1. Introduction
When molecules are incorporated between metal electrodes, charge transfer can occur from one side to the other. The transfer of electrons produces current flow in the opposite direction. Electron transfer is the simplest reaction, and one in which no chemical bonds are created or destroyed [1], [2]. It takes place in all natural processes, including photosynthesis, and is closely related to the arrangement of molecular structure [3]. Hence, the study of molecular structures and the electron transport through them remains significant. Transport mechanisms through molecular layers should be studied and explored intensively, since they find a wide range of applications in molecular-level research [4], [5].
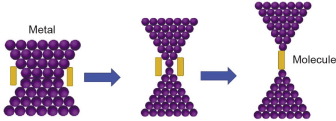
The term molecular junction is an important concept in which a molecular cluster is included between two electrodes, and electrons are transferred across it. However, a specific definition of a molecular junction has not yet been established. In the review article published by McCreery [6], it is stated that the entire structure of a molecular electronic component should be considered as an electronic system in order to understand the true concept of a molecular junction. A single molecular junction can be created using the break-junction technique, which is depicted in Fig. 1. Here, when a metal is stretched in the presence of a suitable molecule, the weak point breaks and the molecule is attached to the leads, forming a molecular junction. The left and right leads have a continuous band structure, whereas the band structures are discontinuous for the central cluster between the leads.
 Fig. 1. Schematic diagram of a single molecular junction formed by stretching according to the break-junction technique.
Fig. 1. Schematic diagram of a single molecular junction formed by stretching according to the break-junction technique.Since there is a difference in the chemical potentials of the left and right leads, electrons are transferred across the molecular junction in order to maintain equilibrium, as shown in Fig. 2. However, the electrons must cross an energy barrier to pass from the contact to the molecule [7]. Transport can be dispersive or ballistic. In dispersive transport, electrons collide with each other and it is the energy that is transferred. In contrast, in ballistic transport, electronic transport is considered to occur in a way that is comparable to the motion of a bullet—that is, in a straight line. The basic idea used to study electron transport across a junction is the Landauer approach [8]. Maassen and Lundstrom [9] have demonstrated that the Landauer approach can be used to explain both the dispersive and the ballistic transport mechanisms.
 Fig. 2. Charge transfer occurs across a molecular junction to maintain equilibrium.
Fig. 2. Charge transfer occurs across a molecular junction to maintain equilibrium.As shown in Fig. 2, when a bias is applied due to the difference in the carrier population, electrons start to flow. This flow can be mathematically written as follows:(1)where I represents the electric current, q represents the electric charge, h is Planck’s constant, is the transmission, is the number of channels, is the Fermi–Dirac occupation distribution on both the electrodes, and is the chemical potential on both the leads [9], [10]. With this equation, the total current can be obtained. In addition to the Landauer approach, methods such as the Boltzmann transport equation [11], [12] and the non-equilibrium Green’s function (NEGF) [13], [14] can be used to study electronic transport. Self-assembling molecules can be considered to be perfect candidates for the assembly of molecular components, since such molecules are able to adopt a defined arrangement without guidance or management from an outside source [15]. In self-assembly, subatomic particles blend together to form complex structures with minimized degrees of freedom [16]. Dithiols, which are organosulfur compounds with two thiol functional groups, are widely used for self-assembly because of their ability to form strong self-assembled monolayers [4]. Successful studies have been performed highlighting the adsorption of dithiols over various metals such as gold [7], [8], [9], [10], [11] and silver [12], [13].
Researchers have developed molecular transistors by coating gold wires [17]. Transistors are the most important component of an integrated circuit (IC), as they act as a switch in accordance with the applied voltage. Solid-state molecular transistors have already been designed using the Coulomb blockademethod and Kondo effect [17]. Diodes are another crucial component of an IC, and are used in the smooth running of a transistor; thus, the fabrication of molecular diodes is inevitable. Polyphenylene-based chains and carbon nanotubes (CNTs) are the proposed building blocks for molecular-based components [18]. Capacitors are used to code information by storing charge, and wires are necessary for interconnection between various components in an IC.
A detailed review of the development of these key elements is given in Section 2. Section 3 then discusses various molecules that are suitable for application in moletronics. Section 4 provides a brief discussion of the properties of graphene and recent research on graphene-based molecular devices, and Section 5describes research trends in molecular electronics. Finally, Section 6 provides concluding remarks.
2. Molecular integrated circuit components
2.1. Molecular transistors
Transistors control the flow of electrons by customizing the voltage that applied. A transistor consists of three terminals; two of the terminals act as the source and drain, and the third acts as the gate. The flow of electrons from the source to the drain can be controlled by the gate. For the theoretical formulation of molecular transistors, it is necessary to have an exact idea of how to align the Fermi levels of the electrodes with the molecular orbitalenergies of the molecules [19]. Ghosh et al. [20] have predicted the utilization of electrostatic regulation of the molecular orbital energy of a single molecule for the evolution of solid-state molecular transistors. Ahn et al. [21] have suggested a similar approach, in which electrostatic modulation of the internal charge density can be done by using an external node to control the charge transfer across the metal electrodes and the molecules. By aligning the molecular energy levels and the Fermi level of the leads, transistor action can be achieved. Jin et al. [22] have recently demonstrated that the work function of the electrodes can be tuned along with the entire range of molecular energy levels.
Once the transistor is well-stacked, transistor action should be acquired. Scientists have developed a special type of molecule called rotaxane, which acts as a molecular switch [23]. These molecules can change their structure, and thereby the conductance, in accordance with the applied voltage. When a voltage is provided, the rotaxane molecule changes from its stable state to another state, just like the ON/OFF states of a switch. Chen et al. [24] have described the potential application of a rotaxane molecule as a switch on a crossbar circuit. They sandwiched a rotaxane molecule between platinum and titanium, and caused this junction to develop at each of the cross points. Within a small area of about 1 µm2, Chen et al. were able to construct an 8 × 8 crossbar circuit consisting of 64 such switches. In the same year, Long et al. [25]established the adsorptive capacity of rotaxane molecules over titanium dioxide nanoparticles, which led to further development in molecular transistors. A sample illustration showing the action of a rotaxane molecule is given in Fig. 3[26].
 Fig. 3. The action of a rotaxane molecule. A single macrocycle (red) can undergo translational motion along an axle (blue) with stoppers (green). (Reproduced from Ref. [26])
Fig. 3. The action of a rotaxane molecule. A single macrocycle (red) can undergo translational motion along an axle (blue) with stoppers (green). (Reproduced from Ref. [26])An alternate method of attaining transistor action is to take advantage of the conformational degrees of freedom [20]. To stop current from flowing through molecules to a metal electrode, the molecules can be tilted away from contact, or the conjugation can be destroyed by twisting, as shown in Fig. 4 [20]. Papadopoulos et al. [27] have confirmed that electronic transport across a junction can be controlled by changing the conformation of the side groups.
 Fig. 4. A molecule is tilted by an angle ɵ, in order to control the flow of current across a junction. Metal electrodes are shown in brown and the gate is shown in blue. (Reproduced from Ref. [20])
Fig. 4. A molecule is tilted by an angle ɵ, in order to control the flow of current across a junction. Metal electrodes are shown in brown and the gate is shown in blue. (Reproduced from Ref. [20])The experimental verification of molecular components is a difficult procedure; most such verifications have been performed using scanning tunneling microscopy (STM) [28], which can also provide information on conductivity [29]. By changing the molecular structure and lattice positions of the metal atoms, charge transfer can be bolstered up [30]. Sotthewes et al. [31] have recently demonstrated the measurement of transport properties through a single octanethiol molecule. By varying the length of the molecule via compression and expansion, they were able to control the conductance of the metal-molecule-metal junction. In their paper, Sotthewes et al. [31] thoroughly explain the methods by which they attached the octanethiol molecule to the macroscopic lead with the help of STM (Fig. 5).
 Fig. 5. The attachment of an octanethiol molecule between a platinum (Pt) atom chain and the apex of an STM. (a) The current-time trace recorded when the octanethiol molecule is attached at the tip of STM; (b) the molecule is adsorbed onto the Pt atom chain; (c) the molecule is adsorbed onto both the Pt atom chain and the tip of the STM. (Reproduced from Ref. [31])
Fig. 5. The attachment of an octanethiol molecule between a platinum (Pt) atom chain and the apex of an STM. (a) The current-time trace recorded when the octanethiol molecule is attached at the tip of STM; (b) the molecule is adsorbed onto the Pt atom chain; (c) the molecule is adsorbed onto both the Pt atom chain and the tip of the STM. (Reproduced from Ref. [31])Joachim et al. [32] used a single molecule of C60 to determine the electrical resistance. They identified the tunneling mechanism by the displacement of an STM tip directly through the highest occupied molecular orbitals (HOMOs) or lowest unoccupied molecular orbitals (LUMOs). As a result, electrons were able to tunnel through the energy barriers of the molecule sample. The transport mechanism depended on the applied bias voltage. However, even when the bias voltage was insufficient, tunneling occurred with the aid of the tail of the broadened energy level. When molecules were adsorbed on the surface, their energy levels broadened and shifted.
A great deal of research is being performed to improve current conductance across the metal-molecule junction of a molecular transistor [31], [32], [33]. Tersoff et al. [34] have already reported that the contact resistance at the nanotube-metal junction plays a greater role in charge transfer than the channel conductance. The Schottky barrier at the junction limits the current flow and hence the conductance. Javey et al. [35] have provided a valuable idea to reduce the strength of this barrier for a smooth flow of electrons by establishing a palladium contact on the semiconducting single-walled carbon nanotubes (SWCNTs). Palladium is known to have a high work function and an exceptional wetting interaction with CNTs. Furthermore, by altering the geometry of CNTs, their metallic and semiconductor behavior can be manipulated. Hence, electronic properties can be determined by differing the geometry, as has been pointed out by Ebbesen et al. [36].
Single-electron transistors (SETs) have the advantages of low power dissipationand faster modes of operation. A conducting state can be changed to a non-conducting state by the addition or removal of a single electron. The use of a Coulomb blockade helps to control an individual electrode from charging by influencing one electron at a time [37]. A variety of works have been carried out on Coulomb blockade effects on SETs [38], [39], [40]. When the tunneling resistance is lower than the quantum resistance, , and the energy required by an electron to charge a junction with capacitance C, where is greater than the thermal energy, the single-electron tunneling effect occurs [40]. Here, h represents Planck’s constant, e depicts the absolute value of the electronic charge, T is the absolute temperature, and is the Boltzmann constant. However, when the voltage is lower than a Coulomb blockade is observed with quenched electron tunneling. Ali et al. [40] have reported the feasibility of silicon for Coulomb blockade structures. In 2007, after the discovery of graphene, Sols et al. [41] pointed out the occurrence of a Coulomb blockade in graphene nanoribbons. Developments in SETs are still proceeding, and are likely to lead to advancements in molecular electronics.
Another significant phenomenon is the Kondo effect [42], [43], [44]. When a magnetic impurity exists, conduction electrons are scattered in a metal, which causes variation in the electric resistivity with temperature. This phenomenon occurs when there is an interaction between a single magnetic atom and the many electrons in a non-magnetic metal [45]. Liang et al. [42] have explained the effect of Kondo resonance on SETs with a divanadium molecule as the spin impurity. They also discovered that by altering the gate voltage, Kondo resonance could be reversibly tuned. Furthermore, they specified the existence of Kondo resonance up to a temperature of 30 K, and when the energy gap between the molecular and the Fermi levels is greater than 100 meV. The Kondo effect could enhance the conductance across the electrodes below a Kondo temperature, Tk [43]. Very recently, Mitchell et al. [43] have shown the combined effects of quantum interference and the Kondo effect on the conduction of current in an SET. They have shown that a Kondo blockade can stop conductance, as shown in Fig. 6 [43]; hence, by the application of reverse gate voltage, they were able to manipulate the tunneling current.
 Fig. 6. A Kondo blockade stops the current flow between the drain and the source. (Reproduced from Ref. [43])
Fig. 6. A Kondo blockade stops the current flow between the drain and the source. (Reproduced from Ref. [43])The effect of quantum interference on molecular transistors has been widely studied [45], [46], [47]. In 2012, Guédon et al. [48] reported the possibility of destructive quantum interference in the charge transport mechanism. They performed research on different molecular wires that were self-assembled over a gold surface. In 2017, Chen et al. [49] performed a study on the effect of nuclear vibration on quantum interference, and suggested that quantum interference effect transistors can survive vibrations in both the steady and transient states. However, a credible and authentic foundation has yet to be established for the manufacture of a stable and reproducible molecular transistor.
2.2. Molecular diodes
Diodes are components that conduct current only in one direction, when they are forward biased. They are composed of semiconductor materials, and have a p-n junction and two terminals for the connections. There are two fundamental types of molecular diode: rectifying diodes and resonant tunneling diodes (RTDs).
2.2.1. Molecular rectifying diodes
For the fabrication of molecular diodes, an electron-donating group (which is equivalent to an n-type semiconductor) and an electron-withdrawing or -accepting group (which resembles a p-type semiconductor) should be available. In the case of an electron-donating group, the electron density is high, whereas for an electron-withdrawing group, the electron density is low [18]. In 1974, Aviram and Ratner [50] discussed the possibilities of a rectifier; that work provided a foundation for current work on molecular diodes. Their design involved a donor π-system and an acceptor π-system linked together by a σ-bonded tunneling bridge. With this, they were able to appropriately align the LUMO and HOMO so that electrical conduction occurred in only one direction, which resembled diode action. The introduction of a σ-electron system established an insulation barrier between the donor and the acceptor groups. A hemiquinone molecule (chemical structure shown in Fig. 7) was used to identify this behavior [51]. Since then, this model has been used by many researchers.
 Fig. 7. Structure of a hemiquinone molecule. Dark grey: carbon; red: oxygen; light grey: hydrogen. (Reproduced from Ref. [51])
Fig. 7. Structure of a hemiquinone molecule. Dark grey: carbon; red: oxygen; light grey: hydrogen. (Reproduced from Ref. [51])The rectifying actions of diodes have been widely explored. In 2002, Ng et al. [52] developed a more defined model for molecular diodes, which accurately portrayed their rectifying effect. These researchers used simple diblock conjugated molecules covalently attached to a gold surface, which acted like a p-n junction. For rectification, the structure should be highly asymmetric in order to exhibit physical variation between the forward and the reverse bias [53]. According to Ellenbogen and Love [18], two energetically accessible molecular levels are required for molecular rectification. In addition, they assume that the potential drop arising at the electrode-molecule interface and inside the molecules influences rectification. Kornilovitch et al. [54] have presumed the independence of rectification on the work function difference between the two electrodes. On the other hand, Liu et al. [53] have considered the contingency of an asymmetric potential drop either at the metal-electrode contacts or across the molecules for rectification. Nijhuis et al. [55] have demonstrated that by inculcating self-assembled monolayers of alkenethiolates with an Fc headgroup (SC11Fc) along with a silver-bottom electrode and a liquid-metal top electrode (Ga2O3/EGaIn), a high degree of rectification can be accomplished. Through intense analysis, Nijhuis et al. [55] showed that only the HOMO of the Fc is essential for a high degree of rectification. They considered their molecular rectifier to be a double-barrier junction if—and only if—the HOMO is not bounded by the Fermi levels of the electrodes. A schematic representation of the tunneling junction taken from their work is given in Fig. 8[55]. Nijhuis et al. [55] were able to produce a rectification ratio of around 100, which surpassed the work of Armstrong et al. [56], who were able to produce a rectifying ratio of only about 22 with a double-barrier junction.
 Fig. 8. A schematic representation of the tunneling junction over a silver-bottom electrode with a liquid-metal top electrode. (Reproduced from Ref. [55])
Fig. 8. A schematic representation of the tunneling junction over a silver-bottom electrode with a liquid-metal top electrode. (Reproduced from Ref. [55])Novel research by Metzger [57] has provided a detailed perspective on unimolecular electronics, and has established a basis for electrical rectification by a molecule. His recent review on unimolecular electronics summarizes current works being performed on molecules for improving their rectifying action [58]. As explained in detail in Section 3, various molecules are being used to obtain the best result with rectification. As discussed in Refs. [56], [57], [58], [59], the hexadecylquinolinium tricyanoquinodimethanide molecule is widely used in research for this purpose, although its chemical synthesis is not fully understood [60] due to insufficient isolation between the donor and acceptor groups in the molecule. Given these circumstances, Lenfant et al. [60] have devised a rectifier with only a donor group and an alkyl spacer chain; in this way, they tackled the problem of isolation between the two groups. Contrary to the methods that have been adopted by researchers to metalize molecules in order to improve conductivity and establish an ohmic interconnection, Lenfant et al. [60] succeeded in utilizing the inherent tunneling facet of the molecules, which is intensified by the source and the drain. Electron transport from the cathode to the anode is predominantly controlled by the molecules. Vilan et al. [61] have demonstrated that the regulation of the electrical properties of the metal-semiconductor junction can be controlled by the molecules without the actual transfer of electrons across the junction through the molecule. They used a series of tartaric acid derivatives for this demonstration. However, the development of more dependable methods to assemble rectifying diodes remains an open field of research.
2.2.2. Molecular resonant tunneling diodes
In RTDs, electrons can tunnel through resonant states at different energy levels. These diodes can be used as oscillators and switches [62]. For the smooth and uninterrupted working of diodes, RTDs have proven to be a sterling candidate for high-functionality circuits [63]. For molecular RTDs, methylene groups, which are aliphatic, are attached to both sides of a benzene ring, thereby creating a potential barrier. As a result, the electrons must tunnel through the barrier; however, since the kinetic energy of the electrons does not align with the unoccupied energy levels of the benzene ring, the transistor remains in the OFF state. By varying the applied voltage, a resonant state can be obtained, in which the kinetic energy of the incoming electrons aligns with the unoccupied energy levels; this turns the transistor ON [18]. The structure of a molecular RTD is shown in Fig. 9 [64], and its band diagram is depicted in Fig. 10 [18]. From a quantum mechanics perspective, an RTD has two potential barriers and a quantum well. Further research into RTDs can reveal quantum aspects of mesoscopic systems [63]. This concept was first patented by Tsu [65] in 1993. Seminario et al. [66] have conducted theoretical studies and have reported that electron conduction occurs through the LUMO. They performed their studies on π-conjugated oligo (phenylene ethynylene), which showed the characters of an RTD. Behavior resembling that of an RTD has been reported in molecules containing a nitroamine redox center [67]. Later, in 2008, Lake et al. [68]incorporated DNA molecules as a bridge between two CNT leads. They were able to attach a GCCG segment of the DNA to the CNTs with the aid of amide linkers.
 Fig. 9. Structure of a molecular resonant tunneling diode (RTD). (Reproduced from Ref. [64])
Fig. 9. Structure of a molecular resonant tunneling diode (RTD). (Reproduced from Ref. [64]) Fig. 10. A band diagram of a molecular RTD depicting the OFF state. MOs: molecular orbitals. (Reproduced from Ref. [18])
Fig. 10. A band diagram of a molecular RTD depicting the OFF state. MOs: molecular orbitals. (Reproduced from Ref. [18])Strong theoretical formulations must be framed in order to elucidate the transfer mechanisms so that further studies and augmentations are possible. The Hartree–Fock self-consistent field theory [69] and the density functional theory (DFT) [66] are the main theories used in developing a theoretical framework for molecular electronic devices. According to theory, for a stable molecule, the HOMO–LUMO gap (HLG) should be large. DFT [62], [70], [71] can be used to scrutinize parameters such as peak voltage, the temperature dependence of the system, and the source of resonance. After the experimental authentication of CNTs, Dragoman and Dragoman [72] discussed the possibility of using semiconducting SWCNTs, which yield a better performance than the usual RTDs with semiconductor heterostructures. They were able to engineer a CNT-based RTD with a barrier height that was controlled by the direct current (DC) voltage applied to the gate and the length by controlling the inter-electrode distance. Two years later, Pandey et al. [73] pointed out the possibility of CNT-based molecular RTDs. They linked pseudopeptide as a bridge between two semiconducting CNT leads, as shown in Fig. 11 [73]. Very recently, Bayram et al. [74] developed AlN/GaN double-barrier RTDs by metal–organic chemical vapor deposition (MOCVD) on sapphire, and obtained a negative differential resistance (NDR) of 4.7 V. They have shown that MOCVD can be beneficial for attaining epitaxial surfaces and for tunneling studies.
 Fig. 11. A CNT-based RTD comprising a pseudopeptide between two CNTs, one on each side. (Reproduced from Ref. [73])
Fig. 11. A CNT-based RTD comprising a pseudopeptide between two CNTs, one on each side. (Reproduced from Ref. [73])Research into stable electron transport is vital for an understanding of molecular electronic device configurations, and for the future of durable diodes. This paper provides a succinct description of the research that has been carried out so far.
2.3. Molecular capacitors
Capacitors store charge when connected to a power source. Few studies have been performed on the manufacture of molecular capacitors. Porphyrins are one group of molecules that are capable of storing charge [75]. Charges stored in a capacitor represent the 1 or 0 states. Hence, molecular memories can be developed using the concept of molecular capacitors. Usually, in order for charges to be stored, each memory element contains a monolayer of about 1 × 106 molecules [76]. It has been established that charges can be stored on molecules that exhibit redox behavior [77], [78]. Porphyrin-based elements could be used to write/read a memory cell, as depicted in Fig. 12 [78]. A detailed review on porphyrins has been done by Jurow et al. [79], in which they elucidate the importance of these molecules in the fabrication of molecular devices such as molecular capacitors. A new field of study includes the concept of supercapacitors, which help to store a large amount of electric charge within a short span of time [80]. Recent research carried out by Merlet et al. [81]resulted in the achievement of a clear depiction of how negative and positive ions are formed inside porous carbon; furthermore, a high capacitance of 125 F·g−1 was obtained.
 Fig. 12. (a) The charge storage molecule, where X represents the surface-attachment group; (b) the redox-based read/write process, where P represents porphyrin. (Reproduced from Ref. [78])
Fig. 12. (a) The charge storage molecule, where X represents the surface-attachment group; (b) the redox-based read/write process, where P represents porphyrin. (Reproduced from Ref. [78])Electrochemical double-layer capacitors (EDLCs) store the charge at the electrode/electrolyte interface [81], [82]. Because the capacitance directly depends on the surface area to store charge, a higher cross-sectional area and a smaller separation should be made possible. However, studies [83] have shown that the charge storage capacity also depends on the pore structure. Chen et al. [84] have performed the fabrication of a metal-insulator-molecule-metal (MIMM) device in which hafnium dioxide (HfO2) was used as a dielectric, deposited by atomic layer deposition (ALD). They found that the leakage current improved considerably after the implementation of HfO2. In addition, they have reported the charging and discharging of the molecular layer. The molecules used were the redox-active porphyrin monomer and a redox-active porphyrin-ferrocene combined monomer.
Developments have been achieved in cylindrical molecular capacitors with the use of CNTs [85]. Research carried out by Madani et al. [86] in 2017 has provided a theoretical framework for the study of cylindrical molecular capacitors by incorporating a single-walled boron nitride nanotube (SWBNNT) inside another SWBNNT. This created an inner positive charge distribution and an outer charge distribution, which could easily resemble capacitor action. The development of molecular capacitors requires further research to be performed on dielectrics and the dielectric constant. A foundation for this work was laid 60 years ago by Jansen [87], who implemented a theoretical quantum mechanical approach to the static dielectric constant. Coulomb blockade and Kondo effects should be explored further for trapping electrons and for their ultimate application as a molecular capacitor. Current understanding of the molecular mechanism of molecular capacitors is impoverished and requires further research.
2.4. Molecular insulators
Insulators play a major role in every scenario in which it is necessary to control or limit the flow of current through any electronic component or IC. When it comes to molecular insulators, many molecules with specific functional groups can be used. Aliphatic organic molecules are the best example of a molecular insulator in which only σ bonds are available, which causes disruption to the flow of current in the presence of an applied voltage [88]. The conductive pathcan easily be broken by inserting these molecules between the electrodes. Regarding band theories, certain classes of materials act as insulators due to electron–electron interactions; these are known as Mott insulators. Fabrizio and Tosatti [89] have examined the nature of these types of alternate non-magnetic insulator behavior using the Jahn–Teller model. Mayor et al. [90] have demonstrated the suppression of tunneling through both π-channels and σ-channels by destructive quantum interference. Novel research by Garner et al. [91] has depicted the suppression of tunneling through σ-channels by destructive quantum interference. These researchers selected a silicon-based molecule for their study and used the Landauer–Buttiker scattering formalism to model single-molecule junction conductance. Fig. 13 illustrates the basic concept of destructive quantum interference taken from their work [91].
 Fig. 13. (a,b) The transmission of an electron through an energy barrier in which transmission decreases exponentially with length, L. T(E, L) represents the transmission probability. (c) The transmission of an electron through the molecule and (d) the exponential decrease in the transmission probability with n repeated units. (e, f) The destructive quantum interference effect due to the contribution from π-channels and σ-channels. (Reproduced from Ref. [91])
Fig. 13. (a,b) The transmission of an electron through an energy barrier in which transmission decreases exponentially with length, L. T(E, L) represents the transmission probability. (c) The transmission of an electron through the molecule and (d) the exponential decrease in the transmission probability with n repeated units. (e, f) The destructive quantum interference effect due to the contribution from π-channels and σ-channels. (Reproduced from Ref. [91])Electrons can be trapped inside the defects of a material. A study by Meunier and Quirke [92] has explained the formation of space charge in a polyethylene molecule: Microscopic defects led to space charge. These researchers were able to establish a relation between the molecular properties of the material and the electron trap. Straight-chain hydrocarbons are good molecular insulators. Molecular semiconductor-doped insulator (MSDI) heterojunctions have been developed in order to obtain nonlinear current-voltage characteristics [93]. Since insulators cannot be avoided in the fabrication of electronic components, it will be necessary to discover and scrutinize more self-assembling controllable insulating molecules.
2.5. Molecular wires
Wires are essential for the interconnection of various electronic components over a substrate; thus, the concept of molecular wires is crucial when dealing with molecular electronics. Molecular wires can be classified into two main types: saturated chains and conjugated chains [94]. In saturated molecules, atoms are connected with single bonds. Alkanes are the simplest saturated hydrocarbons; however, they are considered to be poor conductors since their HLG is very large [94]. In conjugated molecules, the atoms are connected together by alternate single and double bonds. These molecules have smaller HLG gaps than alkanes and are efficient for the long-range transport of electrons [94]. Before the downscaling of electronic components, carbon and aluminum were mainly used for interconnections [95]. These were followed by the concept of quantum wires, which are conducting wires in which the transport mechanism is controlled by quantum effects.
CNTs are widely admissible materials for molecular quantum wires [96], and the astonishing properties of SWCNTs make them ideal applicants for molecular wire manufacture [72]. These can be applied in quantum information devices and photoluminescence measurements [97]. SWCNTs have a thickness of one atom, a circumference of a few tens of atoms, and a length of many microns [98]. Holmes et al. [99] have performed research into silicon nanowires, and were able to develop wires several microns long with a uniform diameter of 40–50 Å. Tans et al. [96] have experimentally proven that conduction through SWCNTs takes place through discreet electron states.
To improve the performance of nanoscale electronic devices, power dissipation should be minimal. Although studies related to phonon conduction provide some ideas about power dissipation [100], such studies are limited. A brief review on the properties of phonons in CNTs was published by Dresselhaus and Eklund [98] in 2000. Later, Zou and Balandin [101] suggested a model for phonon heat conduction through a semiconductor nanowire. They theorized a decrease in lattice thermal conductivity; this alteration in their findings paved the way for the manufacture of nanoscale devices. Not long afterward, in 2008, Mingo et al. [102] put forth the idea of calculating phonon conduction probabilities completely from first principles. They computed phonon transmission through single defects in SWCNT. In 2017, Krittayavathananon et al. [103] reported the application of acetaminophen, which is an electrochemical species, to improve the connection between a CNT and an electrode. This was achieved by applying a potential across the electrodes. Porphyrin molecules can also be used as molecular wires [104]. The current work of Algethami et al. [105] has shown that fused oligo-porphyrin nanowires can improve conductance through molecular-scale circuitry. Recent studies on templates to facilitate the synthesis of porphyrin-based molecular wires are garnering attention [106].
Molecular wires allow the transfer of signals between two terminals. They are intended to transfer the excitation energy or charge from one unit to the other [107]. Wagner et al. [108] have proposed a photonic wire that transfers excitation energy by having the input chromophore absorb a photon; this results in the emission of a photon by the output chromophore, with the two chromophores being on the either end of the lead. Similarly, Mirkin and Ratner [109] have demonstrated electronic molecular wires that can transfer holes or electrons across active sites [107]. It has been established [108], [109], [110] that the charge transfer that takes place across a molecular wire is the result of intramolecular electron transfer across the sites through the linking molecule.
3. Suitable molecules for molecular devices
The most important driver of the development of molecular electronics is the identification of suitable and available molecules. A number of molecules have already been studied and determined to be suitable for the preparation of molecular components [111], [112], [113], [114], [115]. Among these, hydrocarbons have been extensively selected as appropriate molecules [92], [116], [117].
Benzene can potentially be employed to model the interactions of aromatic π-systems [118]. Such systems generate a cloud of delocalized electrons by merging the π-electrons together, forming a circle that results in a resonant hybrid, which makes current conduction much easier [119]. However, the most widely used molecular families for molecular devices are oligo phenylene ethynylene (OPE) [120], [121], oligo phenylene vinylene (OPV) [122], and oligo thiophenes (OT) [123]. OPE derivatives are conjugated molecules with a rod-like shape [124], which can be used as molecular wires up to around 5 nm in length [125]. Research has been performed on thiolacetyle-terminated OPE on a gold surface [126]. Since thiol molecules are very reactive, acetyl groups are attached to protect the molecules from the reactive surroundings. Alkanethiols and arenethiols are extensively used in molecular studies in which they are positioned between gold (Au) electrodes [127], [128]. In addition, OPE can be functionalized internally and at the terminal points, which makes it perfect for application in molecular bridges and wires in donor-bridge-acceptor molecules [129]. OPV derivatives can also be used as molecular wires. It has been argued that OPV molecules are better suited for this purpose than OPE molecules because of their greater degree of planarity [130]. Table 1 provides a summary of the relevant properties of alkanes and the oligo families [131]. The HLG is higher for alkanes, lower for oligo groups, and lowest for OT, making the latter a better candidate for the manufacture of conducting wires, since a smaller HLG indicates greater conduction.
Table 1. A summary of the properties of different molecules.
| Molecule | Length (Å) | Eg (eV) | Ref. |
|---|---|---|---|
| 1,8-Octanedithiol (C8H18S2) | 12 | 7–8 | — |
| 1,10-Decanedithiol (C10H22S2) | 14 | 7–8 | — |
| 1,12-Dodecanedithiol (C12H26S2) | 17 | 7.11 | [131] |
| Oligo phenylene ethynylene (OPE) | 19 | 3.5 | [131] |
| Oligo phenylene vinylene (OPV) | 20 | 3.1 | [131] |
| Oligothiophene (OT) | 14 | 2.9 | — |
Length: the length of the chains made by the molecules: Eg: the HLG in electron volts.
As mentioned earlier, porphyrin molecules are well suited for molecular electronic applications, and particularly for information storage. A porphyrin molecule has 11 π bonds in its core, which facilitates high conduction [79]. The macrocycle associated with porphyrins such as phthalocyanins is excellent, with useful electrochemical properties and various applications in many scientific fields [132], [133]. Liu et al. [77] have demonstrated the possibility of porphyrin-based molecules attached to a Si(100) substrate exhibiting a redox reaction, resulting in storage functions. Saiki et al. [134] have observed capacitor-like electric behavior when a voltage is applied in β-DiCC[Ni(dmit)2], which is a charge transfer salt with redox activity. Here, (dmit) is 1,3-dithiol-2-thione-4,5-dithiolato and DiCC is 3,3′-dihexyloxacarbocyanine (Fig. 14) [134]. Rodríguez-Salcedo et al. [135] recently used the DFT approach on the same salt to examine its redox activity, and showed that the ionic form is more stable than the neutral form.
 Fig. 14. Structure of β-DiCC[Ni(dmit)2]. Red represents [Ni(dmit)2] and blue represents DiCC. (Reproduced from Ref. [134])
Fig. 14. Structure of β-DiCC[Ni(dmit)2]. Red represents [Ni(dmit)2] and blue represents DiCC. (Reproduced from Ref. [134])Another important molecule for the manufacture of molecular-based components is deoxyribonucleic acid (DNA). Many studies [116], [136], [137], [138], [139], [140] have been performed on DNA and have identified its potential application for molecular electronics.
The problem of fabricating contacts in a molecular circuit has always been troublesome. Zhou et al. [137] have developed a method of evaporating metals through a stencil mask in order to ensconce nanoscale contacts into an SWCNT. Using this method, they were able to minimize the damage caused to the contacts and other components by conventional methods such as electron-beam or optical lithography. The advent of techniques such as STM has made it relatively easy to approach single molecules. However, attaching molecular wires to contacts remains a laborious task. It is critical that contact points be conductive; otherwise, current will be lost at the terminals [115].