1. Introduction
The recovery of value from industrial wastes will help avoid the environmental impacts associated with the improper disposal of them. Research has been carried out on recovery of value from industrial wastes coming from various industrial activities. Within this context Hussain et al. (2021) claim the synthesis of Na-Zeolites using as a source of silicon and aluminium the rag fly ash produced from the incineration of waste fabric coming from a textile industry. According to the researchers the zeolites showed a Pb2+ removal of 98% efficiency from polluted waste water at 100 mg/L initial metal ion concentration. In the metal mining industry, the kaolinite-type pyrite flotation tailings have impurities such as iron and titanium. Cui et al. (2018) have published that the calcination (800 °C) of those tailings results mainly in a metakaolin product. The researchers let us know that they have synthesized the zeolite 4A by using the metakaolin as a source of silicon and aluminium. Spent fluid catalytic cracking (FCC) catalyst is a petroleum refinery by-product from fluidised-bed catalytic cracking units. Leone et al. (2018) assert that they have recovered cerium and lanthanum by leaching them from the spent catalyst by testing HCl, HNO3 and H2SO4 as leaching agents. The remainder solid leaching residue was used as a source of silicon and aluminium. The researchers claim the synthesis of zeolites A (Si/Al, 1.00) and X (Si/Al, 1.03) that was effective in the removal of Ni2+, Zn2+ and Cu2+ from waste water. Deng and Ge (2015) declare the synthesis of Na-P zeolite, by using the fly ash residue coming from the coal-fired power generation as a source of aluminium and silicon. Fly ash is first fused at 550 °C with Na2CO3. Then the fused product is treated hydrothermally at 80 °C with 2M NaOH. The researchers claim a Na-P adsorption capacity of 99 mg g-1 Cu2+ from contaminated water.
Passivation is the unit operation that allows the material to be protected from corrosion and by this means it rises life and improves quality and appearance. The surface passivation of aluminium is obtained by oxidizing a surface layer of the material. Anodizing is a technology to passivate the outer layer of the aluminium by immersing the rack of parts in a bath containing the electrolyte. The aluminium parts are given a positive electric charge and it becomes the “anode”. Once the electrical current flows through, oxygen gets free and it goes to the “anode” oxidizing it and builds up an aluminium oxide layer (Al2O3). Therefore, aluminium anodizing is an electrolytic passivation process that converts the metal surface into a corrosion-resistant, anodic oxide film (Al2O3).
The aluminium anodizing industry uses aluminium alloys that contain sodium, potassium, calcium, magnesium, and iron that are partially removed during the electrolytic processes. The spent chemical baths and rinse water undergo a physicochemical treatment. The unit operations in water treatment plant are: coagulation with no addition of any chemical, since the effluents already have aluminium hydroxide, sedimentation, flotation with a polymer and mechanical filtration using a filter press.
The final dewatered anodizing residue (AAR) contains potentially valuable elements but is commonly discharged to landfills. Under acidic conditions, aluminium and other elements could leach from the AAR and migrate to soil, groundwater, and surface water, which could cause environmental impacts.
At pH levels between 4.0 and 4.5, aluminium is bioavailable in natural waters in the monomeric form and is toxic to the aquatic life at low concentrations (Gensemer and Playle, 1999). Under acidic conditions, aluminium can migrate from the AAR sludge matrix to the environment and adversely affect surface water resources. Within this context, Kluczka et al. (2012) studied the potential risk to the aquatic life in water and bottom sediment of fish-breeding ponds, due to the aluminium toxic forms. The researchers conclude that the aluminium bioavailable in the water in the range of Al, 26–34 μg/dm3 and concentrations of interchangeable aluminium in sediment in the range of Al, 5–34 mg/g, may cause a potential hazard impact on the aquatic life of the fish. Kluczka et al. (2017) evaluated the risk of soil pollution due to aluminium bioavailable and heavy metals existing in the aluminium sludge that is used as fertilizer. The assessment was carried out in very weak acidic and acidic agricultural soils, fertilized with aluminium sludge resulting from the water purification facilities and municipals sewage treatment plants. The researchers reveal that 10% of the total aluminium in the sludge was in available form. They conclude that this percentage could be regarded as a danger for the environment.
Depending on aluminium levels, it may affect the enzymatic activity in plants and prevent the uptake of minerals, nutrients, and water (Bojórkez et al., 2017).
The present work focusses on industrial waste generated from aluminium anodization. The general objective of the work is the recovery of aluminium and other ions in the AAR by synthesizing zeolites. Zeolites are composed mainly of aluminium, silicon, and alkali or alkaline earth ions. The recovery of those elements through the synthesis of zeolites constitutes an important contribution to the management of AAR for the aluminium anodizing industry as a whole.
Two recovery processes were considered: (1) metal electrowinning and (2) recovery of aluminium and associated ions by synthesizing a particular zeolite. Freeman (1995, pp.382) describes electrowinning as the method to recover metal from wastewaters before precipitation to reduce sludge. In our case, the sludge is already formed. Therefore, the method might be tested in the acidic anodizing stream saturated with aluminium before it is pumped to the wastewater treatment plant. Hannula et al. (2019) studied the recovery of copper from low concentration and complex wastewater solutions by electrowinning. The scientists pointed out as the main disadvantage in using electrowinning to produce high-quality metal deposits energy efficiently from complex and dilute wastewaters is the low reaction rate of the process. In this case, it might be necessary first to acidic leachate the metal from the sludge and this might require a great amount of acid to have the aluminium in an acidic solution and then attempt the electrowinning technology. The low recovery reaction rate might add obstacles that might make unapplicable the electrowinning method for metal recovery from sludge. The second alternative is based on the recovery of aluminium and associated ions from the AAR by incorporating them in the zeolite structure, which has tetrahedral aluminium and silica units bonded through oxygen and requires cations to maintain electric balance. Zeolites have a wide range of applications in industry, medicine, agriculture, soil remediation, and as a molecular sieve in water treatment to remove cationic dyes and heavy metals. Within this context, Pereira et al. (2018) report an environmental application of zeolite A in the removal of cationic dyes from textile wastewaters. They assert that the zeolite shows high adsorption capacity for the malachite green with an equilibrium value of 55 mg/g. The adsorption capacity lowers on the removal of methylene blue and safranine. Rodríguez (2006) studied the catalytic activity of zeolites ZSM-5 and Beta in the cracking of plastic residues. The researcher reports that zeolite ZSM-5 enables more extensive and more selective conversion of heavy fractions of polyolefins to lighter products (C1-C5), whereas zeolite Beta is more selective for medium distillate fractions (C6 -C12). Gorimbo et al. (2018) investigated the potential of clinoptilolite in metal-polluted industrial effluent treatment. They report that the selectivity of the clinoptilolite for the adsorption of Ni2+, Cd2+ and Pb2+ from contaminated water is Pb2+ > Cd2+ > Ni2+. All these considerations were decisive in the outcome of aluminium reclaiming from industrial sludge, by means of zeolite synthesis.
At an Ecuadorian facility, the cylindrical aluminium alloy ingots (Standard Industrial Codes 6063, 6005, and 1100) are extruded and subjected to the following chemical processes: impurity removal process by degreasing with detergents; acid etching (before caustic etching) in an ammonium difluoride bath; deoxidizing in a sulphuric acid solution at strength 12%, plus 0.5% hydrogen peroxide at room temperature, to remove aluminium oxides; anodizing; and dyeing. Dyeing requires electrodeposition of metallic salts and sealing, which causes hydration of the aluminium oxide layer and covers the product with metal salts. Spent baths and water rinses are pumped to an industrial wastewater treatment plant, where after physicochemical treatment and dewatering of the wet residue in a filter press, an aluminium anodizing residue (AAR) is produced that is stored and finally disposed of in a landfill.
Efforts are made to use aluminium industrial residues as an aluminium source in the production of aluminium sulphate that is used as an aluminium-based coagulant in drinking water and wastewater treatment. Within this context, Patel and Sc Thesis (2008) studied the reclamation of aluminium from aluminium-anodizing sludge at Finex, aluminium passivation facility operating in New Zealand. The researcher claims by using the aluminium sludge the production of alum at 4 °C extractions by treatment of the sludge with sulphuric acid followed by crystallization with anhydrous potassium sulphate. The investigator declares a maximum yield of 92.6% as a crystalline alum. In the same line, the EPA's Water Engineering Research Laboratory developed a project of aluminium acidic reclamation from the aluminium-anodizing sludge produced as a result of finished extruded architectural aluminium. Thereupon, Saunders (1988), as part of the research team, affirms that the sulphuric acid extraction of aluminium from the aluminium-anodizing dewatered residue yields a commercial-strength solution of aluminium sulphate with a concentration of 8% as Al2O3. In the same direction, as the aluminium-anodizing sludge environmental restrictions for disposal and corresponding cost are increasing, Saunders et al. (1984) investigated, at the Research Laboratory of the U.S. Environmental Protection Agency, innovative processes to implement for use in the treatment of sludge resulting from the aluminium passivation processes. The researchers claim that by using the sludge solids content of 21% or higher it is viable the production of commercial-strength aluminium sulphate solution with a minimum concentration of 8.3% Al2O3. Finally, they assert that the reclamation of aluminium-finishing sludge as aluminium sulphate solution is an economical alternative to waste disposal with an estimated pay-back ranging between 14 and 21 months. Saito et al. (1985) assert the production of alum by acidic extraction of aluminium from aluminium-anodizing sludge. Besides the researchers claim the synthesis of zeolite Types A and C, by using the etching waste. The experimenters inform that the pH of the zeolite's suspension falls in the range of 11.5–12.5, and there was identified in the etching zeolite suspension a maximum heavy metals concentration (as Pb) of 10 mg/L. The presence of heavy metals in the effluent opens an environmental issue that has to be taken into account.
Several studies have been examined the economic reuse of AAR. Based on a high-resolution scanning electron microscopy (SEM-EDS) image, a NaP GIS zeolite has been synthesized using AAR as amorphous raw material (Fig. 1, Fig. 2) and ultrapure silicone solution as silicone-enrichment reagent and a 1.5M NaOH solution as the mineralizing agent (Peñafiel and Martínez, 2019). Creating a 5% AAR in mixture with clay enables the fabrication of bricks. The properties of the bricks are improved by the addition of synthetic gamma-alumina nanoparticles, but it is expensive. (Marques et al., 2012). In fact, the synthesis of nano γ–alumina (Nano γ-Al2O3) based on AAR to produce bricks, demands additional energy and operating costs, as the main unit operations are calcination at 800 °C, leaching with HCl at 90 °C, precipitation, filtration and calcination at 700 °C (Khodadadi Darban et al., 2013).
 Fig. 1
Fig. 1 Fig. 2
Fig. 2This work replaces the ultrapure silicon solution, due to its cost by commercial sodium silicate (CSS) that makes more viable the project. These aspects make the research to have a particular approach focussing on synthesis of zeolites using as raw material wet AAR.
In this study it was hypothesized that AAR could be used to synthesize a zeolite, and the hydrothermal method was used to test this hypothesis. Experimental issues examined included the need to enrich the AAR with silicon and the required AAR and CSS proportions, temperature requirements, and reaction time.
The research is relevant to and congruent with concepts of sustainable development, circular economy, cleaner production, pollution prevention, and social and environmental responsibility.
2. Materials and methods
2.1. Aluminium-anodizing residue sampling
The facility “Corporación Ecuatoriana de Aluminio S.A. CEDAL”, installed in the city of Latacunga, Ecuador, produces a monthly average of approximately 200 tons of AAR as dewatered sludge cakes. Composited samples were taken from 5.5% of the monthly production (11,000 kg of AAR) by subsampling 1000-kg bags of dewatered chemical waste.
Composited 185-g samples were taken from the surface, middle, and bottom of each of the 11 bags using a steel sampling auger. A total composited sample of 2 kg was stored in 4 Ziploc bags that were safely sealed.
2.2. Aluminium-anodizing residue transferring to the research lab
A certificate was obtained from the Ministry of the Environment (Ministerio del Ambiente) of Ecuador that allowed a 2-kg sample to be transferred to the Polytechnic University of Valencia (Universitat Politécnica de Valencia), Spain, where the experiments were conducted.
According to the Ecuador's Environmental Law (Acuerdo Ministerial 061., 2015) (AM 061, Art. 80), the AAR is regarded as special industrial residues, which are defined as non-hazardous but capable of causing an environmental and health impact due to the large amount of discharge and/or because they are impossible to degrade. Such wastes must be recovered, reused, and/or recycled to improve industrial waste management and avoid disposal in municipal landfills. In this environmental context, direct or indirect recovery of aluminum and associated ions has attracted the attention of entrepreneurs as a way to meet the law and prevent environmental and societal harm.
2.3. Experimental design for zeolite synthesis
The low measured silicon content of the AAR required enrichment with silicon for zeolite synthesis using CSS. The moisture content of the CSS was determined using a halogen moisture analyzer. Five gel samples were prepared and coded as 1A, 2A, 3A, 4A and 5A; replicates of each sample were prepared and coded as 1B–5B. A 1.5M NaOH solution was used as the mineralizing agent in a constant volume of 40 mL for each gel sample. Weights, reaction time, and treatment temperature for each gel sample are given in Table 1. The supernatant pH and the pH of various rinses were measured as part of the experiments. The liquid-solid separation after hydrothermal synthesis is done at 4400 rpm.
Table 1. Experimental design.
| Item | 1A | 1B | 2A | 2B | 3A | 3B | 4A | 4B | 5A | 5B |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.5M NaOH solution, mL | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| U-AAR/U-CSS, w/w | 2.6:1.0 | 2.6:1.0 | 2.6:1.0 | 2.6:1.0 | – | – | 3.8:1.0 | 3.8:1.0 | 3.8:1.0 | 3.8:1.0 |
| D-AAR/U-CSS, w/w | – | – | – | – | 0.83:1.0 | 0.83:1.0 | – | – | – | – |
| T, °C | 85 | 85 | 85 | 85 | 85 | 85 | 85 | 85 | 85 | 85 |
| t, hr | 4 | 4 | 8 | 8 | 4 | 4 | 4 | 4 | 8 | 8 |
| Centrifugation, rpm | 4400 | 4400 | 4400 | 4400 | 4400 | 4400 | 4400 | 4400 | 4400 | 4400 |
| Rinse, mL/rinse | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Quality, rinsing water | DI | DI | DI | DI | DI | DI | DI | DI | DI | DI |
-- Not applicable.
U-AAR Undried anodizing aluminum residue.
D-AAR Dried anodizing aluminum residue.
U-CSS Undried commercial sodium silicate.
DI Deionized water.
2.4. Zeolite identification
Synthetic zeolite samples were examined using X-ray powder diffraction (XRD). The diffraction angles (2θ) of the synthesized samples were compared with the diffraction angles for zeolites in the database of the International Center for Diffraction Data (Treacy and Higgins, 2001).
2.5. Analytical methods
The moisture content of the AAR and the CSS was determined using the Mettler Toledo Halogen Moisture Analyzer, Model HX204; reference substances were used to calibrate the instrument. Relative humidity and temperature of the laboratory environment were measured using an ALLOSUN Thermo-Hygrometer, Model ETP101. Measurements suggested by the equipment supplier to avoid moisture absorption were 21.9 °C, an atmospheric pressure of 966 hPa (0.95 atm), and a relative humidity of 51%. The moisture content was determined on 28 AAR samples, and relative standard deviation (RSD) was used as a measure of precision in quantitative studies.
The pH values of the AAR were determined with substrate suspensions in (1) ultrapure milliQ water, (2) 1M KCl solution, and (3) 0.01M CaCl2.2H2O solution, using a Mettler Toledo S220 SevenCompact pH/Ion benchtop meter and calibration buffers of pH 4, 7, and 10.
The surface morphology of the AAR was investigated using a JEOL JSM-6300 Scanning Electron Microscope (SEM) with an Oxford Instruments Energy Dispersive System (EDS), and a BAL-TEC sample coater/sputter coater, model SCD 005 for SEM-EDS sample preparation.
The chemical composition of the AAR was determined by two methods, (1) Flame atomic absorption spectroscopy (FAAS), using a PG Instruments AA500 FAAS, and (2) SEM-EDS, using the SEM-EDS and sample coater described in the previous paragraph. Calibration curves were prepared with certified standard chemicals for FAAS.
The mineralogy of the AAR was determined by X-Ray diffraction, using a Bruker D8 ADVANCE for XRD with CuKα radiation. The AAR diffractograms were registered for the 2θ interval between 5° and 50° with a diffractogram step angle of 0.03° and an accumulation time of 2 s/step.
The synthesized zeolites were characterized for morphology, chemical composition, and mineralogy using the same instruments described in the previous paragraphs and under the same testing parameters.
3. Results and discussion
The average moisture content of raw AAR was 68.6% (n = 28), and the pH was mildly alkaline (7.4–8.5).
The SiO2/Al2O3 molar ratio of raw AAR using the FAAS method (0.09) is higher than the ratio determined by SEM-EDS method (0.04). In any case, the ratio is too low, being necessary for the silicon enrichment of the AAR to get a zeolite with a molar Si/Al ratio around the unit. The AAR had high concentrations of aluminum and moderate amounts of silicon, alkali, and alkaline earth elements (Table 2).
Table 2. AAR metal oxide content determined by SEM-EDS and AAS (wt%, g/100g).
| Method | F2O | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | CaO | K2O | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|---|
| SEM-EDSa, average, g/100 g | 23.3 | 3.31 | 0.85 | 74.5 | 1.86 | 0.99 | 7.1 | 0.74 | ||
| FAASb, Average, g/100 g | 1.81 | 0.58 | 70.2 | 3.91 | 1.58 | 0.8 | 0.22 |
- a
-
Scanning electron microscopy-Energy Dispersive System.
- b
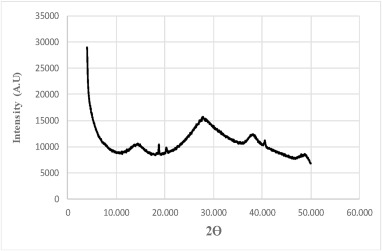
A crystal is formed by atoms, molecules, or ions that are periodically arranged in space. A non-crystal or amorphous material is composed of these particles randomly dispersed in space. The X-rays that hit a crystal scatter in a few specific directions satisfying the Bragg's law with strong intensities of the scattered beam (diffraction), giving rise to high-intensity narrow peaks, whereas in an amorphous material the scattering of the X-rays occurs in all directions not satisfying Bragg's law, giving a broad curve in a wide 2Ɵ range. According to this explanation, the AAR-XRD diffractogram of Fig. 1 shows an X-ray scattered by an amorphous material. It is observed curves in a broad range of Bragg's angles, rather than a high sharp maximum intensity and narrow peaks. It is observed a few very low-intensity peaks that could not be regarded as part of a crystal phase. The amorphous AAR material does not have long-range atomic order and therefore produces only broad scattering features. A crystal, a liquid or amorphous solid, and a monoatomic gas show the curves depicted in Fig. 3. Fig. 1 illustrates the curve of an amorphous AAR solid.
 Fig. 3
Fig. 3Fig. 2 is coherent with the conclusion that the AAR does not show a crystalline phase at all. It is not observed any geometrical shape but only a shapeless AAR material.
The CSS had an average moisture content of 48.0% and an average chemical composition of 29.17% sodium, 2.6% aluminum and 16.2% silicon.
The X-ray diffractograms of the synthesized products shown in Fig. 4 and Fig. 5a, Fig. 5b, Fig. 5c demonstrate the crystalline structure of the samples and contrast markedly with the amorphous pattern of AAR in Fig. 1. For academic comprehension all the diffractograms of the blends 1A–5A and their replicates are plotted in a vertical position, to evidence that in all cases there are correspondence among the various peaks of the synthesized mineral product. It is noticed that there are in some peaks small numerical differences in the first or second decimal of the 2Ɵ degrees from one gel sample to another, however the non-fractional part of 2Ɵ Bragg angle is the same for all the peaks of the whole samples.
 Fig. 4
Fig. 4Table 3 shows that the diffraction peaks position as 2Ɵ of the XRD data for all the samples coded 1A–5A, match with the Bragg's angles of the pattern LTA dehydrated zeolite (Treacy, and Higgins, 2001). The angles 2Ɵ of all the peaks of the replicates, series B, also match with the pattern. However, the relative intensity of the peaks do not match with the relative intensities of the pattern. In all cases the relative intensities of the XRD data are higher than the relative intensities of the pattern. The relative intensity was calculated by dividing the absolute intensity of every peak by the absolute intensity of the most intense peak, and then converting to a percentage.
Table 3. Comparison of the diffraction angles 2Ɵ of the synthesized zeolites with the diffraction angles 2Ɵ of the dehydrated LTA pattern.
| 2Ɵ synthesized zeolites | XRD powder pattern for zeolite LTA, dehydrated | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 2A | 3A | 4A | 5A | ha | ka | la | 2Ɵb | dc | Md | Irele |
| 7.22 | 7.27 | 7.27 | 7.27 | 7.27 | 2 | 0 | 0 | 7.20 | 12.278 | 6 | 100 |
| 10.19 | 10.24 | 10.24 | 10.29 | 10.24 | 2 | 2 | 0 | 10.19 | 8.682 | 12 | 49.6 |
| 12.48 | 12.53 | 12.53 | 12.58 | 12.53 | 2 | 2 | 2 | 12.49 | 7.088 | 8 | 22.4 |
| 16.13 | 16.18 | 16.18 | 16.18 | 16.18 | 4 | 2 | 0 | 16.14 | 5.491 | 24 | 20.1 |
| 21.69 | 21.74 | 21.74 | 21.79 | 21.74 | 6 | 0 | 0 | 21.72 | 4.093 | 6 | 1.3 |
| 4 | 4 | 2 | 21.72 | 4.093 | 24 | 8.4 | |||||
| 24.03 | 24.03 | 24.08 | 24.08 | 24.03 | 6 | 2 | 2 | 24.04 | 3.702 | 24 | 13.7 |
| 26.17 | 26.17 | 26.17 | 26.22 | 26.17 | 6 | 4 | 0 | 26.17 | 3.405 | 24 | 0.6 |
| 27.15 | 27.2 | 27.2 | 27,2 | 27.2 | 6 | 4 | 2 | 27.18 | 3.281 | 48 | 9 |
| 29.98 | 29.98 | 30.02 | 30.02 | 30.02 | 6 | 4 | 4 | 30.01 | 2.978 | 24 | 4.6 |
| 8 | 2 | 0 | 30.01 | 2.978 | 24 | 6.4 | |||||
| 34.22 | 34.22 | 34.26 | 34.22 | 34.22 | 6 | 6 | 4 | 34.26 | 2.618 | 24 | 8.4 |
| 44.25 | 44.21 | 44.25 | 44.3 | 44.3 | 12 | 0 | 0 | 44.26 | 2.046 | 6 | 2.2 |
| 47.37 | 47.32 | 47.37 | 47.37 | 47.37 | 8 | 8 | 6 | 47.41 | 1.917 | 24 | 1.2 |
| 10 | 8 | 0 | 47.41 | 1.917 | 24 | 0.6 | |||||
| 49.86 | 49.76 | 49.76 | 49.76 | 49.76 | 10 | 8 | 4 | 49.82 | 1.83 | 48 | 0.4 |
- a
-
Miller indices.
- b
-
Diffraction angles.
- c
-
Distance between planes of atoms that give rise to diffraction peaks (d-spacing).
- d
-
Multiplicity Mhkl of the reflection hkl; number of lattice planes (hkl), which are equivalent by the symmetry.
- e
-
Relative intensity.
Belaabed et al. (2016) have published the synthesis of a highly crystalline LTA zeolite using pure chemicals Sodium silicate (Na2O·SiO2·5H2O, Sigma-Aldrich) and sodium aluminate (Na2O·Al2O3 anhydrous, Sigma-Aldrich) with peak positions as 2Ɵ scattering angles 7.2°; 10.2; 12.5°; 16.2; 21.7°; 24°. An additional phase of zeolite A is claimed at 2Ɵ 21.02°, 27.18°, and 30.01° by Villaquirán et al. (2016). These scattering angles are shown in Table 3 and Figs. 4 and 5 of the synthesized LTA.
The Bragg's angles of the samples 1A, 2A, 3A, 4A and 5A Shown in Table 3 and depicted in Figs. 4 and 5 (10.19°; 10.24°; 10.24°; 10.29°; 10.24°) have the corresponding relative intensities of 77.6%, 71.2%, 68.3%, 73,9% and 74.4%, whereas the pattern with a 2Ɵ angle of 10.19°, shows a relative intensity of 49.6% (Table 3). The XRD patterns of synthetic LTA zeolite is complex, it contains 900+ reflections within 50° 2Ɵ.
Figs. 4 and 5 illustrate the presence of a crystal as they show sharp diffraction peaks in all cases. It can be seen that the absolute intensities of the samples vary even among the samples for the same angles 2Ɵ.
The diffraction peak intensity depends on the arrangement of the atoms in the entire crystal. The nature of the synthesizing raw material that in this case is an industrial residue rich in aluminium (AAR) differs from the pure chemical precursors used to produce the pattern. This aspect along with the processing conditions, such as reaction temperature, reaction time, or powder sample preparation, might have caused the grains to have preferred crystallographic direction, causing the shifting of the absolute intensities. These variables might lead to the so-called “preferred crystals orientation”.
The “preferred orientation” of crystallites can create a systematic variation in diffraction peak intensities even at the same angle 2Ɵ, creating a systematic error in the observed diffraction peak intensities. Some samples have the same Bragg's angle value as the pattern, other samples have small mismatch in peak position as 2Ɵ (Figs. 4 and 5), this is an acceptable experimental error, that might be caused by the reasons explained before.
Relative intensities graphs give the same shapes as those drawn with the absolute intensities as the formers are only the normalized absolute intensities.
Fig. 5a, Fig. 5b, Fig. 5ca, b, and 5c illustrates the XRD data of the synthesized LTA zeolites main samples compared with the XRD data of the corresponding replicates. As the replicate samples were processed keeping the fixed blend proportions and under the same conditions of reaction time and reaction temperature, of the samples coded A, their resulting position peaks as 2Ɵ A are corresponding to the B position peak as 2Ɵ B of the samples.
 Fig. 5a
Fig. 5a Fig. 5b
Fig. 5b Fig. 5c
Fig. 5cThe Bragg's angles are similar for samples 1A, 1B, 2A, and 2B, but the absolute intensities vary. Main samples (A) show higher absolute intensities than replicates (B). It proves that the intensity is highly sensitive to any variation in process conditions and sample preparation for XRD essays (Fig. 5a).
LTA of the main samples (3A, 4A) show similar scattering angle 2Ɵ in the replicates (3B, 4B). However, the scattering absolute intensities are different among the samples (Fig. 5b). The same findings apply for LTA samples 5A and 5B (Fig. 5c).
Table 4 shows that the synthesized dehydrated zeolite LTA has in its structure high proportions of sodium and silicon, which demonstrates that the alkaline fusion with the mineralizing agent and the enrichment of the AAR with CSS has been successful. It also shows low concentrations of Mg, Ca and Fe, which derive from the AAR, whereas potassium, sulfur, phosphorus, stannum, molybdenum, and chorine were only above detection in all zeolites The high aluminum content (SEM-EDS; average wt% 39.4 and AAS; average wt% 37.2) in the AAR appears in lower proportion in the synthesized product due to the incorporation of high fractions of silicon and sodium.
Table 4. Result of SEM-EDS chemical analysis of synthesized dehydrated LTA (wt%, g/100g).
| Code | O | Na | Mg | Ca | K | Fe | Al | Si | F | S | P | Sn | Mo | Cl | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 44.8 | 12.2 | 0.5 | 0.5 | ˂0.1 | 0.8 | 19.7 | 21.3 | ˂0.1 | ˂0.1 | ˂0.1 | ˂0.1 | ˂0.1 | ˂0.1 | 100.0 |
| 1B | 41.5 | 12.4 | 0.3 | 0.3 | ˂0.1 | 0.5 | 21.3 | 23.2 | ˂0.1 | ˂0.1 | ˂0.1 | 0.3 | 0.4 | ˂0.1 | 100.0 |
| 2A | 41.1 | 13.0 | 0.3 | 0.6 | ˂0.1 | ˂0.1 | 22.2 | 22.7 | ˂0.1 | ˂0.1 | ˂0.1 | ˂0.1 | ˂0.1 | ˂0.1 | 100.0 |
| 2B | 40.5 | 12.3 | 0.4 | 0.7 | ˂0.1 | ˂0.1 | 23.8 | 22.3 | ˂0.1 | ˂0.1 | ˂0.1 | ˂0.1 | ˂0.1 | ˂0.1 | 100.0 |
| 3A | 51.7 | 12.2 | 0.6 | 0.3 | ˂0.1 | 0.5 | 18.3 | 16.0 | ˂0.1 | ˂0.1 | ˂0.1 | 0.4 | ˂0.1 | 0.1 | 100.0 |
| 3B | 56.3 | 11.8 | 0.5 | 0.1 | ˂0.1 | 0.2 | 17.1 | 14.0 | ˂0.1 | ˂0.1 | ˂0.1 | 0.1 | ˂0.1 | ˂0.1 | 100.0 |
| 4A | 55.0 | 10.8 | 0.4 | 0.1 | ˂0.1 | 0.1 | 19.5 | 13.2 | 1.0 | ˂0.1 | ˂0.1 | ˂0.1 | ˂0.1 | ˂0.1 | 100.0 |
| 4B | 53.4 | 12.4 | 0.4 | 0.1 | ˂0.1 | 0.1 | 18.5 | 14.4 | 0.3 | 0.1 | ˂0.1 | ˂0.1 | 0.3 | ˂0.1 | 100.0 |
| 5A | 56.5 | 10.6 | 0.5 | 0.1 | ˂0.1 | 0.1 | 19.8 | 12.1 | 0.2 | ˂0.1 | 0.1 | ˂0.1 | ˂0.1 | ˂0.1 | 100.0 |
| 5B | 53.5 | 10.6 | 0.5 | 0.2 | ˂0.1 | 0.0 | 21.4 | 13.2 | 0.3 | ˂0.1 | 0.1 | 0.1 | ˂0.1 | ˂0.1 | 99.8 |
The successful synthesis dehydrated LTA zeolite (Fig. 5a) demonstrates that the moisture content of AAR and CSS materials contributes to the synthesis and that it is not necessary to add water to the gel samples to synthesize the zeolite. This provides an economical and technical advantage as both materials can be used without dewatering.
The abscissa of the SEM-EDS spectrogram of Fig. 6 shows the peaks of aluminum and silicon, and alkali and alkaline earth elements of AAR. Fig. 7 is an example of the EDS spectrum of the synthesized composite 1A nanostructure. It shows high values of intensity for sodium, aluminium and silicon.
 Fig. 6
Fig. 6 Fig. 7
Fig. 7With respect to the resulting molar ratios, Table 5 shows that samples 1A and 1B have molecular molar ratios SiO2/Al2O3 equal to 2.1 (atomic molar Si/Al ratios, 1.0), which corresponds with the molar SiO2/Al2O3 ratio (1.86) of commercial A (LTA) zeolite produced by the Thai Silicate Company (Supaporn et al., 2006), with molar NaA atomic ratio Si/Al (1.0) marketed by CU Chemie Uetikon AG of Switzerland (Baldansuren, 2008). Tounsi et al. (2009) report the synthesis of Na-LTA zeolite using Tunisian sand and aluminium scrap that has Si/Al atomic molar ratios of 0.98; 1.15 and 1.08. These results are in agreement of those presented in this research. The molar SiO2/Al2O3 ratios of samples 2A and 2B are 2.0 and 1.8 respectively, at same weight ratio (2.6U- AAR: U-CSS), but the reaction time of 8 h. This might mean that 4 h reaction time is enough for the crystallization process. The remainder molar SiO2/Al2O3 ratios are lower than the formers, basically due to the less amount of silicon.
Table 5. Molar ratios of synthesized, dehydrated LTA.
| Molar Ratios | 1A | 1B | 2A | 2B | 3A | 3B | 4A | 4B | 5A | 5B |
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2/Al2O3 | 2.1 | 2.1 | 2.0 | 1.8 | 1.7 | 1.6 | 1.3 | 1.5 | 1.2 | 1.2 |
| Na2O/Al2O3 | 0.7 | 0.7 | 0.7 | 0.6 | 0.8 | 0.8 | 0.7 | 0.8 | 0.6 | 0.6 |
| Na2O/SiO2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Na2O/(K2O + MgO + CaO + Fe2O3) | 6.3 | 10.9 | 9.1 | 6.9 | 7.2 | 10.3 | 11.8 | 13.6 | 9.6 | 9.2 |
| (Na2O + K2O + MgO + CaO + Fe2O3)/Al2O3 | 0.8 | 0.7 | 0.8 | 0.7 | 0.9 | 0.9 | 0.7 | 0.8 | 0.7 | 0.6 |
| (Na2O + K2O + MgO + CaO + Fe2O3)/SiO2 | 0.4 | 0.4 | 0.4 | 0.4 | 0.5 | 0.6 | 0.5 | 0.6 | 0.6 | 0.5 |
| (K2O + MgO + CaO + Fe2O3)/SiO2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 |
| (K2O + MgO + CaO + Fe2O3)/Al2O3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
The values of molar ratios Na2O/Al2O3 and Na2/SiO2 are similar for all the samples. The molar ratio (Na2O/(K2O + MgO + CaO + Fe2O3) is significantly higher in all the cases. This could lead to the conclusion that it has been synthesized a Lynde Type A-Na zeolite. Cations except sodium seems to have little numerical importance in the remainder molar ratios.
The water rinse supernatant, called the mother liquor (ML) in all cases had high pH values (>12). In subsequent rinses the pH value dropped slightly (Table 6). This may affect the efficiency of the reclamation of AAR aluminium, because of the presence of Al(OH)4- complexes at pH values > 9 (Gregory and Duan, 2009). Besides the presence of aluminium ions in the environment may cause an environmental problem, as it was pointed out previously.