1. Introduction
Heart diseases are a primary cause of death and disability in many nations, accounting for approximately 40% of all human deaths [1]. Ischemic heart diseases (IHD) are a group of cardiovascular diseases that include unstable and stable angina, myocardial infarction and cardiac arrests. The major causes that have proven to lead to IHD are increased blood pressure, smoking, unhealthy eating habits, lack of exercise, alcoholism and abnormality of glucose levels. Injuries caused by ischemic heart diseases damage the cardiac tissues. The cells that are dead form a fibrotic scar gradually, which disturbs continuum of the heart beats by the ventricular muscles thus reducing the blood pumping capacity of the heart [2]. Another significant cause of IHDs is atherosclerosis. It is an inflammatory condition where immune competent cells produce cytokines that are pro inflammatory. In such a condition the number of dead cells and lipoproteins with pro inflammatory properties are in high concentrations which lead to cell death due to inflammation further leading to heart failure and death [3]. There has been an increase in the number of surgical procedures to correct heart problems over the last decade. The number of cases and expenditure in hospitals has also increased. This situation calls for a more reliable, permanent, and natural method. Keeping in mind that a healthy lifestyle must be followed to prevent the occurrence of cardiac diseases, it is also necessary to introduce methods to overcome growing incidences of cardiac disorders [4].
There are several new technologies that are coming up for overcoming and repairing the injured cardiac cells. MiRNAs have been proposed as an alternative to cell therapy for cardiac regeneration. MiRNAs are short RNA sequences that bind to target messenger RNA sequences and prevent their translation into a protein. Where MiR-199a and miR-590 have shown the ability of inducing cardiomyocyte proliferation in animal models, miR-208 in cardiomyocytes regulate the balance between the a- and b-myosin heavy chains. MiR-1, highly abundant in cardiomyocytes has shown to target insulin-like growth factor-1. Increasing miR-1 levels downregulates the insulin-like growth factor-1 pathway, which is an important contributor to cardiac hypertrophy and arrhythmias [5]..
Tissue engineering is also an upcoming technology that is a multidisciplinary field involving biological, chemical and engineering sciences which deals with construction of suitable cellular environments in order to enhance tissue regeneration [6], [7]. It is a new approach for improving the current procedures available for heart diseases [8]. Valves, coronary grafts and myocardium are the three main targets of tissue engineering in the heart [9]. In this field, scientists have used a variety of methods to create tissue-engineered heart valves (TEHVs) and tissue-engineered vascular grafts (TEVGs) [10]. Engineering cardiac tissue in vitro has improved recently in areas such as stem cell isolation methods, culture in bioreactors and synthesis of bioactive materials [11].
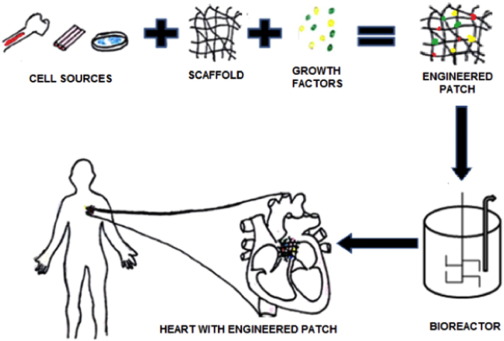
The primary principle involved here is the production of living cells by cellularized grafts that imitate nature and can grow and substitute the damaged cells in the recipient organ through the interaction of cells, scaffold, and growth factors [12] as shown in Fig. 1.
 Fig. 1. Engineering of cardiac tissue using cell sources, scaffolds and growth factors.
Fig. 1. Engineering of cardiac tissue using cell sources, scaffolds and growth factors.Native 3D mimicking cannot be expected out of these constructs, but it can be used at an early stage to study efficacy, safety and ethical concern of the receiving environment [13]. Biomaterials have shown increasing potential as a tool for such techniques [14]. In this review, we recognize the principles, challenges, and recent advancements in the field of cardiac tissue engineering.
2. Cell sources
Engineering a cardiac tissue requires uncontaminated populations of three major cell types: smooth muscle cells, endothelial cells and cardiomyocytes. So far, cells obtained from allogenic, autologous sources as well as stem cell derived cardiomyocytes have been used in trials as shown in Fig. 2. These are introduced into hearts after myocardial infarction (MI) with the goal to improve cardiac function [15].
 Fig. 2. Choices of cells for cardiac tissue engineering.
Fig. 2. Choices of cells for cardiac tissue engineering.2.1. Cardiomyocytes
Cardiomyocytes contribute to most of the volume of a human heart. Thus, earlier approaches were based on exploiting cardiac cells and purifying them to obtain cardiomyocytes [16]. Both neonatal and foetal cardiomyocytes have been used as their replication rate is sufficient and they can aid in scar healing. It has been shown that early-stage cardiomyocytes are preferred over mature cardiac cells due to their greater in vivo survival rate [1]. However, issues such as immunogenicity due to their allogenic source, malignancy, limited availability and ethics of the procedure can pose a major concern [15]. Furthermore, the results of integration of the transplanted cells with host myocardium are contradictory [17]. In addition to these, primary cardiomyocytes have limited access [18] and their microenvironments have not been remodelled entirely. Terminally differentiated cardiomyocytes do not survive as well as neonatal cell types [19] because they are highly sensitive to hypoxic environments [20]. For these reasons, more suitable and apt cell sources are discussed further.
2.2. Skeletal myoblasts
These were one of the earliest cell types tested for cardiac repair. Skeletal myoblasts can be easily obtained through a muscle biopsy from the individual, which obviates the need for immune suppression [21] and can be transplanted to cardiac tissue easily. They multiply at high rates in vitro [8] and are resistant to hypoxia [10]. Satellite cells are triggered upon muscle damage and are able to differentiate and combine with the muscle fibres that are already present to form fresh fibres without tumour formation [1]. Skeletal myoblasts are supplied either by surgery, by percutaneous intramyocardial method [22] or are injected during coronary artery bypass graft (CABG) surgery as in the Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial [12]. The feasibility trial showed disintegration of myoblasts into the myotubules improving the LV functions, which is associated with the property of fatigue-resistance of the cells [17]. MyoCELL®, a skeletal muscle myoblast cell therapy developed by BIOHEART, is in its Phase II/III trial in the USA [23].
A main drawback is that these cells remain committed to the skeletal muscle lineage and do not electro-physiologically couple with the host cardiomyocytes [24]. As a result, they are unable to integrate into the myocardial milieu [17] and have been associated with arrhythmias [25].
2.3. Fibroblasts
Fibroblasts, of mesenchymal origin, belong to a class of connective tissue cells that are spindle shaped and are widely present in all parts of the body [26]. They help in maintaining the extracellular matrix (ECM), mediate the distribution of force via the ECM and cell-cell interactions [27], and aid the development of the endothelial cells [19]. Therefore, they are being extensively used in the culture of cardiac muscle cell and tissue culture [28], [29]. The use of fibroblasts is to enhance vascularization and it has been seen that these cells have angiotensin II receptors. The local angiotensin helps in maintaining the homeostasis of the injured tissues by stimulating the proliferation of more fibroblasts and smooth muscle cells [1], [30].
Under resting conditions, fibroblasts of the cardiac tissue develop contractile features and have features like that of smooth muscle cells. These cells are termed as myofibroblasts. They are not present in normal tissues but appear when there is an injury to the cardiac tissue. These myofibroblasts can be used for rebuilding tissues of the heart. They additionally can secrete ECM component fibronectin and stimulate the release cytokines and growth factors, leading to the repair of injured site [26].
Another application of fibroblasts is to convert them to form cardiomyocytes [15], [31]. This led to better contractile features, eventually leading to localized relief of injured cardiac cells. However certain issues such as the number, safety and the formation of unpredictable cells lines still remain [32].
2.4. Bone marrow derived stem cells
According to a study, the use of bone marrow mononuclear stem cells improved the condition of injured heart by regeneration of the cells thus paving way for its use in cardiac tissue culture [33]. In a particular experiment conducted, bone marrow derived stem cells were transplanted to injured cardiac tissue. Using marker technology, it was found that 68% of the cells had regenerated thus showing the usefulness of bone marrow derived stem cells [34].
Bone marrow consists of various kinds of stem cells including hematopoietic stem cells, mesenchymal stem cells, peripheral stem cells and endothelial stem cells [31], [35]. Hematopoietic and mesenchymal stem cells can be signaled to differentiate to cardiomyocytes. However, they have angiogenic and anti-inflammatory effects on transplantation [36].
The endothelial cells or the progenitor cells have the capacity to improve cardiac function [35]. Studies have shown that they help in the regeneration of cells and microvascularisation with red blood cell perfusion [37]. However, the low numbers limit their use [36]. Studies show that bone marrow stem cells even differentiate to form fibroblast-like cells that form an essential part of cardiac tissue engineering [25].
The benefit of using bone marrow derived stem cells is that they can be derived from the patient thus eliminating the possibility of immune response and making procurement of cells for culturing and surgery easy [15], [30], [35]. Apart from this, they can transdifferentiate and change their function based on the signals [8]. However, the effect of bone marrow stem cell transplant is temporary and the attributed function of transdifferentiation could have been because of the presence of adipose derived cells. Endothelial progenitor cells are still picked when bone marrow stem cells are considered for tissue engineering [25].
2.5. Human embryonic stem cells
Embryonic stem cells, derived from the inner cell mass of blastocyst are used due to their potential to develop into many specialised cell types. Murine embryonic stem cells (mESCs) are easier to cultivate on gelatin-coated petri dishes with the addition of a leukaemia inhibitory factor. Human (hESCs), on the other hand require a far more intricate ECM coating. Furthermore, the rate of beating of mature cardiomyocytes between human ventricular CMs and rodent ventricular CMs is completely different with human cells preferring slower paces of 1–2 Hz [38], [39], thereby making it unsuitable. Such interspecies variances should be taken into consideration. It has been shown that accumulation of lactic acid decreases the pH of the media and thus affects the cardiac differentiation of ESCs [21].
Earlier differentiation protocols were ineffective. In the new modulations, the hESCs are cultured in a medium containing activin A and bone morphogenetic protein-4 (BMP-4) to induce them to form cardiomyocytes. This can be enhanced by adding exogenous Wnt3a as well as by inhibition of Wnt signalling with dickkopf-related protein 1 (DKK1) to increase cardiogenesis [15]. A recent invention is the exclusive use of glycogen synthase kinase 3 inhibitor. It induces mesoderm differentiation by activating Wnt/β-catenin signalling followed by inhibiting Wnt production-4 to promote high levels of cardiogenesis [27]. Electromechanical coupling of the cells with the native myocardium has shown to improve cardiomyocyte alignment and restoration of heart function [18]. It has shown to integrate with host tissue [40] and improve ventricular function in animal MI models.
When hESCs are co-cultured with murine stromal cells and are grown on a two-dimensional layer, the result is a heterogeneous mixture of cardiomyocytes and noncardiomyocyte cells [32], [41]. Due to unavailability of specific cell marking techniques, cardiomyocyte purification is difficult [42]. The allogeneic source of the ESCs requires subsequent immunosuppressive therapy [43]. Furthermore, ESCs have many ethical and legal issues [44] due to the high risk of teratoma formation in animal models [45].
2.6. Parthenogenetic stem cells
Using parthenogenetic stem cells is a fascinating way of significantly reducing the number of possible combinations of surface antigens as the HLA profile of only a single donor is displayed. This also eliminates the ethical dilemma of hESC [28]. In a study, cardiomyocytes derived from mouse parthenogenetic stem cells, when transplanted in a mouse infarct model, integrated electrically with host myocardium and enhanced contractile function [46].
Only BMSCs, myoblasts, cardiac progenitor cells and ADSCs have been active in clinical trials yielding both encouraging and disappointing results [47]. Additionally, the regulatory agencies have not yet examined the cell therapies involving human cardiomyocytes or their progenitors for the quality of differentiation [32].
2.7. Induced pluripotent stem cells
Induced pluripotent stem cells (IPSCs) are somatic cells that are programmed to be pluripotent and have gained importance over the years in the cardiac tissue engineering [48]. Induced pluripotent cells are reprogrammed by the addition of transcription factors kfl-4,Myc c, Oct 4 and Sox 2 [24]. The addition of Flk1, Isl1 or Nkx2.5 have led to the formation of cardiac progenitor cells which have been used in cardiac tissue engineering. Studies have been conducted where IPSCs were successfully used in vivo for the engineering of heart tissue using miRNA for delivery of factors [31]. They are used to regenerate cells of the heart by reprogramming them [27]. Several experiments are being conducted using IPSCs derived from fibroblasts. They have been studied extensively in infracted hearts of rats and pigs to understand the modelling and functionality of these cells [46]. These cells are also used in cell sheets and delivery of cells in vivo and in vitro [49].
On the other hand, cardiomyocytes are generated by growing hiPSCs with endodermal cells which release signalling factors required for differentiation. But, this method results in low yields. A two-step process can generate cardiomyocytes where initially an embroid body is formed which further differentiates to cardiomyocytes. Initially cytokines like BMP-4, activin A, and WNT3A are added and then BMP inhibitors and Wnt inhibitors such as Noggin and DKK1 are included to form cardiomyocytes [41]. Another method involves the formation of a monolayer of IPSCs and sequential addition of activin A and BMP-4 that leads to average yield of cardiomyocytes [42].
The benefit of IPSCs is that the somatic cells are derived from the patient thus eliminating the possibility of immune response. This also eliminates ethical concerns that arise from using hESCs [38]. Moreover, being pluripotent, the cells can be generated in large numbers thus making them easily and readily available [40]. The only concern that arises with using IPSCs is the possibility of development of teratomas. New experiments are being conducted to overcome this disadvantage. Antibodies are being programmed to differentiate cancerous cells and the cardiac tissue cells [36].
Recent studies have used T cells and Sendai virus to produce IPSCs. These cells are said to have the advantages of high rate of multiplication, less invasive to the patient and lower risks for teratoma formation [43].
3. Scaffolds
Scaffolds act as a support for the cells seeded in culture till they secrete their own ECM and form a tissue [50]. Ideally, the scaffold should disintegrate while healing or after. This eliminates the necessity of removal of the material and potential side effects. Disintegrated products must be non-toxic to the cells [51].
3.1. Criteria for scaffolds
In addition to the harvested cells and the microenvironment provided for cell proliferation, the choice of biomaterial and product design plays a vital role in transplantation [50]. An ideal scaffold should imitate the natural myocardial ECM and should possess adequate porosity that promotes vascularization [52]. It should also allow continuous diffusion of oxygen and nutrients to the seeded cells. It must be sufficiently thick to contract with proper strength and beat synchronously with the neighboring cardiomyocytes [53]. The different types of scaffolds used are shown in Table 1.
Table 1. Types of scaffolds
| Scaffold | Sources | Advantages | Disadvantages |
|---|---|---|---|
| Hydrogels | Natural, synthetic and mixed |
1.In-situ injection of cells 2.Eliminates immune response |
1. Vulnerable to breakage before tissue formation |
| Decullarised | Natural |
1. Vascularization 2.Reduced immune response |
1. Decreased rate of cell division |
| Prefabricated | Natural and synthetic | 1.Easy to engineer and manipulate 3D forms |
1. Incompatibility with cellular application 2. Optimal porosity should be maintained |
| Cell sheets | Natural |
1. Easy to scale up in 3D form 2. Easy to manipulate 3. Conduction of action potential |
1. Fragile and difficult to handle 2. Limited thickness |
Though the scaffolds can be diverse, there are certain fundamental criteria that must be met by the scaffold:
Biocompatibility
The transplanted scaffolds must escape two main factors: immunogenicity and coagulability. The host immune response should be minimal for the scaffold to be accepted in vivo. Furthermore, the biomaterial should resist development of blood clots, which may result in proximal or distal tissue infarction [18].
Biodegradability
The scaffold must degrade with time without leaving remnants in the body [29]. The transplanted material should be broken down in vivo by mechanisms such as hydrolysis, oxidation, enzymatic and physical degradation. Bioresorbable polymers degrade into nontoxic products which can be eliminated via metabolic pathways [54]. This process should occur at a specific rate and should finally be replaced by new viable tissue [10].
The use of natural polymers reduces the possibility of transplant eliciting an immune response. Hydrolytically degradable biocompatible polymers were one of the first materials used for cardiac tissue engineering. Later it was realized that the material's mechanical properties had to match host tissue to obtain optimal viable cell-seeded grafts [55].
Mechanical properties
The scaffold must mimic the mechanical properties of the native cardiac tissue and bear the cyclic strains and stresses exerted upon transplantation. As mentioned earlier, the mechanical properties can alter the phenotypical arrangement of the seeded cells. Compared to natural polymers, synthetic polymers provide durability and a much higher strength. However, local inflammation may take place due to bulk degradation [11].
3.2. Hydrogels
In this method, the cells are captured in gel matrix formed by the cross linking of water soluble polymers [24]. They can be natural polymers like collagen or synthetic products or even a mixture of the two kinds. Anchoring materials are added to help the cells adhere to scaffold and grow multifold. An example for the same is the sequence RGD (it is composed of l-arginine, glycine, and l-aspartic acid) of peptides that help in adhesion and proliferation of cells in most cases [56]. As the cells grow, the scaffold loses water and gets highly compressed. These scaffolds are initially used in the multiplication of cardiac tissue cells ex vivo. However, with developing technology, they can now be injected into the body for the delivery of cells [28].
The in-situ injection of cells captured in hydrogel eliminates the possibilities of immunological reactions and increase the efficiency of delivery of cells when compared to cells delivered without a hydrogel [25]. Hydrogels that are injected in situ must be broken down over time. Fibrin is commonly designed to form hydrogels encapsulating cardiac tissue cells. It has been found that fibrin gels have been able to stimulate the release of elastin by SMC. However, they are mechanically weak and are often combined with collagen to form a stronger scaffold [54]. Another commonly used natural polymer is alginate, derived from seaweed. Alginate based hydrogels are commonly used for drug delivery [1]. It has properties that help cells bind to it and lead to a controlled release. Alginate based hydrogels are either crossed linked ionically or photo-crosslinked to cells that captures and releases cells or drugs [57]. Collagen based gels are used as well. Their major advantage is their ability to recruit cells like the endothelial cells that help in regeneration of heart cells.
Matrigel is a mixture of different polymers and growth factors. They are widely being used for their attachment properties to embryonic cells and IPSCs and for their delivery in vivo. However, since they are derived from mouse tumors, they cannot be used in human infarcted tissue [14]. Polyethylene glycol (PEG) based hydrogels are used to trap cells within the matrix using light. The surface can be modified to help in attachment to other cell surfaces. They however do not promote neovascularisation [44]. There is constant effort to make these scaffolds degradable. This can now be done by incorporating lactic acid units. Enzymes and growth factors can be delivered using these systems as well [58], [59]. Another study with hydrogels has shown that a combination of ECM materials with collagen can direct the formation of cardiac cells from embryonic cells. When compared to high ECM containing collagen hydrogels, lower concentration hydrogels have lower capacity to differentiate hESC to cardiac cells [60].
Injectable hydrogels are gaining considerable attention these days. They are prepared by mixing liquid hydrogels and delivered to the body using catheters. This avoids the surgery for scaffold delivery and use of drugs or anaesthesia. The main uses of injectable hydrogels in cardiac tissue engineering are: They remodel and help in repair of damaged cardiac cells, they act as a support for ECM building and matrix for transplanted cells. The major disadvantages of injectable hydrogels include the lack of control of the final delivered transplant and cell number [16]. They are used for delivery of cells, delivery of protein based substances like growth factors and are made in various combinations [22], [44], [61].
3.3. Decellularised scaffolds
This particular type of scaffold utilizes the principle of using the organ itself to provide for the 3D structure of tissue [48], [62]. The heart is taken and the cells are washed away using detergents like Triton X-100 and SDS. Consequently, only the connective tissue of the heart remains along with the ECM. ECM is a very important part of tissue building. It provides support, helps in the multiplication and regulation of cellular function [54]. Thus, using the decellularised skeleton structure and ECM, the seeded cells grow and multiply. Though the blood vessels from the original structure remain intact, the multiplication of the newly added cells are slow and difficult in these conditions [25], [28]. In a particular experiment, decellularised porcine valve was used for human valve replacement. The porcine valve was decellularised using detergent and also treated with DNase and RNase to remove any cells that remained. The scaffold consisted of only collagen and elastin. This scaffold was reseeded with human cells thus reducing the probably of an immune response that happens when a xenograft or allograft is used for transplantation [63]. In another approach, the pericardium was decellularised and then strengthened by cross linking of glutaraldehyde, however there was a possibilty of calcification. Several anti-calcification methods like treating with glutamic acid and nano-coating with titanium can enhance the use of this type of scaffold [14].
3.4. Prefabricated matrices
In this type, the scaffolds are built using prefabricated materials that have the cells of cardiac tissue embedded in them. This type of scaffold building involves more of engineering principles and has proved to be effective in cardiac tissue engineering [64]. The materials used for the matrix can be natural polymers or synthetic materials like poly- l- lactic acid and polyglycolic acid. Studies have shown that introducing gold nanofibres improved the tissue generation capacity [28]. In a particular study, collagen matrix was embedded with bone marrow cells and then transplanted into patient. This treatment improved the patient's condition thus showing that this is a promising method [14]. In another study the scaffolds were made using PEG mesh, coated with poly (4-hydroxybutyric acid) (P4HB). A fastacryl stent was attached to the leaflets of the valve scaffold. Coaptation areas of the scaffold ensured closure. This was then seeded with cells that proliferated and formed tissue [65].
Several methods of fabricating matrices using natural or synthetic materials are shown in Table 2. However, reproducing the exact ECM structure and architecture of organ is difficult using these methods [66].
Table 2. Methods of scaffold fabrication.
| Technique | Necessity of mold | Scaffold recovery | Application | Citations |
|---|---|---|---|---|
| Porogen leaching | Yes | Leaching of porogen with water followed by drying | Porous scaffold | [67], [68] |
| Freeze drying | Yes | Depressurising gas leading to growth and escape of gas bubbles | Obtain various pore sizes | [69], [70], [71], [72] |
| Phase separation | No | Dependent on polymer concentration and quenching temperature | Oriented pore architecture | [73] [74] |
| Electro-spinning | No | Spun fibres are collected on a sheet | Fibrous scaffold, control porosity | [75] [76] |
3.5. Cell sheets
This is a new scaffold free approach employed due to (i) lower cellular concentration inside scaffolds compared to the native heart and (ii) the inflammatory reaction caused by scaffold degradation.
Cells are initially cultured on dishes until they are confluent. These dishes are coated with a temperature-sensitive polymer, poly-N-isopropylacrylamide. The dishes are hydrophobic and cell adhesive at 37 °C. However, on decreasing the temperature to 32 °C, detachment of the monolayer cultured cells along with the extracellular matrix and attachment proteins as an intact layer was seen. When the 2D cell layers are piled, they can quickly attach and form cell-to-cell connections because the ECM and intercellular connections between neighboring cardiomyocytes are preserved [15].
The hiPSC-cardiomyocytes sheets are prepared in layers. When transplanted, they conduct action potentials leading to improved cardiac function, vascularity, and fibrosis [41]. Studies with skeletal myocytes (SkMs) have shown reduction in ventricular arrhythmias. There was an increase in vascularization in multilayered cardiomyocyte sheet patches after sandwiching the ECs between cell sheets. Similarly, the implantation of MSC sheets into infarcted rat hearts showed progress in cell retention number, LV function and reduced dilation [24], [25].
Even though cell sheets are an attractive option as they are composed of fully natural compounds, they are fairly fragile and hard to handle [39]. The layered cardiac cell sheets have a thickness limit of approximately 80 μm, exceeding which, the vascular network formed is unable to supply sufficient nutrients and oxygen to the tissue [21]. Since a thin layer of cells has little strength for contraction, mixed cell sheets of endothelial cells and cardiomyocytes have been used to further improve the vascularization of a cardiac graft [32]. To scale up the production, triple-layered cardiac cell sheets containing cardiomyocytes, endothelial cells and fibroblasts are continually overlaid on the vascular bed and perfused using a bioreactor system with optimized doses of fibroblast growth factor 2. This procedure is carried out in multiple steps to ensure thicker and denser cardiac tissues [53]. However, a study reported that rather than cardiac contraction, cytokine effects were the main cause for the functional recovery of heart with cardiac cell sheets [43].
4. Conclusion
The future of this field is very promising yet there remain drawbacks and many unanswered questions. There are areas in this field that require research and development. The cell types (adult cells, embryonic cells, or induced pluripotent stem cells; heterologous or autologous), matrices (natural or synthetic hydrogels, collagen, polylactic acid-co-glycolic acid, polylactic acid, extracellular matrix) and other factors best-suited to obtaining tissue regeneration are yet to be fully defined. The various cardiovascular products available are shown in Fig. 3. But, given the high risks of cardiac disease and the increasing number of deaths, there is a necessity for better and a more sustainable research in this field. Cardiac tissue engineering has the potential to emerge as good solution to cardiac diseases. The hope to regenerate cardiac tissue becomes stronger as methods and disciplines merge.
 Fig. 3. A summary of cardiac tissue engineered products.
Fig. 3. A summary of cardiac tissue engineered products.