1. Introduction
The finite amount of fossil fuels available is depleting at a fast rate with an urgent need to develop an alternative method to extract valuable chemical compounds that is sustainable [1], [2].
The Global discarding of resources is increasing at an alarming rate. Current technology is available to treat a variety of these resources. However, the majority of the discarded material conversion methods are economically unviable on large scale and have many complications [3].
MSW stands for municipal solid waste, which are resources such as paper, plastic, garden material, textiles, food and many other materials. These resources become MSW at the point that the person with responsibility for these products makes the decision to discard them thus changing its definition under waste regulations to MSW. Over 22.3 million tons of these material were discarded therefore generating the same amount of MSW in England in 2014, each person producing over 420 kg of material annually and only 44.8% of it being recycled [4]. Discarded material composition is shown in Table 1.
Table 1. Discarded material composition in 2011 in England [5].
| Category | Value (wt%) |
|---|---|
| Organic material (e.g. food, garden) | 35.8 |
| Furniture related equipment | 14.9 |
| Paper | 14.0 |
| Glass | 6.8 |
| Dense plastic | 6.6 |
| Card packaging | 5.2 |
| Plastic films | 3.8 |
| Wood | 3.8 |
| Metals | 3.7 |
| Textiles | 2.9 |
Recycling is a solution that reduces resources going to landfill sites whilst reusing the recycled materials to make other products. Some technical issues do arise with recycling, as many materials can only be reused a limited number of times before they can no longer be chemically active to produce products with certain chemical properties [6]. Recycling does leave a carbon footprint due to sorting and transportation processes and taking into account that this process comes with the necessary expenses [7], [8]. This makes recycling a solution which is highly attractive from a short-term viewpoint as it reduces the volumes going into landfill sites and it also has a capability to make other useful products.
Resource management processes, such as pyrolysis and gasification are known as advanced thermal treatment (ATT) methods which provide a solution for resource treatment and can also meet emissions regulations [9]. Other conversion methods, such as combustion, incineration and anaerobic digestion, offer an alternative way to make use of the energy content within the material. Production of toxic compounds such as nitrogen oxides and sulphur oxides are reduced when pyrolysis is used in comparison with incineration [10]. Not only are greenhouse emissions from pyrolysis reduced relative to incineration but the solid residues are of higher quality [11].
Incineration, pyrolysis and gasification are the main ATT technologies. In “waste-to-energy” recovery mechanisms (treating MSW), a combination of the technologies mentioned above can be used together to provide a thermo-chemical process. Combination of the technologies mentioned can result in conversion processes that are more efficient [12]. Combustion is a heat generating process but its efficiency is only around 10% and it produces a large amount of greenhouse gases [13], [14].
Pyrolysis offers a method whereby it produces energy from waste feedstock in the form of three phases: solid biochar, liquid bio-oil and syngas [15], [16], [17], [18]. Biochar is a fine-grained residue which has a high carbon content. It is formed by the thermal decomposition of biomass in the absence of oxygen [19]. The chemical composition of biochar varies because of its heterogeneous nature, containing both stable and unstable components [19].
Bio-oil consists mainly of cyclic-compounds with some straight chain fractions. In comparison to petroleum oil, where a blend of oil compounds are obtained, bio-oil may require less reforming so that it is ready for usage by pharmaceutical or textile companies [20].
Gases/vapours derived from pyrolysis contain carbon monoxide, water vapour and some low molecular weight alkane and alkenes [21]. The contribution of each molecule to the gaseous fraction varies depending on many factors but most significantly on temperature [21].
In comparison with incineration, pyrolytic products contain low amounts of nitrogen oxides and sulphur oxides due to the inert medium the process is being operated in. The solid biochar is also of better quality [11].
2. Principles of pyrolysis
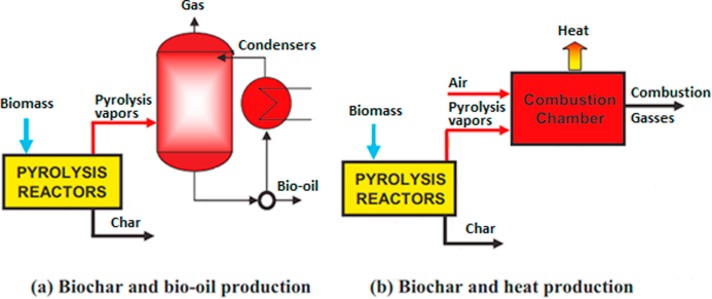
Pyrolysis is a thermal decomposition process that occurs in the absence of oxygen. The chemical reactions that take place in a typical pyrolysis reactor are very complex and consist of many stages [22]. In general, pyrolysis comes in two configurations. The first configuration consists of a reactor, a distillation column a condenser and a reboiler, as shown by the schematic of Fig. 1a. The second configuration consists of a reactor and a combustion chamber as shown in Fig. 1b. The two different arrangements produce different products. The first arrangement produces liquid as well as the solid char and gas. The second arrangement produces no liquid bio-oil because there is no condenser and all of the feed is thermally decomposed to gases which are then used in the combustion chamber to provide heat [22].
 Fig. 1. Flow diagrams for simplified pyrolysis units. (a) Simplified biochar and bio-oil flow diagram. (b) Biochar and gas flow diagram [23].
Fig. 1. Flow diagrams for simplified pyrolysis units. (a) Simplified biochar and bio-oil flow diagram. (b) Biochar and gas flow diagram [23].The majority of pyrolysis processes are conducted at high temperatures of 300–1000 °C but operating temperatures can reach 3000 °C when pyrolyzing polymers [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]. The higher the operating temperature, the more thermal decomposition is able to take place, thus producing a wider range of products but more significantly lower molecular weight molecules that are of high value [10]. The products made using pyrolysis are the result of the type of biomass feedstock used in the reactor. For example, pyrolysis of biomass will produce a different product composition than that from plastic [33].
The rate of heating during pyrolysis significantly alters the products. Slow pyrolysis, which is a slow heating process, allows secondary reactions to take place due to the increased residence time of the feed in the reactor [34].
3. Types of pyrolysis processes
3.1. Pyrolysis processes
Pyrolysis processes are split into the three main categories shown in Table 2below. These processes have different reaction temperatures, heating rates, feedstock sizes and residence times.
Table 2. A summary of the different types of pyrolysis processes and their operating parameters.
| Process | Reaction temp (°C) | Heating rate (°C/min) | Residence time | Feedstock size | Bio-oil yield (%) | Biochar yield (%) | Gas yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Slow pyrolysis | 300–550 | 50 |
5–30 min; 25–35 h |
Whole | 20–50 | 25–35 | 20–50 | [35], [36], [37], [38] |
| Intermediate pyrolysis | 300–450 | 200 | 10 min | Coarse/finely ground | 35–50 | 25–40 | 20–30 | [35], [36], [37], [38] |
| Fast/flash pyrolysis | 300–1000 | 10–1000 | 2 s | Finely ground | 60–75 | 10–25 | 10–30 | [35], [36], [37], [38] |
The operating conditions have a strong impact on the products formed. From Table 2, it is clear that fast pyrolysis uses finely ground feedstock which produces a high bio-oil yield. In contrast, slow pyrolysis uses whole feedstock sizes but produces a much lower bio-oil content. These operating parameters can be manipulated to generate a desired product range depending on the feedstock.
3.2. Reactor configurations
Reactor configurations can be altered to match a particular feedstock and a desired outcome. Such reactors include fixed bed reactors, batch reactors, semi-reactors, rotary kilns, fluidized bed reactors and microwave assisted reactors. The reactors have the same basic principle and that is to thermally decompose the feed material but their differences rely in the design and configuration to adjust to a specific feed material [34]. However, although there are many types of reactor designs, it remains a challenge to utilise such processes in an economically viable way. The high temperatures currently used in pyrolysis processes result in a high operating cost thus reducing the economics of such processes [34].
The type of reactor used in a pyrolysis process plays an important role due to the significant amount of heat that must be transferred across the reactor to enable material decomposition [34].
3.2.1. Fixed bed reactors and batch reactors
A fixed bed reactor, shown in Table 3, heats feedstock externally using an electric furnace. The reactor is kept in anaerobic conditions by flushing it with an inert gas such as nitrogen, which is maintained throughout the process [33]. A discharge of gases and vapours occurs during the process, while biochar is removed after its completion.
Table 3. Pyrolysis reactor designs of a fixed bed reactor from literature.
| Pyrolysis reactor designs | Reference |
|---|---|
 (1-N2 bottle; 2-reactor; 3-heat exchanger; 4-separation unit, 5-water trap; 6-gas flow meter; 7-rotameter) |
Ates et al. [26] |
 Fixed bed pyrolysis system (1-furnace; 2-pyrolysis reactor; 3-thermocouple; 4-temperature controller; 5-N2 pipe; 6-liquid gathering tank; 7-thermometer; 8-condenser; 9-pressure gauge; 10-sampling vent) |
Wang et al. [36] |
A batch reactor is similar to a fixed bed reactor but with no mass transfer occurring to the outside of the reactor [33]. Both of these reactor types are easy to manufacture and operate. However, residence time is long and the char obtained is difficult to remove [33].
3.2.2. Fluidized bed reactors
In-contrast to fixed bed reactors, fluidized bed reactors operate under fast heating rates and they allow a good blending of the feed material. However, the biggest disadvantage of a fluidized bed reactor is that the feedstock must be very small so that the particles can float. This adds complications to the process as a grinder must be installed. Biochar removal from the bed is problematic as most of the biochar phase will be dispersed within the fluid phase [37]. Schematics of fluidized bed reactors within pyrolysis systems are shown in Table 4. This type of reactor is useful to analyse product composition and various blends of feedstock, however, scaling-up can be problematic [37].
3.2.3. Rotary kiln reactors
The rotary kiln reactor is one of the types of pyrolysis reactors that has been successfully implemented on an industrial scale. It operates based on a slow heating rate with temperatures reaching 500 °C and a residence time of 1 h [37]. The only disadvantage of this reactor is that the feedstock requires pre-treatment but it is not complex [37]. Table 5 shows a rotary kiln reactor design which has a more complex configuration than fluidized bed and fixed bed systems.
Table 5. Pyrolysis reactor design of a rotary kiln bed reactor used in literature.
| Pyrolysis reactor design | Reference |
|---|---|
 (1-thermometer; 2-bearing; 3-gear transmission; 4-electrical furnace; 5-rotary kiln; 6-temperature controller; 7-seal; 8-two-steps condenser; 9-filter; 10-accumulative flowmeter; 11-computer; 12-gas sampling device; 13-feed and discharge opening; 14-speed adjustable electrical machinery) |
Li et al. [40], [41], [42] |
In summary, the current reactors used in pyrolysis operate at high temperatures and many are economically unviable. The rotary kiln reactor is the only reactor that has been successfully implemented on an industrial scale but it also operates at high temperature [37].
Many of the reactor designs require shredding of the feedstock to allow the reaction to take place efficiently and effectively. Shredding of the feedstock increases the surface area to volume ratio of the material allowing faster reactions to take place. Therefore, this property can be used to alter the desired output.
4. Kinetics of pyrolysis
The general reaction of pyrolysis is outlined as:(1)
Q is the total amount of heat that is required for the reaction to take place. This term involves heat supplied for moisture vaporisation, Q1 measured in kJ/kg, pyrolysis caloric requirement, Q2, and heat loss from the reactor to the surrounding atmosphere, Q3 [10].(2)where W is the moisture content of the feedstock, %. Water content can be high in certain feedstocks such as food. The higher the water content is, the more heat, thus energy, is needed. Therefore, a drying stage could be adopted prior to pyrolysis [10].(3)where are the specific heat capacities of the char, dry materials and volatile matter, respectively. . are the mass ratios of the char, dry materials and volatile to the feedstock. Depending on the type of MSW, Q2 can be calculated using differential scanning calorimetry (DSC) or differential thermal analysis (DTA) [31]. Q3 can be ignored in heat transfer design. Adequate insulation must be in place to make sure heat loss is negligible. Heating rate (HR) can be described using the following equation:(4)
HR has units of °C s−1. . the temperature difference between the feedstock and reactor wall, the heat transfer coefficient that is inside the reactor, m (kg) is the mass of the feedstock that is heated per m2.
Heating rates reported in literature vary from 4 to 670 °C s−1 [32], [36], [43], [44], [45], [46], [47]. Heating rate has a significant effect on the yield. Higher heating rate values are associated with high volatile matter. Volatile matter is referred to as being the gas and bio-oil phases. High heating rates along with long residence times also generate high gaseous yields [10].
The thermal degradation of organic matter based on agricultural residues, based on isothermal steps, has been described by the following equation [48], [49], [50], [51]:(5)where represents reaction rate; represent the function of thermal degradation; is the reaction rate constant and represented by the Arrhenius formula where the terms A, E, R and T are the pre-exponential factor, apparent activation energy, gas constant and absolute temperature, respectively.
5. Characterisation of pyrolytic products at temperatures below 300 °C
5.1. Discarded food
The UK discarded approximately 7.3 million tons (Mt) of food in 2016. 4.4 Mt of these could have been prevented, as the food was still in the right conditions to be used or consumed. 3.5 Mt of this household food is discarded in sewers or landfill sites. The total amount of household food and the way it is being currently processed are shown in Fig. 2. Pyrolysis might be a great alternative as it forms biochar, bio-oil and syngas which all contain high calorific values [52].
 Fig. 2. The total amount of household discarded food produced by the UK in 2016. The diagram shows the discarding methods and the amount of resource which could have been prevented [52].
Fig. 2. The total amount of household discarded food produced by the UK in 2016. The diagram shows the discarding methods and the amount of resource which could have been prevented [52].5.1.1. Proximate and ultimate analysis
As the products of pyrolysis are highly dependent on the feedstock used, proximate and ultimate analyses provide highly valuable information to analyse why certain products are formed. Proximate analysis focuses on the chemical composition of a material in terms of moisture content, fixed carbon, volatile matter and ash content. Ultimate analysis quantitatively analyses the elemental composition of a material in terms of carbon, hydrogen, nitrogen, sulphur and oxygen. In order to produce accurate results, proximate and ultimate analyses must comply with international standards such as ASTM, ISO, AS and D.
Experimental studies on pyrolysis carry out proximate and ultimate analyses of the feedstock used. This enables possible correlations to be made with the products obtained. Proximate and ultimate analyses of discarded food are shown in Table 6, Table 7, respectively.
| Material | Fixed carbon (%) | Moisture (%) | Volatile matter (%) | Ash (%) | HHV (MJ/kg) |
|---|---|---|---|---|---|
| Walnut shell | 15.90 | 11.00 | 71.80 | 1.30 | – |
| Almond tree pruning | 16.00 | 10.60 | 72.20 | 1.20 | – |
| Almond shell | 9.10 | 10.00 | 80.30 | 0.60 | – |
| Olive stone | 13.80 | 10.40 | 74.40 | 1.40 | – |
| Orange peel | 13.07 | 9.20 | 74.79 | 2.94 | 16.83 |
| Cattle bone | 2.33 | 4.07 | 28.90 | 64.70 | – |
| Hazelnut shell | 7.40 | 11.80 | 77.00 | 3.80 | 18.3 |
| Waste cereal | 19.10 | 5.50 | 73.50 | 1.90 | 17.42 |
| Waste peanuts crisps | 14.10 | 4.60 | 79.10 | 2.20 | 22.13 |
Table 7. Ultimate analysis of various discarded foods [33], [53], [54], [55], [56], [57].
| Material | Carbon (%) | Hydrogen (%) | Nitrogen (%) | Oxygen (%) | Sulphur (%) |
|---|---|---|---|---|---|
| Walnut shell | 45.10 | 6.00 | 0.30 | 48.60 | 0 |
| Almond tree pruning | 51.30 | 6.50 | 0.80 | 41.36 | 0.04 |
| Almond shell | 50.50 | 6.60 | 0.20 | 42.69 | 0.01 |
| Olive stone | 44.80 | 6.00 | 0.10 | 49.09 | 0.01 |
| Orange peel | 39.70 | 6.20 | 0.46 | 53.03 | 0.60 |
| Cattle bone | 32.90 | 8.00 | 3.20 | 55.90 | 0 |
| Hazelnut shell | 46.30 | 2.50 | 0.40 | 50.80 | 0 |
| Waste cereal | 43.00 | 5.89 | 2.16 | 41.4 | 0.12 |
Proximate analysis of various materials, shown in Table 6, clearly show that volatile matter is the major component containing at least 72% by weight of discarded food. However, the only material that significantly differs is cattle bone. It consists predominantly of ash.
Ultimate analyses of almost all feedstock materials used in Table 6 are shown in Table 7. The ultimate analysis shows an almost equal split of carbon and oxygen content with hydrogen content at less than 10% as well as some nitrogen and sulphur content in very low amounts.
5.1.2. Syngas
In-order to show the effect of feedstock on gas yield, Table 8 summarises the experimental results of pyrolyzing discarded food.
Table 8. A summary of pyrolysis of various discarded food feedstock.
The quantities of syngas generated in the pyrolysis of discarded cereals and discarded peanut crisps have different trends with increasing temperature. For discarded peanut crisps the trend is almost constant, not greatly affected by temperature increase above 300 °C, whereas in the case of discarded cereals, the opposite is true. Therefore, from the pyrolysis experiment of Grycova et al. [33], it is clear that the syngas generated is highly dependent on feedstock used as well as temperature in some cases.
An experiment carried out by Aguira et al. [56] on discarded orange peel in a fixed bed pyrolysis reactor shows that the gaseous yield is largely independent of feedstock particle size. The syngas yield variation with particle size was only 2%.
5.1.3. Bio-oil
Purevsuren et al. [53] conducted an experiment on the characterisation of tar from the pyrolysis of animal bones. Their main findings have shown that the major components of tar that was formed included aliphatic chains, with nitrogen functional groups attached forming nitriles, pyridines, amides and many others. The increased presence of nitrogen compounds is clearly observed from the ultimate analysis in Table 7. Aromatic compounds were present but were the minor components. Table 9 shows that bio-oil yield from pyrolyzing animal bones show approximately less than 5% which is explained by the fact that there is low volatile content and high ash content.
Table 9. Summary of bio-oil yield from pyrolysis experiments using various discarded foods.
Pyrolysis of dry orange peel produced bio-oil that is highly aromatic with some aliphatic chains [57]. The composition of dry orange peel is shown in Table 10.
Table 10. Composition of bio-oil formed during pyrolysis of orange dry peel [57].
| # | Retention time (min) | Identified compound | Molecular weight (g/mol) | Condensed formula | Chemical structure |
|---|---|---|---|---|---|
| 1 | 4 | Benzene | 78 | C6H6 |
|
| 2 | 5.53 | Toluene | 92 | C7H8 |
 |
| 3 | 9.43 | 2-methyl-2-hexanol | 116 | C7H16O |
 |
| 4 | 10.01 | Ethylbenzene | 106 | C8H10 |
 |
| 5 | 10.25 | p-Xylene | 106 | C8H10 |
 |
| 6 | 11.18 | Styrene | 104 | C8H8 |
 |
| 7 | 11.74 | 2-Cyclopenten-1-one, 2-methyl | 96 | C6H8O |
 |
| 8 | 12.35 | 1R-α-Pinene | 136 | C10H16 |
 |
| 9 | 13.57 | Benzene 1-ethyl-3-methyl- | 120 | C9H12 |
|
The formation of highly aromatic molecules can be problematic, especially in the case of Benzene, Toluene and Xylene due to their carcinogenic nature. Therefore, bio-oil containing such chemical components will require handling with care and treatment.
5.1.4. Biochar
5.1.4.1. Influence of treatment time on biochar yield
Even though the treatment temperature has a bigger influence on biochar yield, the treatment time also effects the over-all product yield [59], [60]. Table 11 is a summary of reviewed studies along with the reactions’ operating parameters [59], [61], [62]. These studies show that increasing the treatment time has a negative effect on the biochar product yield, with the product yield decreasing by 0.4–58%. The biggest drop in biochar yield is seen in the research of Ronsse et al. [60] where straw biomass shows a biochar yield decrease of 58% with a longer residence time. This can be explained by the fact that a high heating rate results in the degradation of naturally occurring biomass structures and a loss of volatile compounds [63], [64]. Although biochar yield is highly influenced by the type of biomass used, all reviewed studies show a decrease in the biochar yield during a longer pyrolysis residence time [64], [65]. A longer treatment time results in a loss of volatile compounds, which is one of the reasons for lower biochar yield [59], [66].
Table 11. A summary of the experimental conditions used during the pyrolysis of food biomass of four different journal articles [59], [61], [62].
| Type of reactor | GDL-1500X tubular furnace | Tubular stainless steel | Muffle furnace |
|---|---|---|---|
| Reactor size (L) | – | 1.36 | – |
| Type of waste | Rice straw | Straw and dry algae | Rice straw |
| Sample amount | Unknown sample amount as<0.25 mm particles | 70–135 g as 2–15 mm particles | Unknown sample amount as <2 mm particles |
| Treatment time (mins) | 60, 120, 180, 300 | 10, 60 | 120, 240, 460 |
| Heating rate (°C /min) | 5 | 17 | – |
| Treatment temperature (°C) | 300, 400, 500, 600, 700 | 300, 450, 600, 750 | 250, 300, 350, 400, 450 |
| Pressure | Atmospheric | Atmospheric | – |
| Biochar yield with longer residence time | Slight decrease (∼0.4%) | Decrease (∼56%) | Decrease (∼9%) |
| Reference | W. Wu [58] | F. Ronsse [60] | X. Peng [61] |
The residence time also affects the overall biochar content, as shown in Table 12. The residence time has a huge influence on the quantity of volatile matter. Ronsse et al. [60] stated that there is a drop of ∼60% of volatile compounds between the residence time of 10 min and 60 min at a treatment temperature of 300 °C. This finding is confirmed by the results from other studies [59], [62], [66]. At a longer residence time, the amount of fixed carbon increases. Biochar with a high amount of fixed carbon and low volatile matter is very stable, as the biochar has a better resistance to either aerobic or anaerobic degradation [60]. A longer residence time results in more organic carbon structures, as more organic carbon clusters are formed, the char becomes more graphitic, thus more stable [67]. This type of biochar is favoured for soil management and water treatment, as the overall characteristics are better [66], [69].
Table 12. The biochar characteristics of straw biochar made during pyrolysis at two different highest treatment temperatures (HTT) and residence times from the research study of Ronsse et al. [60].
| Biomass material and HTT | Residence time (min) | Volatile matter (wt%) | Fixed carbon (wt%) | Fixed carbon yield (wt%) | Ash content (wt%) | Total C content (wt%) | Total H content (wt%) | |
|---|---|---|---|---|---|---|---|---|
| Straw | 300 °C | 10 | 94.8 | 23.7 | 22.5 | 8.0 | 50.3 | 6.2 |
| 60 | 36.8 | 66.5 | 24.5 | 19.1 | 76.2 | 5.0 | ||
| 450 °C | 10 | 28.5 | 80.6 | 23.0 | 22.4 | 84.1 | 3.6 | |
| 60 | 27.5 | 84.1 | 23.1 | 22.9 | 86.4 | 3.5 | ||
5.1.4.2. Influence of temperature on biochar yield
During the pyrolysis of food biomass, a lot of naturally occurring plant structures degrade, resulting in a huge mass loss. As hemicellulose degrades at 200–260 °C and cellulose at 240–350 °C, there is a reduction of biomass volume during pyrolysis. The mass loss profile is shown in Fig. 3. The first mass drop in this figure (50–100 °C) is due to the evaporation of moisture. The second mass drop (250–350 °C) is related to the degradation of hemicelluloses and celluloses. Lignin, another plant structure, degrades at a much higher temperature, between 300 °C and 600 °C (highest temperature is highly dependent on the biomass) which explains the last mass loss at 500 °C [64], [68], [70], [71].
 Fig. 3. Percentage mass loss of a typical plant feedstock. The mass drops are caused by the degradation of plant structures [69].
Fig. 3. Percentage mass loss of a typical plant feedstock. The mass drops are caused by the degradation of plant structures [69].The highest treatment temperature (HTT) has a major influence on the mass loss profile but it is also the factor that has the most impact on biochar yield. Especially when working with food biomass, a lower treatment time is favoured as high temperatures cause a drastic decrease in the biochar yield [56], [61], [63], [72], [73], [74], [75]. Ronsse et al. [60] researched the influence of treatment time and temperature on the yield of biochar with four different types of biomass. All biomasses showed a great decrease of biochar yield when the treatment temperature was increased. The results of Ronsse et al. [60] are summarized in Fig. 4, where the effect of the treatment temperature is shown. When the temperature was increased at 150 °C increments (from 300 °C to 450 °C) there was an average biochar yield loss of ∼60%.
 Fig. 4. Percentage Biochar yield as a function of temperature [60].
Fig. 4. Percentage Biochar yield as a function of temperature [60].As seen in Table 13, the increasing temperature lowers the H/C ratio which can be explained by the aromatic rings becoming more graphitic. The Brunauer-Emmett-Teller (BET) surface area also increases because the cell structures and naturally occurring pores degrade resulting in fewer, but bigger pores [17], [26], [28].
Table 13. The effect of treatment temperature on food biochar characteristics researched by Ronsse et al. [60].
| Temperature (°C) | Time (min) | pH in solution | C (wt%) | H (wt%) | H/C ratio | Highest heating value (MJ/kg) |
|---|---|---|---|---|---|---|
| 300 | 10 | 6.1 | 50.3 | 6.2 | 1.47 | – |
| 450 | 9.8 | 84.1 | 3.6 | 0.51 | 25.1 | |
| 600 | 10.9 | 90.1 | 2.4 | 0.32 | 26.5 | |
| 750 | 12.1 | 92.2 | 1.6 | 0.20 | – |
To produce a very stable biochar, a low temperature and long residence time are preferred. The optimal treatment temperature in order to make biochar is a temperature of 300 °C or below, as this produces the highest biochar yield (when comparing the yield with that at higher temperatures) and also the best biochar characteristics.
5.2. Plastics
The production and use of plastic products have grown enormously over the last couple of decades due to their wide range of properties. Plastics are long chains of monomers that are mainly derived from fossil fuels but can also be derived from chemical processes. Plastics are categorised into the following groups: Poly-Propylene (PP), Polyethylene terephthalate (PET), Low-density polyethylene (LDPE), High-density polyethylene (HDPE) and Polyvinyl chloride (PVC) [77], [78]. Due to the fact that there is not a proper way to recycle all types of plastics, most of it is dumped in landfills. Pyrolysis of plastic may be an interesting alternative as it provides an opportunity to turn waste into valuable biogas and biofuel [79].
5.2.1. Proximate and ultimate analysis
Plastic comes in a variety of forms to serve different purposes. China is the biggest producer of plastic, reaching 269 million tons in 2015. The biggest disadvantage of plastics is that they are the least likely organic material to biodegrade from municipal solid waste [80]. Therefore, the deposition of plastic into landfill sites is not desirable and incineration has many controversies due to the toxic gases that can be released into the atmosphere [81].
Pyrolysis offers a mechanism of plastic waste conversion that is economical with the least environmental effect [82]. Sharuddin et al. [37] has reviewed pyrolysis of plastics and concluded that it has the potential to produce valuable and economical syngas, bio-oil and bio char. There are many types of plastic and the majority fall into groups as shown in Table 14.
Table 14. Properties of various types of plastic [4], [5], [6], [8], [38], [39], [86].
| Types of plastic | Moisture (wt%) | Fixed carbon (wt%) | Volatile (wt%) | Ash (wt%) |
|---|---|---|---|---|
| Polyethylene terephthalate (PET) | 0.46–0.61 | 7.77–13.17 | 86.83–91.75 | 0.00–0.02 |
| High-density polyethylene (HDPE) | 0.00 | 0.01–0.03 | 98.57–99.81 | 0.18–1.40 |
| Polyvinyl chloride (PVC) | 0.74–0.80 | 5.19–6.30 | 93.70–94.82 | 0.00 |
| Low-density polyethylene (LDPE) | 0.30 | 0.00 | 99.60–99.70 | 0.00–0.40 |
| Polypropylene (PP) | 0.15–0.18 | 0.16–1.22 | 95.08–97.85 | 1.99–3.55 |
| Polystyrene (PS) | 0.25–0.30 | 0.12–0.20 | 99.50–99.63 | 0.00 |
| Others | 0.00–0.16 | 0.04–2.88 | 97.12–99.78 | 0.00–1.01 |
Table 14 clearly shows that the majority of the plastics are highly volatile, thus responsive to thermal decomposition. The moisture, fixed carbon and ash content are very small meaning that the products produced will predominantly be bio-oil and no water processing is required if pyrolysis is carried out for plastic only.
To evaluate the quality of the bio-oil that can be used as fuel in motor engines, Table 15 showcases the properties of the bio-oils that are derived from the pyrolysis of plastic in comparison with the commercial standard values of gasoline and diesel.
Table 15. Physical properties of bio-oils from various types of plastic and standard values of gasoline and diesel [4], [5], [6], [8], [38], [39], [86].
| Physical properties | Type of plastic | Commercial standard value ASTM 1979 | ||||||
|---|---|---|---|---|---|---|---|---|
| PET | HDPE | PVC | LDPE | PP | PS | Gasoline | Diesel | |
| API gravity@ 60°F | N/A | 27.48 | 38.98 | 47.75 | 33.03 | N/A | 55 | 38 |
| Viscosity (mm2/s) | N/A | 5.08 | 6.36 | 5.56 | 4.09 | 1.4 | 1.17 | 1.9–4.1 |
| Density @15 °C (g/cm3) | 0.90 | 0.89 | 0.84 | 0.78 | 0.86 | 0.85 | 0.78 | 0.81 |
| Octane number MON (min) | N/A | 85.3 | N/A | N/A | 87.6 | N/A | 81–85 | – |
| Octane number RON (min) | N/A | 95.3 | N/A | N/A | 97.8 | 9–98 | 91–95 | – |
| Pour point (°C) | N/A | −5 | N/A | N/A | −9 | −67 | – | 6 |
The properties of bio-oils from HDPE, PP and PS, shown in Table 15, have MON and RON values that are within the range of commercial gasoline and diesel standards. However, the bio-oils from plastics have higher viscosity values than the standard. Therefore, bio-oil derived from plastics would require further chemical treatment before it is suitable for commercial use.
5.2.2. Syngas
Syngas is considered to be a by-product when plastic is the feedstock of a pyrolysis reactor. The percentage weight of the gaseous fraction is within 5–20%. To increase the gaseous fraction of the pyrolytic product, high temperatures (over 500 °C) are required. This parameter opposes the conditions needed for high bio-oil yield [87].
The gaseous products from the pyrolysis of LDPE, PS and their mixture were investigated by Onwudili et al. [87]. Their results have shown that more gas is produced when a mixture of plastic feedstock is used in comparison to the individual types of plastics. The gaseous fraction produced in their investigation did not exceed 13% at a temperature of 350 °C [87].
Williams et al. [38] conducted experiments on HDPE, LDPE, PP, PS, PET and PVC individually and their results show that the composition of the gas components produced during pyrolysis of each type of plastic were hydrogen, methane, ethane, ethene, propane, propene butane and butene. However, it was noted that when PET was used as feedstock in pyrolysis, gases such as carbon dioxide and carbon monoxide were produced in higher amounts relative to other types of plastics. Hydrogen chloride was produced when PVC was used as feedstock.
Jung et al. [88] investigated the use of PET and PP plastic as feedstock for pyrolysis. Their findings show that each of PET and PP individually had produced gases that have high calorific values of approximately 42 and 50 MJ/kg. Therefore, the pyrolysis gases produced using PE and PP have a great potential to be used as a heating source in pyrolysis plants.
Furthermore, as the majority of different types of plastics used in pyrolysis produce gases containing ethene and propene, these can be used as a chemical feedstock for the production of polyolefins if separated from other gas components. The high calorific value of pyrolysis gas enables it to be used to generate electricity using gas turbines or in direct firing in boilers [37].
Quantification of each type of syngas produced from pyrolysis at temperatures below 300 °C is not available in literature but its composition may include the gases shown in Table 16 depending on the operating parameters of the reaction.
| Gas |
|---|
| Hydrogen |
| Carbon monoxide |
| Carbon dioxide |
| Methane |
| Ethane |
| Propane |
| Propene |
| Butane |
| Butene |
5.2.3. Bio-oil
Many experiments in literature [38], [87], [88] have been carried out to characterise the chemical composition of the bio-oil produced from the pyrolysis of plastics. Bio-oil is the major product produced from the pyrolysis of plastics, therefore, it has been widely focused on in literature. The type of plastic used as a feedstock in the reactor ultimately dictates the composition of the products. Table 17 shows the possible chemical composition of the pyrolytic products of plastics. It must be noted that the distribution of the compounds varies depending on the composition of the feedstock and the operating parameters.
Table 17. Chemical composition of the products produced from the pyrolysis of plastics [38], [87], [88], [89].
| PET | HDPE | PVC | LDPE | PP | PS |
|---|---|---|---|---|---|
| 1-Propanone | 1-Methylcyclopentene | Azulene | Benzene | 2-Methyl-1-Pentene | Benzene |
| Benzoic acid | 3-Methylcyclopentene | Naphthalene, 1-methyl- | Toluene | 3-Methylcyclopentene | Toluene |
| Biphenyl | 1-Hexene | Biphenyl | Xylene | 1-Heptene | Ethylbenzene |
| Diphenylmethane | Cyclohexene | Naphthalene, 1-ethyl- | Dimethylbenzene | 1-Octene | Xylene |
| 4-Ethylbenzoic acid | 1-Heptene | Naphthalene, 1-(2-propenyl)- | Trimethylbenzene | C4–C13 hydrocarbon | Styrene |
| 4-Vinylbenzoic acid | 1-Octene | Naphthalene, 2,7-dimethyl- | Indane | Over C14 hydrocarbon | Cumene |
| Fluorene | 1-Nonene | Naphthalene, 1,6-dimethyl- | Indene | Benzene | Propylbenzene |
| Benzophenone | 1-Decene | Naphthalene, 1,7-dimethyl- | Methylindenes | Toluene | 2-Ethyltoluene |
| 4-Acetylbenzoic acid | 1-Undecene | Naphthalene, 1,4-dimethyl- | Naphthalene | Xylene | Naphthalene |
| Anthracene | 1-Tridecene | Naphthalene, 1,6,7-trimethyl- | Methylnaphthalenes | Ethybenzene | Diphenylmethane |
| Biphenyl-4-carboxylic acid | C4–C13 hydrocarbon | 9H-Fluorene | Ethylnaphthalene | Indene | Anthracene |
| 1-Butanone | Over C14 hydrocarbon | Naphthalene, 1-(2-propenyl) | Dimethylnaphthalene | Biphenyl | 1,2-Diphenylethane |
| m-Terphenyl | Benzene | Phenanthrene, 1-methyl | Acenaphthylene | – | 2,2-Diphenylpropane |
| – | Toluene | Fluoranthene, 2-methyl- | Acenaphthene | – | 1,3-Diphenylpropane |
| – | Xylene | 1H-Indene, 2,3-dihydro-5-methyl- | Trimethylnaphthalenes | – | Phenylnaphthalene |
| – | – | Naphthalene, 2-phenyl- | Fluorene | – | Diphenylbenzene |
| – | – | – | Tetramethylnaphthalene | – | Triphenylbenzene |
The majority of the plastics will produce a variety of compounds; some are alkanes, alkenes, aromatics and others shown in Table 17. The major concern is that benzene is produced and benzene is a known carcinogen [90]. Therefore, the products must be properly controlled and used before they come into contact with humans.
The chemical characterisation of pyrolytic products with plastics as feedstock, shown in Table 17, may require a high temperature to achieve. However, these chemical compounds may be achievable by altering parameters other than temperature such as heating rate, pressure and residence time [37].
Paradela et al. [91] conducted a research study on co-pyrolysis of biomass and plastic wastes. Their findings show that when plastics were pyrolysed up to temperature of 450 °C, a higher heating value of 44.7 MJ/kg was achieved. This value is similar to that of heating fuel oil. The major parameters that showed a high influence are as follows: alkane content increases with increased temperature and reaction temperature strongly influences product phase distribution.
Ma et al. [92] carried out an experiment on the low temperature pyrolysis of PVC. They have found that at temperatures below 300 °C the yield consisted of a solid residue while oil and gas were the minor phases. The most influential parameters were found to be similar to that of Paradela et al. [91]. The molecular content of PVC includes chlorine, which is toxic, and after decomposition due to pyrolysis over 90% of the chlorine is found to be in the gaseous phase. Therefore, the gaseous phase must be treated carefully.
5.2.4. Biochar
5.2.4.1. Influence of treatment time on biochar yield
Pyrolysis of plastic in literature differs in the operating parameter conditions and there is some contradiction between the articles’ conclusions. The reviewed articles with experimental conditions are shown in Table 18 [79], [87], [93], [94]. Studies with a higher treatment temperature (>450 °C) have been shown to have a decreasing biochar yield when the feedstock residence time is increased. Whereas studies with a significantly lower treatment temperature (<450 °C) show that doubling the residence time could result in 6 times the biochar yield relative to the high temperature experiments, as shown by Onwudili et al. [87], [94].
Table 18. A summary of the experimental conditions used during the pyrolysis of plastic biomass from four different journal articles [79], [87], [93], [94].
| Type of reactor | Unstirred semi-batch reactor | Batch reactor | Autoclave | Parr Mini Bench Top Reactor, 4651 m stirred pressure reactor |
| Reactor size (L) | 3.5 | 20 | 0,025 | 0,3 |
| Type of waste | PE, PP, PS, PET & PVC | Plastic plates (mostly made of PS) | LDPE | LDPE & PS |
| Sample amount | 100 g as 3 mm particles | 1000 g as 5 cm2 particles | 4 g as 40 µm particles | 10 g as 2 mm particles |
| Treatment time (minutes) | 30 | 60, 75, 120 | 30, 90 | 60, 120 |
| Heating rate (°C /min) | 20 | 10 | – | 10 |
| Treatment temperature (°C) | 460, 500, 600 | 400, 450, 500 | 370 – 450 | 300, 500 |
| Pressure | Atmospheric | Atmospheric | – | 0.8–4.3 MPa |
| Biochar yield with longer residence time | Decreased (∼25%) | Decreased (∼4%) | Increased (∼35%) | Increased (∼8%) |
| Reference | A. López [79] | R. Miandad [93] | L. Tiikma [94] | Jude A. Onwudili [87] |
Treatment temperature and heating rate have a bigger impact on the biochar yield than the treatment time. When reviewing journal articles [93], [94], [95], [96], López et al. [79] used a high heating rate in combination with a high treatment temperature, which resulted in a large decrease in the biochar yield.
The particle size also plays a major role in the biochar yield. Many journal articles have shown that there is a strong correlation between biochar yield and particle size [63], [64], [97], [98], [99]. The bigger the biomass particles, the higher the biochar yield is. Scott et al. [99] stated that the highest biochar yield of plastic biomass is obtained when large particle sizes are used along with low temperature and slow heating rate pyrolysis with a long residence time [100]. When comparing this statement with Table 18, the statement doesn’t seem to be accurate because other research studies show that higher particle sizes can also result in a decrease in biochar yield. This can be explained by the high treatment temperature and fast heating rate [79], [87], [93], [94].
Research conducted by López et al. [79] has shown that the treatment time has a big impact on the H/C-ratio. Especially in the first 15 min there is an increasing amount of carbon and a decreasing amount of hydrogen in the biochar, as shown in Table 19. After the first 15 min, all the characteristics stay relatively the same. The treatment time did not have a big impact on the biochar characteristics in pyrolysis performed at 500 °C [79], [80], [81], [82], [83], [84], [85], [86], [87].
Table 19. The biochar characteristics of plastic biochar made during pyrolysis with 500 °C as HTT, as stated by López et al. [79].
| Temperature (°C) | Time (min) | Moisture (wt%) | C (wt%) | H (wt%) | H/C ratio | Highest heating value (MJ/kg) |
|---|---|---|---|---|---|---|
| 500 | 0 | – | 83.8 | 14.0 | 2.0 | 47.4 |
| 15 | 0.4 | 94.4 | 3.7 | 0.5 | 39.4 | |
| 30 | 0.2 | 93.7 | 3.5 | 0.4 | 38.2 | |
| 120 | 0.3 | 94.1 | 3.5 | 0.4 | 38.2 |
5.2.4.2. Influence of temperature on biochar yield
In order to break down plastic, a relatively higher treatment temperature is favoured during pyrolysis, as the degradation of plastic starts at 400 °C and it is fully pyrolyzed at 500 °C [79]. The degradation can be registered by looking at the mass loss. López et al. [79] conducted research into pyrolysis of different kinds of plastics. The results in terms of mass loss rate can be seen in Fig. 5. As seen in the figure, every plastic (except for PVC) has one peak. This peak shows the degradation temperature and speed per type of plastic. The first PVC peak is explained by the evaporation of HCl, the second peak is the decomposition of the remaining polyene [79]. During the full degradation of plastics, bio-oil and syngas are the most common products. The higher the pyrolysis temperature is, the higher the syngas yield will be [93], [96].
 Fig. 5. Rate of mass loss of different types of plastics due to pyrolysis. PVC is the only plastic type that has two peaks. The average decomposition temperature is 450 °C as found by López et al. [79].
Fig. 5. Rate of mass loss of different types of plastics due to pyrolysis. PVC is the only plastic type that has two peaks. The average decomposition temperature is 450 °C as found by López et al. [79].While a higher temperature is needed for full decomposition of plastic mass, biochar yield decreases with increasing temperature. The required temperature to fully break down plastic is so high that the biochar yield during pyrolysis very low or not even present. The common products that are formed appear as bio-oil and syngas [93], [96]. To get the highest biochar yield during the pyrolysis of plastic, a very low treatment temperature and a long residence time are favoured. The lower temperature ensures that less plastic gets converted into bio-oil or syngas and the longer treatment time increases the biochar yield [87], [94], [96].
Another temperature related influence on the biochar characteristics is the heating rate. The heating rate plays a major role in the formation of pores. When comparing the same biochar made with other heating rates, the biochar made with low heating rates have fewer pores [69], [101]. The pores are also smaller when comparing them with pores of biochar made during a high heating rate process. When the heating rate is increased, the volatile compounds evaporate, creating new pores or enlarging the already existing pores [58], [102]. However, a very high heating rate (>100 °C/min) negatively influences the biochar pore structure, as natural biomass structures degrade resulting in destruction of the pores present [103]. An overview of four different heating rates and some of the biochar pore structure reported in literature is given in Fig. 6 [62].
 Fig. 6. The influence of heating rate on the pore definition of biochar, where a high heating rate results in a biochar with very defined and big pores [62].
Fig. 6. The influence of heating rate on the pore definition of biochar, where a high heating rate results in a biochar with very defined and big pores [62].The overall biochar characteristics strongly depend on the HTT and heating rate. For the optimal pore formation, a heating rate of 10 °C/s is favoured, as the structure of micro- and macro-pores are almost the same at a heating rate of 100 °C/s, but the lower heating rate makes sure no internal structure cracks occur [62]. Looking at Table 20, increasing the temperature has some negative effects on biochar characteristics. As the temperature increases, the H/C ratio decreases meaning that the (aromatic) carbon-rings are getting more graphitic. When the structure becomes more graphitic, the amount of random cross-links will decrease, meaning there is a decrease in natural occurring pores. The higher heating value also decreases, this goes hand in hand with the decreasing H/C ratio [79].
Table 20. The effect of temperature on the biochar composition as found by Lópezet al. [79].
| Temperature (°C) | Time (min) | Moisture (wt%) | C (wt%) | H (wt%) | H/C ratio | Higher heating value (MJ/kg) |
|---|---|---|---|---|---|---|
| 460 | 30 | 0.1 | 92.0 | 3.9 | 0.5 | 38.5 |
| 500 | 0.2 | 93.7 | 3.5 | 0.4 | 38.2 | |
| 600 | 0.1 | 91.7 | 2.3 | 0.3 | 36.8 |
The best biochar yield and characteristics are obtained during a long residence time, lower heating rate and low temperature pyrolysis [63], [79], [87], [93], [94].