1. Introduction
Manufacturing places broad emphasis on speed, accuracy, flexibility, and minimizing waste nowadays. This is why there have been increasing interests in the area of additive manufacturing, most commonly known as three-dimensional (3D) printing. While conventional manufacturing methods such as machining are rooted in removal of material from bulk form (i.e. subtractive manufacturing), the essence of 3D printing is to build up an object layer by layer adding material only where necessary [1], [2]. Near-net-shape capability, or printing parts close to the designed profile, with the exception of support structures, uses as little excess material as possible. This drastically reduces by-product waste as compared to subtractive manufacturing. This in turn reduces the lead-time and tooling required for product completion, leading to savings in production costs [3]. The capability of 3D printing extends to cover a wide range of material types, including polymers, ceramics, metals, etc. [4], [5].
One mainstream direction for 3D printing is that of biomedical applications, specifically in creating scaffolds for medical implants [6], [7], [8], [9], [10]. This paper focuses on the fabrication of scaffolds for orthopaedic (bone) implants by utilizing powder-bed based metallic 3D printing [11]. The first and foremost requirement for the orthopaedic implants is to fill 3D defect cavities. Traditionally, metallic orthopaedic implants have been produced by investment casting or forging. Although different prosthetic implant sizes can be produced through the conventional means, they cannot achieve the same level of patient-customization as 3D printing. With 3D printing, the shape and design of implants can be individualised to ensure best fit to their recipients. This can even be done by direct data input from computed tomography (CT) or magnetic resonance imaging (MRI) scans. Beyond efficiency, the near-net-shape capability of 3D printing drastically reduces wastage of material as compared to traditional subtractive manufacturing methods. This will help to balance out the equipment setup costs in the long run [12].
Hutmacher derived four essential characteristics of a biodegradable bone scaffold, which were found to be transferrable to a metallic orthopaedic implant as well: (i) biocompatibility leading to a natural cell growth rate on the scaffold; (ii) similar mechanical characteristics with existing tissue at implant area; (iii) suitable porosity for cell ingrowth and channels for nutrient and waste transportation; (iv) attractive surface morphology for cell attachment and proliferation [13]. Biocompatibility of a scaffold mainly depends on the materials used and the fabrication process. Titanium and its alloys, and various other metals, such as cobalt chromium (CoCr) alloys and stainless steel 316L (SS316L), are known to have excellent biocompatibility [14]. While this review will be focused on titanium alloys, which were most widely used for orthopaedic implants because they have a lower modulus of elasticity that is closer to that of host bone and are more biocompatible than CoCr alloy or SS316L. On the other hand, titanium alloys are notch-sensitive, which predisposes it to cracks if the implant is not well supported [15].
In addition, it is important for an orthopaedic implant to mimic mechanical characteristics of bone to maximise its usefulness in the body. Dissimilar mechanical properties between the implant and bone may lead to many undesirable effects. One such phenomenon called stress shielding is caused by the differences in elastic modulus or stiffness, leading to the existing bone being overly relieved of load [16]. This leads to bone resorption which may cause the implant to loosen from the bone [17], which might affect the fixation and longevity of the implant within the body [18]. A solution to this is to use cellular or porous titanium structures, which have closer mechanical properties to actual bone [19]. Another crucial reason to use cellular structures is to mimic the structure of native bone to promote bone regeneration and ingrowth into the implant, which has so far not been observed on solid structures [20], [21]. Studies have also shown that the surface types of these implants play a role in regulating bone cell responses and bone healing [22]. Rough surfaces obtained through sandblasting and/or acid-etching are favoured [23]. Chemically modified implant surface by using hydrogen chloride (HCl) and sodiumhydroxide (NaOH) also believed to provide a better fixation of the implant and improve the long-term stability of the implant [24].
In general, it is very difficult or impossible to rely on traditional manufacturing methods to craft a cellular structure throughout an orthopaedic implant. 3D printing makes this relatively easy as it is builds up a form layer by layer, including the internal cellular cross-sections. It is hence possible to produce intricate cellular implants tailored to biomedical applications. To produce a useful implant, factors such as topological design of pores, porosity, mechanical properties, and interfacing with natural bone have to be carefully considered [25]. Design of cellular scaffolds can also be made more anatomically-suitable by applying image data from medical databases. This allows satisfactory replication of natural bodily functions such as transport of nutrients and waste [12].
This review is divided into the following four major sections: metallic powder-based 3D printing techniques, hierarchical design of metallic cellular scaffolds, microstructural characterization and mechanical properties, and in vitro and in vivo studies of 3D-printed cellular bone implants. This review will gather findings from across the fields related to 3D printing for bio-implants, and serve to compare the different methodologies used to eventually arrive at the most reasonable direction for each of the above sections. In the first section, the working principles behind two common powder-based 3D printing techniques, selective laser melting (SLM) and selective electron beam melting (SEBM), will be examined and compared. These methods involve using a high energy beam to melt the shape of cross-sections into layers of metallic powder, building layer upon layer into the desired product [26], [27]. The advantages and disadvantages of each method with regards to biomedical applications will also be mentioned. The next section will briefly touch on the needs for metallic scaffold implants and present regular and irregular interconnected pore cellular designs. This will lead up to the following section which will present the mechanical behaviour obtained from testing entire cellular structures. A study into the microstructures of these 3D printed cellular structures will be made and the differences resulting from different manufacturing processes will be compared. The final section will look at the performances of 3D-printed bio-implants with regard to in vitro cell culture and in vivo animal testing. Challenges and future perspectives on 3D-printed cellular scaffolds for orthopaedic implants will be given in the end.
2. Metallic 3D printing systems
SLM and SEBM are the two prolific powder-bed based 3D printing techniques for metals nowadays. In both processes, high energy beams are utilized to melt cross-sectional shapes into layers of metal powder [26], [28], fusing powder particles into a large form, with each layer representing a “slice” of the final product. After every “slice” is formed, the build platform moves downwards by the distance equivalent to a layer's thickness and a fresh layer of metal powder is uniformly spread on top of it. The process repeats so that the cross-sections build up cumulatively until the build is finished. At the end, the excess, unmelted powder is removed by high-pressure blowing, leaving behind the near-net-shape build as defined in the computer-aided design (CAD) model [29]. The unused powder can be recycled for subsequent rounds of fabrication. Both processes are capable of fabricating complex designs such as cellular structures with high accuracy [30].
The main difference between SLM and SEBM is the power source - SEBM utilizes electron beams while SLM employs laser beams [31]. This leads to a difference in operating environment as it is necessary to use a high vacuum in SEBM to maintain the strength of the electron beam. Metallic powder-based 3D printing, specifically SEBM, has proved to be advantageous in manufacturing complex cellular titanium builds suitable as bio-implants. Titanium, being an extremely reactive metal, is highly susceptible to the formation of impurities simply through contact with molecules of oxygen, nitrogen, and other atmospheric gases. Such impurities increase the difficulty of manufacturing these titanium parts to meet the mechanical properties required of an implant, for example unsatisfactory ductility. The integrity of titanium implants can be preserved under the high vacuum of the SEBM process. Another benefit of 3D printing is the high flexibility of its builds. Various properties, such as porosity, strength, and ductility, can be adjusted to achieve the optimum in replicating the function of bone in a body [32].
2.1. Selective laser melting
The set-up of an SLM system consists of a laser source, powder containers and delivering and layering apparatus, build platform, and computer systems for process parameter controls [33]. The build platform is heated to a temperature usually below ~ 200 °C and maintained at this temperature throughout the process. The desired metal or alloy in powder form is loaded into a tank. This powder, of mean particle size ranging between ~ 20–60 μm, is delivered onto the build platform, and recoated into layers with the thickness of a few powder particles, typically 30–100 μm [34]. A flat, even layer is crucial to the accuracy and reliability of the SLM process and hence, the morphology and granulometry of powders used must be scrutinized. This is to ensure that powder particles are able to be spread effectively and uniformly at operating temperatures to make up the desired thickness. As a result, metal powders produced for SLM and other powder-based 3D printing processes should ideally possess good sphericity and small size distribution range to enhance their overall flowability[35]. Additionally, oxygen must be removed from the system to prevent oxidation. This is usually achieved by introducing either purified argon or nitrogen into the build chamber [36]. After all the above conditions have been met, a laser beam is focused onto the powder bed, tracing the areas to be melted. To achieve an optimal effect depending on the powder material and build specifications, various laser parameters can be altered. A variety of lasers can be used such as CO2, Nd: YAG, and fibre lasers. These vary in wavelength and energy density, and may have different levels of absorptivity with different materials [33]. The laser power, scanning speed, and hatching pattern could be optimised in order to achieve a specific build definition [30].
These processes and apparatus are similar to that used in selective laser sintering (SLS), another commonly used rapid prototyping method, although the parameters set may differ. While SLS has been a commonly used method in rapid prototyping for polymers and metals, there has been a shift in preference towards SLM over the years. Unlike SLS, SLM allows full melting and solidification of metal powder to produce components with density and mechanical properties comparable to that of bulk material. This way, benefits of manufacturing with bulk material such as strength can be obtained without the material waste and lead-time consumption associated with traditional subtractive manufacturing methods [33]. Accordingly, more intricate and complex designs such as lattices become achievable.
2.2. Selective electron beam melting
SEBM technique was developed by a Sweden-based company Arcam AB, who named their commercial machine electron beam melting (EBM). Similar to SLM, EBM involves a system consisting of powder hoppers, rake, build platform, and an energy source used to melt powder. However, EBM adopts an electron beam as the energy source. Electrons emitted by a tungsten filament are accelerated by a high voltage of 60 kV to a high velocity before being focused into a high energy beam by electromagnetic lenses [37]. Unlike SLM, EBM must work under a high vacuum due to some inherent features of electron beams. Additionally, certain metals or alloys, being highly reactive, are susceptible to gathering impurities when exposed to air. Impurities afflicted by contact with oxygen or other chemical entities in air can be prevented using a vacuum chamber and this way, the integrity of a 3D printed titanium part can be ensured [38]. As with SLM, there is a strong correlation between powder quality and the fabrication process. A good powder for metal printing is defined by good flowability, compactness of packing, and heat transfer characteristics, all of which can be maximized by using fine powder with spherical morphology. Powder size distribution must also be taken into consideration as this can have a strong effect on build density, surface finish, and mechanical characteristics [39].
Table 1 lists the main differences between SLM and SEBM techniques. As compared to SLM, SEBM is faster in producing fully dense builds with a high energy electron beam allowing full melting of powder particles and a faster scanning speed. Completely molten particles are also key to aid metallurgical bonding between layers [38]. SEBM is also the preferred method to fabricate Ti-6Al-4 V as interstitial elements can be minimized due to the highly clean environment [40].
Table 1. Comparison between SLM and SEBM processes.
| SLM | SEBM | References | |
|---|---|---|---|
| System schematic |
 |
 |
[28], [41] |
| Power source | Laser beam (up to 1 kW) | Electron beam (3 kW) | [40] |
| Operating environment |
|
|
[40] |
| Powder material | Metals, polymers, ceramics, composites, etc. | Metals and metallic-based composites | [29] |
| Powder layer thickness | 20–100 μm | 50 μm | [40] |
| Powder particle size | ~ 20–63 μm | ~ 45–105 μm | [27], [42] |
| Melting method |
|
|
[40] |
| Build rate | Slow | Fast | [40] |
3. Hierarchical designs for metallic cellular scaffolds
Patients with joint damage, such as hip or knee joint, that causes pain and interferes with daily activities despite treatment may be candidates for a replacement surgery (arthroplasty). Osteoarthritis, or degenerative joint disease, is the most common reason for the total hip or knee arthroplasty. Arthroplasty is a surgical procedure in which the diseased parts of the joint are removed and replaced with artificial parts (prosthesis). The goals of arthroplasty include increasing mobility, improving the function of the joint, and relieving pain. The history of the different prostheses and arthroplasty procedures were reviewed in Learmonth et al. [15].
Cement-free implants were designed to provide adequate initial stability and to encourage bone to osseointegrate onto or into the implant. Most of the bone tissues are composed of a porous environment [43], [44]. Hence, in the conventional cement-free implants, their surfaces must be further processed to provide a sturdy fixation. This is done by application of porous coatings such as sintered beads, fibre mesh or thermal spray processes [45] that would allow bone ingrowth to fix the implant in place. Results showed sufficient bone ingrowth. However, many of these designs were associated with a high rate of stress-shielding and bone loss. Patients occasionally complained about thigh pain, presumably due to elastic mismatch between the rigid implant and the biologically flexible bone tissue [15]. Concluding the clinical related outcomes, orthopaedic implants must be able to withstand regular loading but have mechanical properties that match the stiffness of the local host bone to reduce bone resorption induced by stress shielding. This can be done by having cellular designs that decrease the stiffness and increase the implant's surface area that encourages bone ingrowth [46], [47]. Effective permeability is determined by the presence of interconnected pores, the 3D pore arrangement and the porosity [12] in the implant. High permeability assists in transportation of cellular nutritional and waste matter, and allows for bone ingrowth, leading to a sturdier and longer-lasting fixation [48]. However, the requirements of strength and permeability must be balanced because they both depend, in opposite ways, on the amount of material (and density of structures) that makes up the implant.
Hierarchical objects contain structural elements which themselves have structure [49]. A rigorous hierarchical design is required to maximise the functionality of implant in the body. Design of cellular structure for orthopaedic implants includes a careful selection of the materials, the cellular structure design, and the manufacturing process. These design considerations can boost the affinity between bone tissues and the implant surface which determines the effectiveness of bone cells infiltration and bone ingrowth [12]. It is advisable to archive all unit cells designed (including different sizes of the same design), and this can be done through image- [50], [51] or CAD-based [52], [53], [54]methods. These archives can be tapped for designs to be agglomerated into full scaffold architectures [12].
3.1. Topological design of cellular structures
Cellular solids are composites in which one phase is solid and the other is empty/fluid [49]. The solid phase consists of a lattice, which is a connected network of struts. Cellular solids are characterized by a typical unit cell with certain symmetry elements [55]. The unit cell in millimetres or micrometres scales allows the cellular solids to be viewed both as structures and as materials [55]. Hence, the macroscopic properties of cellular solids, such as the elastic moduli and compressive strength, are governed by both material and structural properties [56]. This review will only focus on designs with interconnected pores (i.e. open cellular structures), as these designs allow transport of cells into the implant [57], [58], [59]. Open cellular structures may originate from many different designs. These designs may also vary in porosity, pore size, strut thickness, shape and orientation of unit cells, etc., to mimic the macroscopic properties of actual human bone structure [7].
A large variety of cellular designs have been fabricated through 3D printing techniques [60], [61]. In light of growing interest in the area of 3D-printed porous metallic orthopaedic implants, there has been an enormous drive to optimise the structural design to (i) provide mechanical properties similar to natural bone to minimise stress shielding; (ii) to boost load-bearing ability; as well as (iii) to aid bone cell ingrowth [38]. These can be achieved by carefully altering the design's porosity (and gradient in porosity if applicable) and pore shape to create a strong structure which encourages bone ingrowth [25]. In the multitude of different structure designs, there are a few guidelines for desired effects. In general, the stiffness and strength of solids decrease with the increasing porosity and in turn the permeability of solids is enhanced. Degradation in mechanical properties can be drastic with the decrease in strut size of the cells [38]. When comparing between two pore shapes, e.g. spherical vs. cylindrical, spherical pores were found to be stiffer while cylindrical ones tend to be more permeable [12].
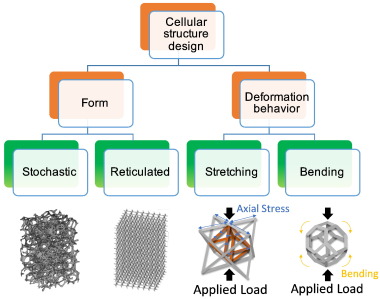
3.1.1. Stochastic and reticulated cellular scaffolds
Cellular designs can come in many forms, either stochastic (irregular) such as expanded foams, or reticulated (regular) such as lattice structures or meshes with constantly repeating unit cells [62]. The stochastic open cellular foams have pores of random shapes and sizes and their unit cells do not repeat regularly. Open cellular foam structures were manufactured in Ti-6Al-4 V using a CAD model obtained from CT-scanning existing aluminium alloy foams [63] or by inverting a scanned model of a container filled with spherical glass beads [64]. The stochastic foams' structure is often heterogeneous, causing the foams to be strong in some regions, while weak in the others. The weak regions drag down the mechanical performance significantly [55]. Drastic degradation in mechanical behaviour in stochastic foams, especially at relative densities less than 0.1%, shows a quadratic (or higher order) scaling relationship between elasticity and density as well as strength and density [65]. An improvement in mechanical properties can be attained from structures that contain unit cells arranged in an ordered hierarchy [65].
Reticulated lattice is yet another variant of cellular structures. Unlike stochastic structure, it consists of repeating unit cells leading to a highly regular form. One method of designing a cellular scaffold to be 3D printed involves choosing the unit cell design from an archive. To date, many different unit cell designs have been produced and studied and they vary from the most basic cube or triangular prism, to the more complex octagonal prisms or rhombic dodecahedrons [66]. 3D printing technologies have accelerated cellular solid designs of extremely low density with a periodic microarchitecture. Nonetheless the design of these topologies is limited by the processing-related restrictions. Table 2 compiles some typical cellular structures.
3.1.2. Bend- and stretch-dominated unit cells
Cellular solids can be classified into bend- and stretch-dominated topologies because they deform by either the bending or the stretching of the cell struts [71]. Most of the cellular structures [71], especially the stochastic foams [65], belong to the bend-dominated types. A bend-dominated unit cell structure consists of b struts and j frictionless joints, satisfying the Maxwell's criterion of M = b − 3j + 6 < 0. The cell struts bend upon loading. A stretch-dominated unit cell structure satisfies the Maxwell's criterion of M = b − 3j + 6 ≥ 0. In general, it is structurally stronger than the bend-dominated type. The struts of stretch-dominated cellular solids are loaded in tension or compression largely without bending [56]. The stretch-dominated cellular solids show a linear scaling behaviour of the strength and the stiffness with the apparent density [65]. Fully triangular 3D structures, such as the tetrahedral-truss and octet-truss unit cell, belong to the stretch-dominated type. Designing orthopaedic implants using stretch-dominated cellular structures would allow both to exploit the topological advantages and to gain enhanced strength of materials [56]. Fig. 1illustrates different cellular designs from varying cellular forms and deformation behaviour as stated above.
 Fig. 1. Examples of cellular structure design in terms of cellular forms and their deformation behaviour [65], [72].
Fig. 1. Examples of cellular structure design in terms of cellular forms and their deformation behaviour [65], [72].3.2. Feasible scaffold design
Arabnejad et al. [25] conceived a strategy to identify feasible design limits for different lattice structures fabricated by SLM. It took into account the following constraints: (1) bone ingrowth requirements (i.e. pore size and porosity) and (2) manufacturing limitations (i.e. strut thickness), to encourage bone ingrowth. These requirements can be visually represented on a graph plotting strut thickness against pore size, as shown in Fig. 2. Different porosities and unit cell sizes are represented as diagonal lines in the graph. It is worth noting that different feasible design graphs will be obtained varying unit cell topologies and manufacturing techniques. By using tetrahedron and octet topologies (two representative stretch-dominated unit cells) as examples, we modify the graphs presenting the manufacturing limitations of both SLM and SEBM processes, with minimum printable strut thicknesses of 200 and 400 μm, respectively. For bone ingrowth, minimum porosity of the scaffold should be at least 40% for sufficient cell infiltration [73], and pore size should be within 50–800 μm for adequate permeability [18], which will be discussed in detail in the following sections. The feasible design space is defined as the triangular shaded areas which satisfy all the above constraints [25]. Obviously, the feasible design space of SLM is much bigger as compared to SEBM due to its thinner minimum strut thickness. Moreover, stretch-dominated cellular structures will usually have a relatively narrower design space in comparison to bend-dominated ones because they may have complicated diagonal struts.
 Fig. 2. Feasible design space (triangular shaded areas) and constraints for (a) tetrahedron and (b) octet truss cellular structure using SLM [25] and SEBM.
Fig. 2. Feasible design space (triangular shaded areas) and constraints for (a) tetrahedron and (b) octet truss cellular structure using SLM [25] and SEBM.4. Microstructure and mechanical properties of metallic cellular scaffolds
4.1. Microstructure of cellular Ti-6Al-4V struts
Among all the biocompatible metallic materials such as CP-Ti, Ti alloys (α + β type and β type), CoCr, stainless steel 316L, Ti-Ta, Ni-Ti, etc., Ti-6Al-4V is considered as the optimal materials for making orthopaedic implants due to its excellent biocompatibility and corrosion resistance, low density, and suitable mechanical properties. There are two species of Ti-6Al-4V alloys in terms of oxygen contents, i.e. Grade 5 (Owt% < 0.20%) and Grade 23 (Owt% < 0.13%), which are typically called Ti-6Al-4V and Ti-6Al-4V ELI, respectively. They consist of two phases of different crystal structures: α-Ti (hexagonal close-packed) and β-Ti (body-centred cubic). In addition, hexagonal α′ martensite will occur when Ti-6Al-4V is subject to rapid cooling process [11]. The addition of Al leads to solid solution hardening which strengthens the alloy, and stabilization of the α phase. By contrast, V helps to improve ductility at room temperature as well as stabilize the β phase [26]. The microstructure of both SLM and SEBM-built Ti-6Al-4 V cellular struts consists of columnar prior β grains and within these prior β grains, it was observed to be dominated by α΄ thin platelets [74], [75], [76] as shown in Fig. 3. Differently, due to the elevated build temperature involved in SEBM, those α′ martensite could be partly decomposed into ductile α/β dual-phase microstructure in some specific builds. It means that SEBM-built cellular scaffolds may have better fracture toughness when compare to the SLM-built counterparts. However, it is worth noting that the microstructure of struts may not affect much on the mechanical properties of cellular scaffolds but their entire topology due to the high porosity.
 Fig. 3. (a) Optical and (b) transmission electron microscopy images showing strut microstructure of a SEBM-fabricated Ti-6Al-4V mesh array. (c) An optical image showing strut microstructure of a SLM-fabricated Ti-6Al-4V lattice structure [74], [75]. Figures adapted and reprinted from Ref. [74] and [75], with permissions from Elsevier.
Fig. 3. (a) Optical and (b) transmission electron microscopy images showing strut microstructure of a SEBM-fabricated Ti-6Al-4V mesh array. (c) An optical image showing strut microstructure of a SLM-fabricated Ti-6Al-4V lattice structure [74], [75]. Figures adapted and reprinted from Ref. [74] and [75], with permissions from Elsevier.4.2. Compressive properties
It is of particular importance to have the implant's mechanical properties similar to the natural bone at the implantation site. This will help to prevent stress shielding where excessive implant stiffness leads to bone resorption [77]. The compressive properties of bone depend on age and location within the body [78], [79], [80]. It has been established that cortical and cancellous bones have compressive strengths in the ranges of 100–230 MPa and 2–12 MPa, respectively. Their Young's moduli are in the ranges of 3–30 GPa and 0.02–0.2 GPa, respectively [81]. For bone defects, metallic implants are preferred for their high load-bearing abilities. However, the Young's moduli of traditionally-manufactured metallic bio-implant are usually much higher, e.g. it is ~ 114 GPa for fully-dense titanium alloy implants [82].
Porous implants are primarily used in orthopaedic applications to patch up cracks or cavities. Studies have shown that Ti-6Al-4V mesh gave a lesser stress shielding compared to solid samples of the same dimensions [18]. Gibson and Ashby [83] derived the relation between the mechanical properties of cellular solids and the relative density, ρr:(1)where ρ* and ρs are the densities of the cellular structure and the corresponding solid material, respectively. Herein, the porosity is equal to (1 − ρr). Elastic modulus of bend-dominated cellular structures, E⁎, is:(2)where Es denotes the bulk elastic modulus of the solid constituent material property and C1 is a constant ~ 1 [55], [84]. In general, ρr < 0.8 for cellular materials (structure consisting of connected struts). For ρr > 0.8, the material should be considered a solid interspersed with small holes [84]. This analysis can be extended to the failure strength (σ*) of bend-dominated cellular solids as well:(3)(4)where σy , s is the yield strength of the bulk solid and C2 is a constant ~ 0.3 [55]. Eq. (4) includes a correction for cellular structures with relative density > 0.3 [64]. Elastic modulus and failure strength of stretch-dominated cellular structures [55] are:(5)(6), respectively. Nevertheless, regardless of bend- or stretch-dominated, metallic cellular solids would buckle before they yield when ρr ≤ 0.01 [55]. Buckling of a strut in a cellular solid mostly depends on its slenderness ratio (t/L), which is directly linked to the ρr. The ‘buckling strength’, σel∗ is:(7)where C3 is a constant that depends on the details of the connectivity of the strut [55]. From the Ashby's charts in Fig. 4, it is clearly seen that a linear scaling relationship between the relative mechanical properties and the relative density for the ideal stretch-dominated cellular solids, which is in good agreement with Eqs. (5), (6). By contrast, ideal bend-dominated structures lie along a trajectory of slope 2 in the relative modulus vs the relative density log-log plot while a trajectory of slope 1.5 in the relative strength vs relative density log-log plot, which are consistent with Eqs. (2), (3). The Ashby's chart is very useful to identify if a cellular structure is bend-dominated or stretch-dominated and to predict its mechanical properties with varying porosities.