1. Introduction
Nowadays, the hydrocarbon industry is a large part of the global economy, as well as it is main source of energy, so there is a great interest in optimization of processes and solution of problems of oil industry (Kinitisch et al., 2007). In particular, asphalt fractions in oil contribute to various problems that are present in such segments of the oil industry as mining, transportation, storage and refining (Novaki et al., 2016). This is aggravated if being considered that the deposits of lighter hydrocarbons are being depleted and the vast reserves of heavy hydrocarbons become major sources of extraction of these precious materials (Rogel et al., 2015).
Efficiency for the hydrocarbons recovery processes starts from their extraction at the site, where the presence of asphaltenes can lead to their solid deposits, generated in the walls of the reservoir (Zhang et al., 2007). Their flocculationand precipitation decrease extraction capacity of light oil fractions causing blockages in pipes and pieces of equipment, preventing extraction of lighter hydrocarbons by a simple way and generating losses of energy resources (Novaki et al., 2016, Zhang et al., 2007).
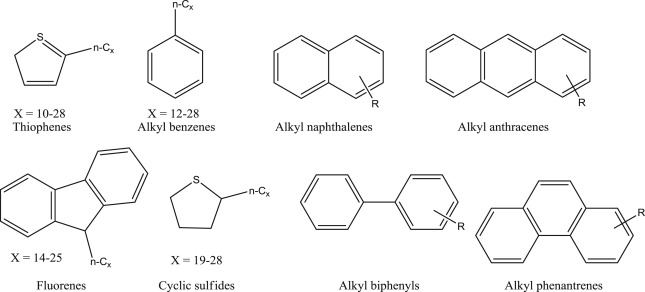
The asphaltenes are, by definition, a family of organic chemicals originating from crude oil, representing the heavier components and, therefore, possessing higher boiling points. Some authors define them according to their solubility. Asphaltenes are insoluble in solvents such as n-heptane and n-pentane and tend to have a higher solubility in aromatic solvents such as toluene (Speight and Long, 1996). Fig. 1 shows some structural fragments of asphaltenes. They frequently have such heteroatoms as sulfur, oxygen, nitrogen and, in some cases, depending on the extraction deposit area, metals such as vanadium and nickel (Speight, 1984). The fact that a crude oil containing a significant percentage of asphaltenes does not mean their necessary flocculation. Oils with a low content of asphaltenes can cause serious problems of deposition, especially more polar asphaltenes (Nassar et al., 2015b). Several studies confirm that more polar and metal-containing (especially vanadium) asphaltenes are those that cause most deposition problems (Nassar et al., 2015a).
 Fig. 1. Asphaltene structural fragments.
Fig. 1. Asphaltene structural fragments.In this review, we analyze most of these conditions and generalize main methods for decreasing negative features of asphaltene presence in oils.
2. Main current strategies for asphaltene removal
There are two strategies for solving problems related to asphaltenes: their conservation and rejection. The conservation of asphaltenes is based on being kept in crude oil, but decreasing the viscosity of the medium by distinct methods. Some applied techniques are as follows: the addition of organic solvents (Argillier et al., 2005) and precipitation inhibitors (Taborda et al., 2016). In case of precipitation inhibitors, a great knowledge of the chemical nature of the crude is required (Rogel et al., 2015); however, the addition of organic solvents is usually very expensive due to the large amount of solvent needed to achieve the required viscosity. On the other hand, the rejection of asphalts consists of the asphaltene removal from the medium to be used in other processes. The advantages of the first method are that it is usually more practical: it is not necessary to know the exact composition of the raw material and crude oil does not require further treatment. Sometimes, solvent deasphalting (Álvarez et al., 2015), ultrafiltration and selective adsorption (Osaheni et al., 2012) are used. Application of solvent deasphalting is usually lesser practical than the other two techniques and more expensive due to use paraffinic and naphthenic solvents, although this technique may be combined with extraction emulsion thus reducing the amount of these solvents (Yeung, 2009). Meanwhile, the ultrafiltration has the disadvantage that the membranes easily become dirty and saturated with asphaltene agglomerates (Nwadinigwe et al., 2015).
Selective adsorption is a practical strategy for removal of asphaltenes, as it can be designed directly in flow systems of existing refineries (Janssen et al., 2012). During this process, the asphaltenes can be adsorbed and then desorbed to regenerate the adsorbent and, in some instances, they can function as catalysts (Pourabdollah et al., 2011). Among the most investigated adsorbents for remediation of asphaltenes, some minerals as montmorillonite, metal substrates, nanoparticles (Nassar et al., 2011) and metal oxide composites, among others, are used. However, there are a number of factors affecting the adsorption capacity of the materials, such as the nature of asphaltenes, their concentration in the medium, temperature, pH, presence of metals, etc.
3. Most common natural adsorbents and factors influencing on their adsorption capacity
3.1. Use of minerals
Among the materials that have been used for adsorption of asphaltenes, mainly minerals found in nature have been investigated because of their low cost and high availability. However, one of the problems in the oil fields is that clays and rocks, sometimes found there, adsorb asphaltenes causing some losses in resulting crude oil extraction. One of the oldest records (González and Middea, 1987) in this area is the feldspar, which demonstrated an adsorption capacity of 0.94 mg/cm2. Studying the mechanism of its adsorption of asphaltenes, it was indicated that fine particles of feldspar with polar fractions of adsorbed oil play an important role in stabilizing water-in-oil emulsions formed in some secondary processes of oil recovery. In a related research using quartz (González and Middea, 1987), its adsorption capacity does not alter its surface charge or the superficial aspects after being desorbed. Therefore, this mineral is not affected by the presence of adsorbed organic species.
The important materials to be analyzed here are also minerals such as Bedford limestone calcite (97.3% SiO2, 1.7% Al2O3, 0.5% MgCO3), Berea sandstone (93.1% Al2O3, 3.86% FeO, 0.54% MgO, 0.25% Fe2O3, 0.11%), and those on the basis of dolomite CaMg(CO3)2 (Castro et al., 2009). These materials are the most common in nature and present a good adsorption capacity for heavy hydrocarbons, using standard conditions (pressure and temperature). In some studies, the adsorption isotherms of heavy hydrocarbons are reported (Castro et al., 2009). The following adsorption capacities for asphaltenes were noted: the dolomite having highest adsorption capacity with 13.1 mg/g followed by Bedford limestone calcite with an adsorption capacity of 12.8 mg/g and Berea sandstone with 6.8 mg/g. Main influencing factors on adsorption capacity are the morphology and major surface area of the Berea sandstone compared with two other aforementioned materials.
Other important and frequently used natural materials are siltstone and montmorillonites (Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O (López-Linares et al., 2009). Two montmorillonites, one with calcium atoms and another with sodium atoms, were compared with synthetic microporous kaoliniteAl2Si2O5(OH)4, revealing that these materials, after being submerged for 300 min, have the order in their adsorption capacity as shown below: macroporous kaolinite (2.30 mg/g) > Ca-montmorillonite (1.2 mg/g) > Na-montmorillonite (0.80 mg/g) > siltstone (0.75 mg/g). These capacities are obviously much lower than those analyzed by Castro et al. (2009).
3.2. Main important factors
López-Linares et al. (2009) indicated that some of the most important variables are the textural properties of these materials and also properties of natural asphaltenes used for analysis of their adsorption. The most important factor is the surface area of solids. However, it mainly depends on the pore volume and pore diameter of the adsorbent, possibly indicating that adsorbed asphaltenes provide a substrate followed by the adsorption of molecules to reach saturation, either pore volume and chemical equilibrium. These last two variables limit the adsorption of asphaltenes on the material surface. It is also mentioned that size of asphaltene molecule adhered on the surface does not limit the ability of the substrates. In a related report, Cosultchi et al. (2005) studied Na-montmorillonite with a solution of known asphaltene concentration of 2.5 wt.% and 6.4 wt.% of polar species containing metals in their structures. The micro and macroscopic diffusion data indicate that it is a slow and continuous process. Due to the macroscopic morphology of clays and viscosity of microemulsions (Hannisdal et al., 2006), the process tends to be continuous but slow. The viscosity does not limit the adsorption capacity, but directly influences the time needed for clays to reach its maximum adsorption capacity. Maximum adsorption was observed after 40 h; however, in the first 2 h, 70% of the total adsorption capacity of the material is obtained.
Jada et al. (2006) reported the effects of asphaltenes in dependence on particle size and the surface charge of two montmorillonite clays in an aqueous mediumin order to establish any correlation among these variables and their adsorption capacity. The effects of pH in the applied water were also examined, confirming that the reaction mechanism of absorption of asphaltenes involves interactions between surface groups of asphaltenes such as carboxylic and carboxylate ones to the silanol and aluminol groups of the mineral. The pH in the water contributes to the saturation effect of the material surface thus diminishing its ability to adsorb asphaltenes, consistent with the above explanations by Gonzalez and Taylor (2016). The adsorption capacity was shown to be greatly affected by the pH more than by moisture.
On the other hand, referring specifically to the kaolinite, better results of its ability on adsorption by surface area were reported by Wang et al. (2016)showing an adsorption capacity of 3 mg/m2 giving a more accurate value. However, this report notes that the adsorbed amount depends not only on the variables mentioned above, but also on the asphaltene/kaolinite ratio. Another less-impact factor was found to be heterogeneity of asphaltene solutions. It was also mentioned that the repeated contact of asphaltene solutions with kaolinite caused the adsorption of asphaltenes in solution of up to 98%. This seems like a great result; it was indicated that the kaolinite, after being immersed several times, does not show a selective adsorption in respect to any particular species. However, the repeated exposure of kaolinite to the solution of asphaltenes greatly increased its adsorption capacity after each immersion. The surface composition data, analyzed by XPS, show that the kaolinite surface remained without alterations despite adsorptions. Further, strong adsorption of asphaltenes was found to occur preferentially on asphaltenes already adsorbed on the kaolinite surface. This proves a great ability of asphaltenes tending to have strong interactions with other asphaltenes and producing agglomerations contributing to a double layer on the surface. Finally, Wang et al. (2013) showed that the maximum thickness of asphaltenes adsorbed on kaolinite, with a total surface coverage, is estimated to be 11 nm based on the XPS depth profile. It was also indicated that no preferential adsorption of nitrogen or rich sulfur species at the interface between the asphaltenes and kaolinite was registered.
Both kaolinite and illite (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2O)] were studied in a related report (Wang et al., 2013). Having as main target the surface charges and wettability, those interactions that occur between surface groups of asphaltenes and silanol (-(Si-OH)-)/aluminol (-(Al-OH)-) mineral groups, were studied in respect to their mechanisms. Another factor, not mentioned in other works, is the hydrophobicity of the observed clay materials which leads to a modification of properties of the mineral surface, such as surface charge, composition, and surface energy (Gan and Liu, 2008). The asphaltenes containing carboxylic and carboxylate groups have greater adsorption of asphaltenes by interaction of these groups and those of silanol and aluminol in the mineral. The data indicate that the maximum adsorption of asphaltenes depends on the surface charge in the clay; the latter contradicts the findings of Wang et al. (2013) because of non-consideration of the interaction of adsorbed and free asphaltenes.
In some cases, we have observed that some variables for these minerals predominate over other variables. Jada et al. (2009) raised the possibility of mineral saturation with water, which improves their adsorption capacity. Meanwhile, Wang et al. (2016) noted that the clay particles, as kaolinite, in contact with water, change the state from hydrated to dehydrated. This could be explained why in the report mentioned above (Wang et al., 2016) no morphological change is seen on the surface, if the effect was caused by water absorbance in the surface of kaolinite, which plays an important factor for adsorption of asphaltenes. These data, observed for wetting of materials, were thoroughly studied by Gonzalez and Taylor (2016) who indicated that water is involved in the behavior of asphaltenes. For example, deposition delays of asphaltene aggregates were noted, meaning that their agglomeration is avoided, producing micro-emulsions instead. It was also established that the adsorption of asphaltenes on mineral surfaces is reduced in the presence of water.
In the processes, where steam is used, the adsorption is affected by this condition; the adsorption of organic molecules in vapor phase is reduced in the presence of pre-adsorbed water (Rankin et al., 2012), so it is interested to get to know and determine if the same effect is applicable for liquid phase adsorptionof asphaltenes by materials. For some materials, it has been reported to reduce even four times its adsorption capacity. The water adsorbed in the surface of a material generate a monolayer reducing the ability to adsorb asphaltenes in the material. It is believed to be the first demonstration of the effect of pre-adsorbed water on the adsorption of material in the solution. The highest adsorption of asphaltenes occurs at very low relative humidity suggesting that its presence is inversely proportional to the adsorption of asphaltenes on different mineral surfaces. In addition, surface water films have been observed in samples; this may suggest that the adsorbed water inside the material generates interactions leading to an improved adsorption. However, in this case a possibility exists that asphaltenes and water are competitive to the same (silanol) surface sites. These results added a support for the existence of films separating water from asphaltenes on the solid surfaces of natural sands and can also serve to explain the differences between data adsorption materials for asphaltenes reported in the literature.
4. Adsorbents on the basis of metals
Surfaces of metals and metal oxides are of great interest, since, in several occasions, their ability to adsorb can influence the materials used in the extraction processes. In this respect, Alboudwarej et al. (2005) studied the interaction of asphaltenes (specifically those with a chain of 7 carbons) on stainless steel (304L), aluminum powder and iron (Fig. 2). These metals were found in the oil and refineries and are used directly in the extraction of oil in the reservoir, containing a lot of hydrocarbons of high molecular weight. The trend to an adsorption type I (Langmuir adsorption isotherm type) was observed in all cases, with adsorption ranging from 0.25 mg/m2 to 2.7 mg/m2and stainless steel being the highest adsorption capacity. Meanwhile, aluminumand elementary iron counted with an adsorption of 1.35 mg/m2 and 0.45 mg/m2, respectively. Related results with stainless steel showed (Abdallah and Taylor, 2008) that hetero atoms and the aromatic rings are linked to metals leading to the formation of metal-asphaltenes. In case of presence of water or moisture in the system, this leads to activation and negatively charge on metal surfaces by forming the FeOH groups on its surface. This decreases bonding with heteroatoms as Fe-O and Fe-N and instead favors the links with Fe-S.
 Fig. 2. SEM microphotograph of powdered metals: (a) stainless steel (304L), (b) iron, (c) aluminum. Reproduced with permission of the American Chemical Society from the Alboudwarej et al., 2005.
Fig. 2. SEM microphotograph of powdered metals: (a) stainless steel (304L), (b) iron, (c) aluminum. Reproduced with permission of the American Chemical Society from the Alboudwarej et al., 2005.Studying formation of Fe-asphaltene bonds or directly with a hetero atom, various evidences exist that several bonds are formed and the exchanges between the Fe atoms and heteroatoms can take place. This interaction is favored in case of N-atom; as a general rule, the links Fe-X (X = N, S, O) tend to be stronger than those formed solely by Fe-C (Nassar et al., 2012). The iron surfaces favor to the S adsorption more than the N, usually allowing crude oils with major quantity of heteroatoms to have more problems in refineries in comparison with hydrocarbons with linear chains. A very relevant issue linked to these metal surfaces is their catalytic ability to oxidize asphaltenes which are discussed in greater detail in the section of temperature effects. However, it is worth mentioning that, in the special case of Fe, it has a greater ability to oxidizebeing linked with heteroatoms such as N and S.
Another metal that has received much attention is nickel with different coatings. The adsorbent was generated by Ma et al. (2005), who performed the adsorption on the basis of nickel-aluminum in flow conditions 25–200 °C in air at normal pressure. It was mainly used in gasoline to be applied in fuel cells. It was found that, as well as the iron (see above), the nickel had higher affinity to sulfur and not so much to the aromatic rings (Owusu-Boakye et al., 2005), thus showing a high selectivity of nickel to sulfur. This is especially useful for the removal of thiophene in commercial fuels; so, the catalytic capacity of nickel allows generate novel research of desulfuration catalysts.
Among peculiar characteristics of nickel as adsorbent, the effect of temperature (see below the section on temperature effects) on its adsorption capacity is known. The higher temperature leads to a significant increase of the asphaltene adsorption, especially sulfur-containing ones. This can be explained by the catalytic activity of nickel, which with the increase of temperature favors to the hydrogenation of olefins and sulfurated compounds (Ma et al., 2005). It is seen that the nickel, unlike iron, promotes the formation of multiple layers; this explains why at higher temperatures it adsorbs more asphaltenes. In addition, the adsorption capacity of the nickel-based adsorbents can be improved by increasing the surface area of Ni and by introducing the active hydrogen atomson the Ni surface (Ma et al., 2005). The adsorption capacity of the nickel was found to be 4 mg/g, but in case of the hydroxylated metal 12 mg/g yield was observed. These characteristics are directly affected by the metal surface morphology and involvement of solvent, used for the tests (normally used n-heptane and toluene); this decreases self-association of asphaltenes and thus the amount adsorbed on the surface.
5. Adsorbents on the basis of metal oxides and salts
Generally, the iron oxides have a great impact on the system especially on different adsorbents in the medium due to their catalytic activity and interactions with aromatic rings and heteroatoms (Nalwaya et al., 1999). Main advantage of the iron oxides as adsorbents is that water can easily remove more polar hydrocarbons adsorbed in the material. It is worth to mention that both iron and its oxides in nanomaterial forms are used for oil removal from water (Kharisov et al., 2014) and other environmental purposes (Kharisov et al., 2012). Also, Fe3O4 and a series of other materials for the adsorption of asphaltenes on such inorganic particles as CaCO3, BaSO4, FeS, Fe3O4, TiO2 and SiO2 (hydrophilic and hydrophobic) were reported by Dudášová et al. (2008). According to these results, the tested materials showed an adsorption capacity from 0.45 to 2.40 mg/cm2. It is interesting that the highest adsorption material is hydrophilicSiO2 with an adsorption capacity of 2.40 mg/cm2 while the non-hydrophilic SiO2resulted only 0.45 mg/cm2. The analysis of all materials used in this report shows the following order and adsorption capacities: hydrophilic SiO2(2.40 mg/cm2) > TiO2 (2.20 mg/cm2) > kaolin (1.90 mg/cm2) > Fe3O4(1.66 mg/cm2) > FeS (1.55 mg/cm2) > CaCO3 (1.16 mg/cm2) > BaSO4(0.83 mg/cm2) > non-hydrophilic SiO2 (0.45 mg/cm2).
The authors above also decided to see how the adsorption capacity of these materials varies for different crude oils. It was found that the adsorption depends more on the kind of particles than on the origin of asphaltenes. This fact is in agreement with the report of López-Linares et al. (2011) describing a weak effect in the system. For this small effect, it was found a correlation between the amount of nitrogen found in the sample of asphaltenes and the amount adsorbed on the particle. On the basis of these results, it appears that the polar interactions between asphaltenes and surfaces are predominant for the adsorption capacity of the material. Calcium carbonate was also reported by Subramanian et al. (2016) showing a similar maximum adsorption capacity for a particular asphaltene of 3 mg/m2. The asphaltene used for this measurement contained a lot of carbonyls, carboxylic acids or derivated groups (Lai et al., 2009), whose electrostatic nature favors surface interactions with calcium carbonate.
As noted in the sections above, the mentioned materials are relatively common and do not need further changes prior their use. However, some of these materials allow us to see what is causing the problems in refineries, for instance in case of stainless steel. The nickel and iron are the important metallic materials in this area, as well as several common oxides and salts.
6. Adsorbents on the basis of nanomaterials
In the previous sections, the features of micro and macroscale materials for asphaltene adsorption are discussed. At the same time, it is well-known that the nanomaterials have recently received great attention due to their unique properties. The nanoparticles have great potential both as adsorbents and for catalysis purposes. However, the nanoparticles still have much pending properties in order to contribute to the oil industry (Adams, 2014). Among the peculiarities of nanoparticles, their high dispersity and large surface area allow improving the capacities of various devices to remove asphaltenes, frequently with high selectivity, and gives the chance to increase the adsorptive properties of several materials (Hashemi and Nassar, 2014). Among the advantages of nanomaterials, a lot of existing synthesis and characterization techniques and obtaining products with unusual morphology, particle size and high purity can be noted. Nanoparticles of transition metal oxides belong to the most investigated and those with the greatest potential to be used as additives in oil reservoirs for enhanced stability and in situ spread of asphaltenes (Hosseinpour et al., 2014). Nanoparticle catalysts could be used to improve and enhance heavy oil recovery under both in situ and ex situ conditions (Mirzayi and Naghdi Shayan, 2014), being also combined with adsorption techniques.
6.1. Nanostructured iron oxides
The nanomaterials, applied for asphaltene treatment and viscosity decrease, are generally nanostructurized metals in distinct forms (in a lesser degree), which can be free or on such supports as, for example, alumina or graphene, and metal oxides (widely represented, especially those on the basis of iron oxides). Thus, asphaltenes found to form aggregates on the iron oxide surface with a diameter of a few hundred nm and about 50 nm height. No clear concentration dependency of aggregate size was observed (Balabin et al., 2011). Iron oxide nanorods were used both as a catalyst as an adsorbent (Al-Ruqeishi et al., 2016). Small nanorods are important to increase the chemical reactivity and heat conductivity in hydrocarbons when heated from an external source of radiation, in order to reduce the viscosity of crude oil. The viscosity was shown to be reduced by 10% when 0.2 g of nanorods were added to 1 L of heavy oil at 30 °C. This reduction increased to 38% and 49% when 0.4 and 0.6 g/L of additives, respectively, at the same temperature were added. On the other hand, when 0.8 g of heavy oil nanorods were added, no appreciable change in viscosity was found, indicating the achieved saturation point of the nanorods, delimited by their surface area.
Among recent studies on distinct Fe2O3 modifications, nanoparticles of iron oxides, magnetite (γ-Fe2O3) and hematite (α-Fe2O3), are investigated (Shayan and Mirzayi, 2015). The effect of the structural differences between these forms and their effect on the asphaltene adsorption, as well as the possibility of formation of monolayers or multilayers on the nanoparticle surfaces, were elucidated (Abu Tarboush and Husein, 2015). In particular, the self-association of asphaltenes and multilayer adsorption of nanoparticles were confirmed. The optimum value of the nanoparticle dose (mass of nanoparticles/volume of solution) was found to be of 10 g/L. The adsorption includes two steps: the fast and the slow steps, adsorbing the greatest amount of asphaltenes in the first hour (90%). Crystalline iron(III) oxide nanoparticles (10 nm) were synthesized (Mirzayi and Naghdi Shayan, 2014) with maghemite structure, showing that their oxidative capacities on asphaltenes start at 203 °C. Their resulting adsorption capacity was found to be 9.09 mg/g, similar to the reported above. These nanoparticles could be used as catalysts to improve the process of refining asphaltenes (Nassar, 2010). Behavior of asphaltenes on the surface of Fe2O3 nanoparticles were also studied by Mejía et al. (2012), revealing the formation of asphaltene aggregates of 10–20 nm in size.
For the iron(II,III) oxide Fe3O4 nanoparticles, an unusual strategy was applied based on their injection in the CO2 flow in order to reduce interfacial tension (Kazemzadeh et al., 2015). Solutions of n-heptane and toluene with two types of asphaltenes were used. It was shown that the higher mass fraction of Fe3O4nanoparticles the lower the intensity of asphaltene precipitation for the mass fractions attempted. The iron system promotes precipitation of asphaltenesindependently on the pressure (Khoudiakov et al., 2005). The oil (with 5% of sulfur content) in these experiments contained heavy hydrocarbons in its composition, possessing high affinity with iron oxide. Fe3O4 nanoparticles were unable to eliminate the asphaltenes completely. Adsorption of the studied types of asphaltenes on the nanoparticle surface was found to be enhanced by the low ratio of H/C and high nitrogen content. In a related report (Nassar and Hassan, 2012), it was shown that Fe3O4 nanoparticles can serve as an excellent adsorbent/catalyst for heavy oil upgrading.
6.2. Nickel-containing nanomaterials
In addition to iron oxide, certain attention is paid to nickel-based nanomaterials. Thus, the mixture of cobalt and molybdenum supported on alumina, was prepared by pyrolysis (Choi et al., 2004), resulting particles with a diameter in the range from 10 to 20 nm. This composite does not have a great capacity for adsorption of asphaltenes, but it is useful as a selectively catalyst in processes with sulfur-containing compounds, as well as in thermal cracking (Carbognani et al., 2008). Nickel and palladium composites in the form of nanoparticles are also known (Franco et al., 2015) and used as bifunctional, taking into account the adsorption capacity of nickel and catalytic capabilities of palladium. Main idea of this work was the evaluation of the rate of adsorption/oxidation and their best conditions. The adsorption capacities for these composites were the following: silice (0.23 mg/m2), Ni silice (0.45 mg/m2), silice palladium (0.43 mg/m2), silice Pd 0.66 Ni 0.66 (0.46 mg/m2), silice with 2% of palladium (0.54 mg/m2), silice with nickel (2%) (0.46 mg/m2). The advantage of the nanoparticles of this type is its ability to disperse and stabilize the aggregates of asphaltenes (Guzman et al., 2016). Avoiding aggregates is the result of the adsorption capacity of the nanoparticles which prevents their flocculation and simultaneous precipitation when the asphaltene content is large. In addition, several methods are being investigated using these metals for in situapplications in deep reservoirs, mostly containing heavy oil and asphaltenes, to reduce viscosity. The microemulsions containing metal nanoparticles of W, Ni, and Mo in the form of ultradispersed colloids (Hashemi et al., 2013). They can even traversing the porous material thus helping to heavy hydrocarbons not to be trapped in the rocks.
The presence of nickel was found to promote multi-layer formation (Franco et al., 2013a, Franco et al., 2013b, Franco et al., 2013c). This fact could be considered as a great help for asphaltene inhibition and removal. It was found (Abu Tarboush and Husein, 2012) that the amount of adsorbed asphaltenes on nickel oxidenanoparticles is 2.8 g of asphaltene per gram of NiO for 2 h. In addition to treating nickel oxide as it is, its adsorption capacities could be improved by generating composites on a matrix of silicon oxide. The following adsorption capacities were observed: 5% Ni silice (322 mg/g) and Ni 15% silice (384 mg/g) at 25 °C. In a related report (Hosseinpour et al., 2014), adsorption of asphaltenes on NiO and/or PdO supported on fumed silice was found to be much higher than for fumed silica in the range of tested concentrations. Nanoparticles significantly decreased decomposition temperature of asphaltenes; as a consequence, use of these nanoparticles significantly improves the thermal decomposition of asphaltenes, decreasing viscosity of heavy oils in situ. In case of other supporting materials, the adsorption capacity of the composite of nickel oxide particles on alumina was found to achieve high efficiency in only 2 min (Franco et al., 2013a, Franco et al., 2013b, Franco et al., 2013c). This nanomaterial could serve as a commercial adsorbent. In particular, it was noted that the nickel oxide in the presence of carbon dioxide in porous media favors adsorption kinetics of alumina.
Comparing the ability of nickel oxide in inert substrates above (alumina and silicon dioxide) in similar conditions, interesting results were obtained. Thus, using silica gel (amorphous), silica gel (commercial), nanoparticles of composite nickel alumina 15% (AlNi15), nanoparticles of alumina with 15% of nickel oxide NiO (AlNi5), nanoparticles of alumina with 5% of nickel oxide (AlNi5), nanoparticles of silice with 15% of nickel oxide l5% (SNi15), nanoparticles of silice with 5% of nickel oxide (SNi5), aluminum oxide, silica gel (crystalline), the following order for the asphaltene adsorptivity was observed: AlNi15 > SNi15 > SNi5 > AlNi5 ≈ silica gel (amorphous) > silica gel (crystalline) > zeolite > alumina > silica gel (commercial). A complete asphaltene adsorption in these materials especially of nickel composites was quickly reached, making this material a candidate for inhibiting the precipitation and deposition of asphaltenes. However, for more concentrated environments, it is possible multilayer-type adsorption. Nanoparticles adsorbing strongly more polar compounds are capable of neutralizing the polar forces that remain active during the weak adsorption between asphaltenes (Iman and Babak, 2015). The tendency to form multiple layers seems to be depended primarily on the concentration and nature of the adsorbent.
6.3. Mixed oxides
Recently studied some mixed metal oxides (MMO) on the basis of aluminum, manganese and copper were studied in combination with graphene oxide (GO). Adsorption capacity of graphene oxide was recently observed (Menzel et al., 2016). It was shown that the graphene oxide may be used as an excellent support because of its geometry and other outstanding well-known characteristics, being a hybrid material with high adsorption capacity. It seems to be more selective to heavy thiophenic hydrocarbon compounds. Its addition improves by 5% the adsorption capacity of the crude oil system, but its strongest impact arose due to the adsorption of thiophenic organosulfur compoundsincreasing up 170%. Graphene oxide can separate adsorbent particles generating more active sites, thus being a better remover than nickel to remove sulfur compounds; however, graphene oxide does not present such strong catalytic ability as nickel (Ancheyta et al., 2005). This work shows an importance of graphene oxide among many others, which displays a wide range of novel materials for the removal of heavy sulfur-containing hydrocarbons and which can be applied in different process steps. In addition to pure GO, its metal-containing composites were also studied: MgAl-MMO (0.987 mg S/g of adsorbent), MgAl-MMO/GO (1.449 mg S/gads.), CuAl-MMO (0.209 mg S/gads.), CuAl-MMO/GO (0.598 mg S/gads.), coal-MMO (0.133 mg S/gads.), coal-MMO/S GO (0.344 mg/gads.) (Menzel et al., 2016).
6.4. Comparative studies
To get a better panorama about the influence nanoparticles' nature on asphaltenes, the conditions in which they are measured, need to be recreated. As noted in the sections above, all variables such as pH, temperature, moisture in the system, solvents, oil type, etc. need to be strictly controlled. So, a series of comparative studies of the nanoparticles began to emerge after the year 2010 on this subject, in particular in several reports of Nassar. In one of them, the nanoparticles of three oxides, NiO, Co3O4 and Fe3O4, were found to present a peculiar behavior, being used in solutions with asphaltene concentrations about 200 mg/L. Their adsorption capacities are 22.3 mg/g (for NiO), 12.4 mg/g (for Co3O4) and 15.1 mg/g (for Fe3O4), meanwhile if the concentration of asphaltenes solutions is 2000 mg/L, higher capacities were observed: 60.1 mg/g (for NiO), 63.3 mg/g (for Co3O4) and 62.0 mg/g (for Fe3O4) (Nassar et al., 2011b). The affinity for surface adsorption by nanoparticles is NiO > Co3O4 > Fe2O3(amorphous) (Nassar et al., 2011a). Later on, on the basis of results the capacities for asphaltene oxidation using the same oxides (NiO, Fe3O4 and Co3O4) (Nassar et al., 2011b), it was shown that a correlation exists between the affinity constant and the catalytic activity: the greater the catalytic activity, constant affinity appears.
In another report (Nassar et al., 2011a), the behavior of six different metal oxides (Fe3O4, Co3O4, TiO2, MgO, CaO, and NiO) was comparatively studied. The adsorption type was compared with a Langmuir model, resulting the following order CaO > Co3O4 > Fe3O4 > MgO > NiO > TiO2, using the same composition of asphaltene solutions. It was also established that the adsorption capacities are as follows 2.7 mg/m2 (CaO) > 1.76 mg/m2 (Co3O4) > 1.7 mg/m2(Fe3O4) > 1.35 mg/m2 (MgO) > 0.58 mg/m2 (NiO) > 0.54 mg/m2 (TiO2). As noted, CaO possesses highest adsorption capacity, but it has a small selectivity and adsorbs both heavy and light hydrocarbons, which is not helpful for asphaltenes isolation. This shows that the quality of adsorption and degree of adsorption are not always related. These studies aimed at analyzing the effect of chemical nature of the nanoparticles on the adsorption of asphaltenes in conditions that can be found in the deposits. Therefore, the delay or inhibition of deposition of asphaltenes and transport of nanoparticles were investigated in a porous medium tank at standard pressures and temperatures.
In a recent work (Hosseinpour et al., 2014), three different types of metal salts and oxides were studied (acidic: WO3 and NiO; amphoteric: Fe2O3 and ZrO2; basic: CaCO3 and MgO (Fig. 3)). It was found that the ability of asphaltene adsorption by another series of nanoparticles decreases in the order NiO > Fe2O3 > WO3 > Mn2O3 > CuO > silica Co3O4 (Fig. 4). In this case, we note that nickel oxide has greater adsorptivity than iron compared with that reported above by Nassar. However, this is because Hosseinpour used petroleum coke and not virgin oil, so this allowed the nickel an increasing capacity to generate multilayers by 11%. The catalytic oxidation of asphaltenes by metal oxides moves from 100 to 150 °C to lower temperatures. This latter is reflected if the activation energy for each material, using them as catalyst for asphaltenes oxidation, is observed as Co3O4 < NiO < CuO ≈ Mn2O3 < Fe2O3 < WO3. As it is seen, the higher is the redox activity of metal oxide, the greater the degree of conversion is achieved. In addition, as interesting observation, Nassar concluded that basic oxides such as MgO and CaO, and amphoteric oxides, such as Fe3O4 and Co3O4, demonstrate a greater adsorption capacity compared to acidic oxides, such as TiO2 and NiO (Franco et al., 2013a, Franco et al., 2013b, Franco et al., 2013c). However, the quality of adsorption is higher for basic oxides except CaO, as already mentioned above. The authors suggested that strong interactions present between the asphaltenes and nanoparticles, likely increasing catalytic effect of nanoparticles for various reactions.
 Fig. 3. HRTEM images of MgO nanoparticles after the asphaltene adsorption at 25 °C and the 2000 mg/L initial asphaltene concentration. Scale bars are (a) 50 and (b) 5 nm. Reproduced with permission of the American Chemical Societyfrom the Hosseinpour et al., 2014.
Fig. 3. HRTEM images of MgO nanoparticles after the asphaltene adsorption at 25 °C and the 2000 mg/L initial asphaltene concentration. Scale bars are (a) 50 and (b) 5 nm. Reproduced with permission of the American Chemical Societyfrom the Hosseinpour et al., 2014. Fig. 4. FESEM micrographs of (a) NiO, (b) Fe2O3, (c) WO3, (d) MgO, (e) CaCO3, and (f) ZrO2 samples before and after asphaltene adsorption. Left, bare nanoparticles; right, samples after asphaltene adsorption at 25 °C and 2000 mg/L initial asphaltene concentration. Scale bars are 500 nm except in panel e, left (scale bar = 1 μm). Reproduced with permission of the American Chemical Society from the Hosseinpour et al., 2014.
Fig. 4. FESEM micrographs of (a) NiO, (b) Fe2O3, (c) WO3, (d) MgO, (e) CaCO3, and (f) ZrO2 samples before and after asphaltene adsorption. Left, bare nanoparticles; right, samples after asphaltene adsorption at 25 °C and 2000 mg/L initial asphaltene concentration. Scale bars are 500 nm except in panel e, left (scale bar = 1 μm). Reproduced with permission of the American Chemical Society from the Hosseinpour et al., 2014.6.5. Use of supporting nanomaterials
The nanomaterials, described above as supports, were also applied alone for asphaltene treatment. Thus, the use of nanoparticles of γ-Al2O3, as typical catalysts/supports, commonly present in heavy oil upgrading, for the adsorption of asphaltenes was reported as a first step in improving heavy oil in situ (Nassar, 2010). It was shown that the effect of the concentration in the adsorption of asphaltenes is smaller in case of the titanium oxide in comparison with other metal oxides such as nickel(II) oxide. This type of technology is dependent largely on the pH in the reservoir (Mohammadi et al., 2011). The titanium dioxide is known to form colloidal dispersions at pH < 4; in basic conditions it can be used without any problem to reducing adsorption and subsequent precipitation of asphaltenes for removal. However, at lower pH nanoparticles of titanium dioxide can serve as a precipitation inhibitor in crude. So, TiO2 can be effectively used to enhance stabilization of asphaltenes, as well as some other materials, such as ZrO2, SiO2.
As noted in this section, the applications of nanomaterials combined with new technologies have great potential to solve main problems caused by asphaltenes. However, in order to compare all these materials, it is necessary to take into consideration the reported adsorption conditions. On the other hand, certain possibilities to misinterpret results exist, as there are a number of factors that were observed which could change in a greater or lesser extent the adsorption capacity of the nanomaterials. These variables are discussed below.