group; The mean value for peak RT of Ti implants was higher than that of Ti-Zr implants (p = 0.219) in the younger group; In the aged group, Ti-Zr implants showed a higher mean value for peak RT than Ti implants (p = 0.250); |
[109] |
| Ti-24Nb-4Zr-7.9Sn and nanotube- Ti-24Nb-4Zr-7.9Sn | cpTi and nanotube-cpTi |
Female white rabbits; 8 cylindrical implants in each group. |
BIC, BA, BV/TV and ratios; Mean trabecular number (Tb·N); Mean trabecular thickness (Tb·Th); Mean trabecular separation (Tb·Sp) |
Up to 12 weeks |
The BIC and BA ratios for the nanotube implants at 6 weeks were significantly higher than those of the other two groups (p < 0.05); At 12 weeks, the BIC were higher for all other groups than for the Ti implants (p < 0.05), whereas the bone area ratio was significantly larger for nanotube-Ti–Nb–Zr–Sn implants than for the other three groups (p < 0.05); The BV/TV, Tb·N, and Tb·Th values were the highest for the nanotube Ti–Nb–Zr–Sn group at both 6 and 12 weeks, whereas this group had the lowest Tb·Sq values. |
[110] |
| Ti-15Mo-1Bi | Ti-6Al-4V |
Female white rabbits; 24 cylindrical implants in each group. |
New bone formation. | Up to 26 weeks |
At 6 and 12 weeks postimplantation, there were no significant differences between groups; At 26 weeks, the bone areas of Ti-15Mo-1Bi were larger than those of Ti-6Al-4V (p < 0.001); The sum of the bone areas of the Ti-15Mo-1Bi implant was ≈ 249% of that of Ti-6Al-4V. |
[111] |
| Ti-24Nb-4Zr-7.9Sn | Ti-6Al-4V |
Female white rabbits; cylindrical implants in each group; |
Pull-out force; BV; Tissue Mineral Density (TMD); |
Up to 12 weeks |
Higher pull-out strength was detected in the Ti-Nb-Zr-Sn group than in the Ti-Al-V group at 12 weeks (p < 0.05); There were significantly increased BV and TMD in the experimental group compared with the control group at 12 weeks (p < 0.05). |
[112] |
| Ti-Zr, SLActive® Straumann Roxolid® |
Ti, SLActive® Straumann |
Male hound-type dogs 54 closure screw implants in each group. |
Mean bone loss; First bone–implant contact (fBIC); |
Up to 8 weeks | There were no statistically significant differences between the mean values (fBIC-x, bone loss/gain) for Ti-Zr and cpTi implants at or between any of the evaluated time points. | [113] |
| Ti-Zr, SLActive® Straumann Roxolid® |
Ti, SLActive® Straumann Ti-6Al-4V (sand-blasted and acid- washed surface) |
Female pigs; 24 implants in each group. |
BA and BIC; Multinucleated giant cell count |
Up to 8 weeks |
No difference in BA was found between groups; Ti-6Al-4V had statistically significantly less BIC than the other groups; Significantly more surface was covered by multinucleated giant cells on Ti6Al4V implants after 4 and 8 weeks. |
[114] |
It can be clearly seen that in most of the studies, alloys exhibit similar biological behavior to cpTi when fixed to animal bones. In one study, Ti-Zr implants produced higher new bone volume formation and greater removal torque value than cpTi implants [8]. Other alloys that are worthy of mention include Ti-Cu, Ti-Nb, and Ti-Nb-Ta-Zr, which exhibited bone tissue biocompatibility without adverse effect on new bone formation [45], [104], [105]. The surface composition also appeared to favor the mechanical stability of the implant in the bone for the Ti-Nb-Ta-Zr alloy, as the amount of bone around the implant of this alloy increased over time, without changing in cpTi implants [1].
Human studies with Ti alloys are scarce and restricted to Ti-Zr, which is already commercially available (Roxolid, Institut Straumann). In vivo studies in humans conducted with small diameter implants of Ti-Zr [106], [107], [108]demonstrated acceptable performance regarding to implant survival and success rates, showing that this material presents osseointegration comparable to that of cpTi. Table 4 shows an overview of the studies in humans with this alloy. Despite the results, most of the studies present a maximum follow up of 1 year, which indicates the need for further long-term research to confirm its clinical performance.
Table 4. Overview of the Ti-15Zr alloy used in human studies.