1. Introduction
Zeolites are natural or synthetic aluminosilicate materials which have versatile applications in petrochemical industry (Yue et al., 2018, He et al., 2021), biomedical industry (Lam & Rivera, 2006), chemicals and detergents (Wu et al., 2020) and water purification (Wang & Peng, 2010) due to their excellent ion exchange abilities, abundance in nature and low price (Mignoni et al., 2008, Mohamed et al., 2008, Reed and Breck, 1956). Historically, in 1756 the Swedish mineralogist Freiherr Cronstedt introduced the name zeolite which means a “stone of borax”. In 1862 the first synthetic zeolite was prepared by St. Claire-Deville (Querol et al., 2002). In 1948 Barrer concluded that a wide range of zeolites could be synthesized from aluminosilicate gels (Barrer, 1948), which opened the field of zeolite synthesis for further exploration and advancement from various alumina and silica sources. By 1958, Milton et al. had successfully synthesized nearly all the commercially important zeolites Including Zeolite A and Faujasite-type zeolites (Zeolite X, Y) (Milton, 1959).
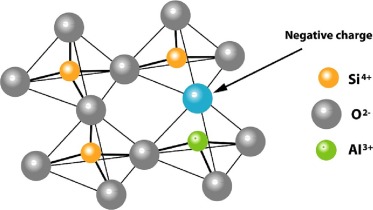
In zeolitic structures, the tetrahedra are linked together to form cages connected by pore openings of defined size; depending on the structural type, the pore sizes range from approximately 0.3–1 nm (Wilson, 2001). The negative charge on the lattice is neutralized by the positive charge of cations located within pores of the material (Fig. 1). An empirical formula representative of a zeolite can be expressed in the following way: M x/n [(AlO2)x (SiO2)y] · w H2O, where M is an extra-framework cation (alkaline or alkaline earth metal) of valence n, y/x represents the SiO2/Al2O3 ratio of the zeolite and w is the number of water molecules.
 Fig. 1. Structure of zeolite framework of tetrahedral [SiO4]4 with a Si/Al substitution ([AlO4]5) yielding a negative charge.
Fig. 1. Structure of zeolite framework of tetrahedral [SiO4]4 with a Si/Al substitution ([AlO4]5) yielding a negative charge.According to International Zeolite Association (IZA), there are 46 natural zeolite minerals, whereas over 250 synthetic zeolites have been prepared, and this number is on the increase (Baerlocher et al., 2007, Brouwer et al., 2020). The IZA Structure Commission publishes and updates the Atlas of Zeolite Structure Types, which allocates a three-letter designation to any known framework structure, regardless of composition. Low silica zeolites (Si/Al = 1–2), medium silica zeolites (Si/Al = 3–10), and high silica zeolites (Si/Al = >10) are the three types of zeolites classified by the International Zeolite Association (IZA) Structure Commission (Baerlocher et al., 2007).
This prospective study aims to summarize the state-of-the art technologiesapplied for zeolite synthesis from natural clay and secondary ash sources in the last decade and provide new insights into their future development. The manuscript consists of three main parts: Part (1) introduces the zeolite and zeolite framework structures briefly. Part (2) devotes for a detailed discussion on zeolite synthesis from natural clay sources and their applications. Part (3) focuses on different zeolite materials and their sorption performance synthesized with the application of waste ash as the precursor.
2. Current status
The global zeolite market is poised for significant growth in the coming years, driven by a variety of factors. According to a recent report by Markets and Markets (2023), the global zeolites market was valued at USD 12.1 billion in 2021. It is projected to reach USD 14.1 billion by 2026, with a compound annual growth rate (CAGR) of 3.1% between 2021 and 2026.
The increasing demand for zeolite-based catalysts in the petrochemicalindustry, growing awareness about the importance of clean water, and rising demand for zeolite-based fertilizers in agriculture are some of the key drivers of this growth. China dominates the global zeolite market, accounting for approximately 40% of the global market share, both in terms of production and consumption (Fig. 2) (Fortune Business Insights, 2023). The demand for zeolites in the detergents industry is expected to remain high, with zeolites being used as a substitute for phosphate, which is an environmentally harmful chemical. The petrochemicals industry is also a major consumer of zeolites, with the material being used as a catalyst in various processes. Additionally, the construction industry is expected to drive the demand for zeolites, with the material being used as a building material for its lightweight and insulating properties. Overall, the zeolite market is expected to experience steady growth over the next few years, driven by the increasing demand for the material in various industries and the dominance of key players such as China, United States, India, and Japan in the global market (Fortune Business Insights, 2023; Markets and Markets, 2023). The growth opportunities for market players in the global zeolite market are expected to be significant in the coming years, particularly with the increasing use of zeolite in the construction industry for applications such as lightweight concrete and insulation.
 Fig. 2. Global market share of synthetic zeolite by types and applications.
Fig. 2. Global market share of synthetic zeolite by types and applications.The commercial availability of zeolites is characterized by a diversity of suppliers worldwide, with variations in chemical composition, pore size, and shape. Notably, Clariant, a Swiss-based company, is recognized as one of the largest producers of zeolites globally, offering a wide range of products, including ZSM-5, beta, Y, CHA, and X. These zeolites have been employed in a plethora of applications, such as catalysts (Artillo et al., 2023, Rutkowska et al., 2017, Sobuś and Czekaj, 2021), adsorbents (Hong et al., 2020, Oshima et al., 2020), and membranes (Lee and Dutta, 2002). Zeolyst International, a joint venture between PQ Corporation and Sumitomo Chemical Company, and Honeywell UOP, a subsidiary of Honeywell International, are the major zeolite supplier specializing in manufacturing high-performance zeolite-based catalysts for the refining and petrochemical industries (Fortune Business Insights, 2023). Other notable suppliers of zeolites include BASF and Arkema. BASF offers a diverse range of zeolite products such as ZSM-5, beta, mordenite, and faujasite for various applications. Similarly, Arkema, a French chemical company, provides different zeolite-based products, including molecular sieves that utilize zeolite for desiccation purposes in insulating glass, spacer bars, and warm edge technology.
In addition to these significant suppliers, several smaller companies specialize in producing specific types of zeolites or customized zeolite-based solutions for particular applications. For instance, Zeochem, Albemarle Corporation, and Tosoh Corporation are some examples of such companies. These firms offer a unique product portfolio and tailor-made solutions for niche applications that require specialized zeolite properties.
3. Zeolite synthesis from natural clays and ash
Synthetic zeolites have received considerable attention since the synthesis allows excellent tailoring of the product for various engineering applications and scientific purposes as the products possess high purity (Buhl and Löns, 1996) uniform particle size, good selectivity and efficiency (Buhl and Löns, 1996) . Nevertheless, the preparation of synthetic zeolites from available alumina and silica sources is expensive because of the large energy and water requirement, raw material costs and long reaction times. Therefore, many studies have been performed to optimize the synthesis procedure and the characteristics of the final product from different raw materials including kaolinite (Bahgaat et al., 2020, Pereira et al., 2018), bentonite (Garshasbi et al., 2017; Srilai et al., 2020a), palygorskite (Youcef et al., 2020), diatomite (Garcia et al., 2016), coal fly ash (Cardoso et al., 2015, Musyoka et al., 2013, Zhang et al., 2020), and biomass ash (Fukasawa et al., 2018, Oliveira et al., 2019).
Zeolite synthesis is typically conducted by creating a super-saturated aluminosilicate solution, which undergoes dissolution, nucleation, and crystallization processes at high temperatures. This is referred to as an in-situ reaction at elevated temperature and pressure in an aqueous solution, as described by Musyoka et al. (2014). When natural clays or ash are used as precursors in the synthesis process, the first step is to extract aluminum and silicon from the raw material, followed by washing and drying the resulting product to remove impurities and organic matter. The next step involves mixing the precursor with an alkali solution, such as NaOH or KOH, at high temperatures to create a reactive aluminosilicate gel in an alkaline fusion or activation process. The alkaline solution dissolves the aluminum and silicon ions in the clay, forming a gel. Subsequently, the gel is subjected to hydrothermal treatment in an autoclave at high temperature and pressure, which facilitates the crystallization of the gel into a zeolite product. The resulting zeolite crystals’ size, shape, and purity can be influenced by the duration of the hydrothermal treatment and the composition of the gel. Finally, the synthesized zeolite undergoes washing, drying, and calcination processes to remove any residual organic matter and stabilize the structure of the zeolite.
Several limitations and factors should be considered when synthesizing zeolites from these precursors. The purification process, which can be time-consuming and costly, is a critical factor that impacts the feasibility of the synthesis method for commercial applications. The efficiency of the purification process and its impact on the final product should be carefully evaluated. Moreover, energy consumption is another significant concern, as the thermal treatment process required for zeolite synthesis demands a substantial amount of energy, which can result in higher production costs and environmental impacts. Another vital consideration is product purity, as impurities such as calcium, iron, or quartz in the raw kaolin can affect the quality of the final product. Therefore, evaluating the purity of the starting material and the effectiveness of any purification methods used is critical. Optimal conditions for zeolite synthesis from clays or ash as a source material require precise control of several factors, including temperature, pressure, reaction time, and solid/liquid ratio. It is worth noting that the optimal conditions may vary depending on the specific source material and desired product properties. As a result, optimization may be necessary to achieve the desired results.
Various synthesis methods have been developed for the preparation of zeolite A from natural clays. These methods include the conventional hydrothermal method, alkali-assisted and sonication-assisted hydrothermal methods, sol–gel method, and multi-step methods. The conventional hydrothermal method and fusion-assisted hydrothermal method have been widely utilized due to their simplicity and efficiency (Basaldella et al., 1993, Holmes et al., 2011). Essential factors in this method include the duration and temperature of calcination, the concentration of NaOH solution in hydrothermal treatment, and the time and temperature during the hydrothermal treatment step. Additionally, careful control of the mixture’s SiO2/Al2O3, Na2O/SiO2, and H2O/Al2O3 ratios is necessary for successful synthesis.
3.1. Synthesis of zeolite a using natural clays
The zeolite LTA (Linde Type A) was developped in the 1950 s by a team of researchers led by Donald W. Breck (Reed & Breck, 1956). The development of zeolite LTA was a major breakthrough in the field of zeolite chemistry and catalysis, as it was the first synthetic zeolite to exhibit high ion exchange capacity and selectivity. Zeolite LTA, also recognized as zeolite A [Na12(Al12Si12O48).27H2O], has a three-dimensional pore structure, which run perpendicular to each other in three dimensions, and it is made up of secondary building units 4, 6, 8, and 4–4 as an arrangement of β-cage [48 66] pseudo-corner share via 4–4 structure units showing α- cage [412 68 86] at the center of the unit cell (Musyoka et al., 2014; Srilai et al., 2020a). The zeolite A structure contains two types of cages, β and α, which can host different cations, and each cage contains water molecules or cation/anion pairs (Fig. 3) (Gerson & Zheng, 1997). This structure provides zeolite materials with high adsorption and ion exchange capacity (Barrer, 1979) making them suitable materials as molecular sieves and adsorbents in refrigeration systems, purification, and water softening (Farag & Zhang, 2012). Generally, the development of zeolite LTA was a significant achievement in the field of zeolite chemistry and paved the way for the synthesis and use of many other zeolite materials with unique properties and applications.
 Fig. 3. Features of the pores in zeolite A (Petrov & Michalev, 2012) SEM image of zeolite A reprinted from Ref (Belviso et al., 2015). Copyright (2015), with permission from Elsevier.
Fig. 3. Features of the pores in zeolite A (Petrov & Michalev, 2012) SEM image of zeolite A reprinted from Ref (Belviso et al., 2015). Copyright (2015), with permission from Elsevier.Kaolin clay is chemically represented as Al2Si2O3(OH)4 is an important material as a silica and alumina source for zeolite A synthesis. Many authors have used Kaolin as a natural source to synthesize zeolite. For example, a pioneer study reported the synthesis of highly crystalline zeolite A synthesis from kaolin, where the transformation of kaolin into reactive metakaolin was performed at 550 to 900 °C. The molar ratios SiO2/Al2O3, Na2O/SiO2, and H2O/Na2O were kept constant at 2.0, 2.5, and 40 during all experiments. The hydrothermal treatment was performed at 85 °C, for 2 to 8 h. The optimum calcination temperature, calcination time, and hydrothermal treatment time were reported as 900 °C, 1 h, and 8 h, respectively (Chandrasekhar, 1996). One of the first systematic studies focusing on the synthesis of Zeolite A from kaolin developed a 3-step process, where an acid treatment was performed between two alkaline treatments. Instead of calcination at high temperatures, kaolin was mixed with certain amount of NaOH and water which was followed by the suspension of the dry product in HCl solution in the second step. After the last alkali treatment where NaOH was applied, the mixture was refluxed with stirring at 212 to 214 °F for 3 h. The following conditions were reported to form good grade of Zeolite A: first alkali treatment: Na2O3/SiO2: 1.6 and H2O/Na2O: 77; second alkali treatment: Na2O3/SiO2: 2.3 and H2O/Na2O: 57. Although it is important that no precursor was used other than kaolin and high calcination temperatures were not needed, the lack of information about the purity, crystallinity, and performance of the obtained material are some factors reducing the impact of this study (Mason, 1962). Chen et al. (2000) conducted research on the process of kaolin calcination and the synthesis of 4A zeolite through the application of microwave energy (Chen et al., 2000). Their investigation demonstrated significant enhancements in the crystallinity, particle size distribution, and Ca exchange capacity of 4A zeolite. Moreover, the activation energy for nucleation and crystal formation was significantly reduced. The utilization of microwave radiation stimulated the formation of new phases and accelerated the crystallization rate.
Another study presented an effective method for zeolite A synthesis using kaolin as the main source of SiO2 and Al2O3 (Bessa et al., 2017). The synthesis process started with the conversion of kaolin to metakaolin at 600 °C for 2 h. The zeolite A was synthesized via hydrothermal method by mixing metakaolin with NaOH solution. The mixture was transferred into a Teflon-lined stainless-steel autoclave where the crystallization occurred via thermal treatment under autogenous pressure and static conditions. The product was washed several times with distilled water until constant pH and dried at 80 °C overnight. It was reported that with increasing the NaOH concentration of solution, the amount of moisture in the material increased until it achieved an ideal concentration of 5.0 M, at which point the moisture content of the zeolite attained a constant value. This substance was utilized for water softening, with removal rates of around 97% in the initial application sessions. Ayele et al. synthesized zeolite A using both raw and purified kaolin via hydrothermal and alkali fusion methods for effective the removal Cr(III) from tannery wastewater (Ayele et al., 2018). They obtained a material with a maximum removal of 99.8% and achieved an adsorbent dosage of 100 g/L. Moreover, maximum adsorption occurred during the first 3 h of adsorption, as revealed by the kinetics study, and the experimental results best fit pseudo-second order kinetics. In another study, the synthesis of zeolite A using raw and purified Ethiopian kaolin was conducted (Ayele et al., 2015). After the mechanical purification of raw kaolin for the removal of quartz and iron, the purified product underwent calcination at 600 °C for 3 h. The final step of the synthesis was mixing of kaolin with NaOH solution for the gel formation and hydrothermal treatment. It was demonstrated that the zeolite A with a SiO2/Al2O3 ratio of 2.04 and Na2O/SiO2ratio of 0.44 had a crystallinity of 90% with a solid/liquid ratio of 1.25 g/25 ml and a reaction time of 3 h. The synthesized Zeolite A exhibited a high thermal stability and cation exchange capacity of 295 mg CaCO3/g. This study shows the potential for synthesizing zeolite A using both raw and purified kaolin as a source material for effective removal of contaminants from wastewater.
In another significant work, zeolite A was synthesized using red and white kaolin sourced from Brazil with no modifications (Pereira et al., 2018). Firstly, metakaolin was prepared via the calcination of kaolin. Afterwards, metakaolin was mixed with 5 M NaOH solution which has a NaOH: metakaolin molar ratio of 8:1. The mixture underwent hydrothermal treatment to form Zeolite A crystals under magnetic stirring. The resulting product was washed with distilled water several times and dried at 110 °C. The cation exchange capacity and specific surface area of synthesized zeolite A were 8 meq/100 g and 62 m2/g, respectively. The zeolites prepared under the optimal conditions were used as adsorbents in the removal of methylene blue, safranine, and malachitegreen from aqueous solutions. The zeolites achieved an adsorption uptake of 0.96 mg/g in short periods of time (1 to 5 min). The equilibrium study revealed that zeolites had a better capacity for malachite green adsorption (55.0 mg/g) than the other two cationic dyes. This study has several strengths, such as the use of raw kaolin without any purification process, and the short time required for adsorption. However, the long duration of calcination (12 h) and hydrothermal treatment (24 h) are some of the factors impeding the scale up and commercialization of the method. Additionally, the study only focused on the removal of cationic dyes and did not explore the potential of synthesized zeolite A for other applications such as catalysis or gas adsorption. Finally, the study did not report the regeneration and reusability of zeolite A, which is an essential aspect to consider when evaluating the potential of the material for practical applications.
Wang et al. developed a novel hydrothermal approach for the synthesis of zeolite A from natural kaolin in three steps (Wang et al., 2014). (1) 3 g of natural kaolin was activated in a 4 M NaOH solution under vigorous stirring. The mixture was put in a stainless-steel autoclave for 3 h at 240 °C for crystallization. The material was then filtered, washed with deionized water, and dried at 90 °C. (2) The dry material was dispersed in water for slurry generation, and subsequently mixed with 60 ml of HCl (0.2 M) for 30 min at room temperature under continuous stirring. (3) The final step was to modify the pH of the filtered solution to 7 by stirring it with NaOH. This procedure resulted in the precipitation of a white gel, which was filtered out. The filtered gel was dissolved in 1.0 M NaOH solution for further hydrothermal crystallization at 90 °C in a sealed Teflon-lined stainless steel autoclave for 3 days. After hydrothermal crystallization, the solid product, was filtered out, washed with deionized water, and dried at 90 °C overnight. The synthesized zeolite A with this innovative approach has more uniform particle sizes and morphologies, better brightness, and higher cation-exchange capacity (299 mg CaCO3/g) than its traditional calcination equivalent (244 mg CaCO3/g). The hydrothermal approach for synthesizing zeolite A from natural kaolin offers several advantages. Firstly, it is a more energy-efficient and environmentally-friendly alternative to the traditional calcination method, which requires high temperatures and consumes significant amounts of energy. Secondly, this approach results in a zeolite A product with more uniform particle sizes and morphologies, better brightness, and higher cation-exchange capacity than its calcination equivalent.
In the industrial market, Na-A zeolite is in high demand, particularly for process stream dehydration. An alternative renewable fuel of Na-A zeolite is utilized in the bioethanol sector to dry the ethanol water combination (>99.3%) for use in cars. As a result of its high adsorption capacity for water molecules, zeolite type Na-A has attracted interest of several researchers. One such study investigated the synthesis of zeolite Na-A using kaolin via hydrothermal treatment method (Abdullah et al., 2016). After the calcination of kaolin at 800 °C for 5 h, the resultant metakaolin was treated with NaOH. The mixture was homogeneously mixed with a rotary evaporator set to 100 rpm and 70 °C and it was transferred to a bottle after 2 h of reaction. Then, it was placed in a Teflon container and went through crystallization inside an incubator at 60 °C overnight. Finally, the mixture was filtered and washed with distilled water until pH 7 to eliminate any unreacted NaOH and dried in an oven at 105 °C for 12 h. The XRD analysis showed that the synthesized zeolite Na-A had a high purity and a high water adsorption capacity of 70 mg H2O/g adsorbent, which was nearly twice as high as commercial zeolite 3A adsorption capacity. The high-water adsorption capacity of the synthesized zeolite Na-A suggests its potential for use in applications such as water treatment, moisture control, and dehydration. However, the method involved a high temperature calcination step, which can consume significant amounts of energy and lead to environmental impacts.
In a recent study, a green, highly efficient, and commercially potential Quasi-Solid-Phase (QSP) activation method was developed to activate the natural kaolin mineral to prepare different zeolites including Zeolite A (Yang et al., 2017). The first step in the synthesis procedure was the QSP activation of kaolin and thermal activation of diatomite. Certain amount of raw kaolin was kneaded with NaOH pellets (Mix-KN), which subsequently kneaded further with water (Mix-KNH) to have a molar ratio of 10:1:3 representing kaolin: NaOH: H2O. After the extrusion of the kneaded mixture, it was heated at 100 °C for 30 min, followed by crushing of the activated material (QSP-T-R) (Fig. 4). The synthesis of Na-A zeolite was performed as following: 5 g of QSP-10–3 (QSP-Temperature-H2O/kaolin) was mixed with 48 g of DI water, and it was aged at 50 °C for 8 h. After ageing, the mixture underwent hydrothermal treatment in a stainless-steel autoclave 95 °C for 2.5 h. The product (QSP-A) was filtered, washed with DI water, and dried at 100 °C overnight. One of the significant advantages of the QSP activation method is that it achieved a depolymerization rate of 84.7% for Si and 69% for Al through the application of mechanical forces during the kneading and extrusion steps. This high depolymerization rate indicates that the QSP method can effectively break down the kaolin mineral structure, making it more amenable to zeolite synthesis. This method can potentially be extended to the synthesis of other types of zeolites, opening possibilities for diverse applications in catalysis, adsorption, and separation processes.
 Fig. 4. Schematic Illustration of Quasi-Solid-Phase Activation Process. Reprinted from ref (Yang et al., 2017). Copyright (2017), with permission from American Chemical Society.
Fig. 4. Schematic Illustration of Quasi-Solid-Phase Activation Process. Reprinted from ref (Yang et al., 2017). Copyright (2017), with permission from American Chemical Society.Palygorskite (PAL) is a natural mineral with a high SiO2 content in addition to Al2O3, Fe2O3, CaO and oxides of other metals. Despite its high silica content, impurities in the structure hamper the zeolite synthesis from PAL. To address this problem, Jiang et al. applied acidic dissolution prior to alkaline treatment to remove the impurities in PAL (Jiang et al., 2012). 5 g of PAL was soaked in a HCl solution with various concentrations at 80 °C. After the filtration, washing and drying, the newly formed PAL was reacted with 5 M NaOH and NaAlO2 solution (3.12 g NaAlO2 in 20 g H2O) in a stainless-steel autoclave for the hydrothermal treatment at 80 °C for various times. The optimum HCl concentration in the acidic dissolution and crystallization time were demonstrated as 4 M and 5 h. Zeolite A prepared under these conditions had a crystal size of 2 µm, while its calcium binding capacity (CBC) was reported as 318 meq/100 g. In addition, the influence of SiO2/Al2O3 molar ratio was investigated by the addition of different amount of NaAlO2. The results indicated that zeolite A could be synthesized in a SiO2/Al2O3 ratio of 1.53 – 3.05 with similar CBC. This method has several advantages, including improved purity, tunability of the SiO2/Al2O3 ratio, and high calcium binding capacity. By applying acidic dissolution prior to alkaline treatment, impurities in PAL are removed, resulting in a purer product. The SiO2/Al2O3 ratio can be adjusted by varying the amount of NaAlO2 added, providing flexibility in the synthesis process. However, there are also some potential disadvantages to this method, including long processing times, high reagent costs, high temperature and pressure requirements.
Another recent study utilized Algerian palyogorskite to synthesize zeolite LTA with a two-step synthesis route (Youcef et al., 2020). The investigated five parameters were as following: (1) Acid concentration in the acid activation, (2) NaOH concentration in hydrothermal treatment, (3) NaAl2O3 amount, (4) nucleation time, and (5) crystallization time. After the acid activation in HCl solution at 85 °C for 48 h, the powder was mixed with NaOH solution (1, 2, 3, 4 and 5 M). Then, NaAl2O3 solutions with different concentrations were added as the Al source. After the nucleation, the gel solutions went through crystallization in a closed reaction system at 90 °C. The best synthesis conditions were reported as 4 M HCl, 3 M NaOH, 3 g NaAl2O3 (in 20 ml H2O), 3 h, and 24 h as parameters (1), (2), (3), (4), and (5), respectively. The quantitative analysis showed that the product synthesized under these conditions had a zeolite A phase of 98.43%. This method offers flexibility in controlling the synthesis parameters, which allows for the customization of the final product for specific applications. However, there are also some potential disadvantages to this method, such as the long nucleation (3 h) and crystallization steps (24 h). Another limitation is the use of high concentrations of HCl and NaOH, which may lead to safety concerns and higher reagent costs. The use of closed reaction systems at high temperature and pressure also requires specialized equipment, which may add to the cost of the synthesis.
Ma et al., (2010) performed zeolite A synthesis from bentonite via alkali fusion-assisted hydrothermal method (Ma et al., 2010). A mixture of Al(OH)3, Na2CO3and bentonite was fused at 830 °C for 3 h. After the addition of H2O, the mixture was placed in a closed reaction system for hydrothermal treatment at 90 °C for 6 h. To determine the effects of SiO2/Al2O3 and Na2O/SiO2, the composition of raw materials was varied throughout the synthesis process. The highest crystallinity and calcium exchange capacity for zeolite A was reported as 82.7% and 302 mg CaCO3/g, which was prepared with the molar ratios of SiO2/Al2O3, Na2O/SiO2 and H2O/Na2O 1.5, 2.0 and 50, respectively. The advantages of this method include its simplicity and low cost compared to other methods of zeolite synthesis. Moreover, the synthesis method allows for control over the composition of the starting materials, which can be tailored to obtain the desired properties in the final product. However, the high-temperature fusion step may require specialized equipment and pose safety risks. The use of Na2CO3 may also introduce impurities into the synthesis process, which can affect the purity and properties of the final product. Additionally, the low crystallinity and calcium exchange capacity of the zeolite A product (82.7% and 302 mg CaCO3/g, respectively) obtained in this study may not be sufficient for certain applications.
Srilai et al. useda two-step hydrothermal method for zeolite A synthesis using bentonite as raw material (Srilai et al., 2020a). The purpose of the first step was to form hydrous sodium aluminosilicate, where bentonite was mixed with an 8 M NaOH solution. Afterwards, the mixture underwent hydrothermal reaction in a stainless steel autoclave at 200 °C for 3 h, followed by filtration, washing and drying. The solid product was mixed with 1 M HCl solution and stirred for 30 min at room temperature, then it was washed with DI water, filtered, and dried. In the second step, the dry solid went through another hydrothermal treatment at 90 °C for 72 h with the addition of different alkali reagents including NaOH, KOH, Na2CO3 and K2CO3 solutions of 1 M concentration. While the best alkali reagent was reported as NaOH, the relative crystallinity of the Zeolite A prepared with 1 M NaOH was reported as 91.11%. The limitation of this method is that the first step involves a high-concentration NaOH fusion, which may cause safety concerns and increase the cost of the synthesis process. Additionally, the long duration of the second hydrothermal treatment increases the synthesis time and energy consumption. Finally, the method does not provide information for the calcium binding capacity of the synthesized zeolite A, which is an important parameter for many applications.
The use of Zeolite has gained significant attention for its high potential in various industrial and scientific applications over the past two decades. This is due to its well-defined structure that includes channels and cages, enabling it to filter and adsorb molecules selectively. Due to the negatively charged framework, it exhibits a high cation exchange capacity, rendering it a valuable material for various industrial and scientific applications such as water softening (Ayele et al., 2018), membrane separation (Chen et al., 2021, Sen et al., 2018), adsorption (Djeffal et al., 2017), ion exchange (Ayele et al., 2018; Mosai, 2021, Mosai et al., 2019), and catalysis (Đặng et al., 2017; Yang et al., 2017). The Si/Al ratio of 1 has been established as the optimum condition for producing zeolite A. Moreover, the unique pore structure and size of Zeolite A make it particularly suitable for the adsorption of small molecules such as water, carbon dioxide, and nitrogen. It also exhibits excellent thermal and chemical stability, which enables its use in harsh environments. While the method of synthesizing zeolite A (Fig. 5) using natural clays as a source material has several advantages, such as the abundance and low precursor price, high cost and energy consumption due to increased calcination temperature, and risk of greenhouse gas emissions, difficulty in the scale up of the small-scale experiments are some of the challenging topics need to be considered in the optimization of the process. Table 1 summarizes the relevant parameters for synthesizing zeolite A from different natural clays.
 Fig. 5. Experimental flow chart of Zeolite A synthesis.
Fig. 5. Experimental flow chart of Zeolite A synthesis.Table 1. Synthesis of zeolite A from different natural clays.
|
Raw material |
Synthesis conditions |
Product |
Ref. |
||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2/ Al2O3 | Initial Treatment | Contents | Ageing | Heating | |||||
| T(°C) | Time (h) | T(°C) | Time (h) | ||||||
| Kaolin | – |
44 g kaolin + 48 g NaOH were calcined at 214 °F for 19 h. Product (20 g) + H2O (63 g) + HCl (35 g HCl/ 70 g H2O) |
Product (20 g of starting material) + 5 g of HCl + 313 g H2O + (39 g NaOH in 115 g H2O) | – | – | 214 °F | 3 | Zeolite 4A | (Mason, 1962) |
| Kaolin | – | Calcination of kaolin at 900 °C for 1 h. | Metakaolin was mixed with NaOH for molar ratios SiO2/Al2O3:2, Na2O/SiO2:2.5, and H2O/Na2O: 40 | – | – | 85 | 8 | Zeolite 4A | (Chandrasekhar, 1996) |
| Kaolin | – | Calcination at 600 °C for 2 h. | 1.0 g of metakaolin + 12 ml of NaOH 2.8 mol.L-1 | – | – | 100 | 4 | Zeolite A | (Bessa et al., 2017) |
| Kaolin | 1.6 | 1.25 g of raw kaolin + 1.5 g of NaOH calcined at 600 °C for 1 h. | Calcined product + 12.5 ml water. | 50 | 1 | 100 | 3 | Zeolite A | (Ayele et al., 2018) |
| Kaolin | – | Calcination at 750 °C for 3 h. | Calcined kaolin + 3 M NaOH | 60 | 2 | 90 | 5 | Zeolite A | (Rahman et al., 2019) |
| Kaolin | 1.6 |
1) Purification of kaolin by separation, sedimentation, ultrasonic suspension, and magnetic separation. 2) Calcination of kaolin at 600 °C for 3 h. |
Treated kaolin + 3 M NaOH. | 25 |
3 |
50 | 3 | Zeolite A |
(Chebude & Díaz, 2015) |
| Kaolin | 1.27 | Calcination at 600 °C for 12 h. | Metakaolin + 5 M of NaOH solution (molar ratio of 8:1). | – | – | 80 | 24 | Zeolite A | (Pereira et al., 2018) |
| Kaolin | – | Calcination at 800 °C for 5 h. | 1 g metakaolin + 13.3 ml of 2.5 M NaOH. | 25 | 2 | 60 | 12 | Na-A | (Abdullah et al., 2016) |
|
Kaolin |
1.4 |
1) 3 g of activated kaolin + 16 ml of NaOH 4 M. 2) The mixture was heated for 3 h at 240 °C. 3) Product was mixed with water 60 ml of HCl (0.2 M) for 0.5 h. |
The formed gel was dissolved in 1 M of NaOH solution and placed in a sealed Teflon-lined stainless steel autoclave | – | – | 90 | 72 | Zeolite A | (J. Q. Wang et al., 2014) |
| Kaolin | – |
1) 50 g kaolin + 75 g of NaOH + 10.2 ml of H2O. 2) Heating at 100 °C for 30 min |
5 g treated kaolin with 48 g of H2O. | 50 | 8 | 95 | 2.5 | Na-A | (J. Yang et al., 2017) |
| Kaolin | 1.6 | The raw kaolin was purified by separation, sedimentation, ultrasonic suspension, and magnetic separation. |
1) 1.25 g of kaolin was dry-mixed with 1.5 g NaOH and calcined at 600 °C for 1 h. 2) The calcined product was ground and mixed with 12.5 ml of water. |
50 | 1 | 100 | 3 | Zeolite A | (Ayele et al., 2018) |
| Bentonite | 2.44 | The bentonite washed with deionized water. Particles < 1 μm were separated from clay slurry by homogeneous dispersion and gravity separation. The mixture was sonicated. | The sonicated bentonite without any addition. | 25 | 24 | 100 | 24 | Zeolite A | (Sen et al., 2018) |
| Bentonite | 4.45 | Bentonite was mixed with Al(OH)3, Na2CO3 and reacted at 830 °C for 3 h. | Activated bentonite and water | – | – | 90 | 6 | Zeolite A | (Ma et al., 2010) |
|
Bentonite |
4.70 |
1) The bentonite mixed with 8 M NaOH and heated at 200 °C for 3 h. The mixture washed with H2O and dried. 2) The product was mixed with 1 M of HCl solution and stirred for 30 min. |
The formed gel was mixed with 1 M NaOH. |
– | – | 90 | 72 | Zeolite A |
(Srilai et al., 2020a) |
| Palygorskite | 8.50 | 5 g of PAL and 3 M of HCl (5 g/20 ml) at 80 °C for 48 h. | Treated PAL with 20 ml NaOH (5 M) and NaAlO2 | – | – | 80 | 5 | Zeolite A | (Jiang et al., 2012) |
| Palygorskite | 4.50 | The PAL was treated with 4 M HCl solution (50 g.L−1) for 48 h at 85 °C. | PAL with3 M NaOH solution (1 g.20 ml−1), 3 g of NaAl2O3 and 20 ml H2O. | 25 | 3 | 90 | 24 | Zeolite A | (Youcef et al., 2020) |
3.2. Zeolite Y syntheses from natural clays
Zeolite Y is a type of zeolite with a faujasite structure, which is characterized by having a SiO2/Al2O3 molar ratio>3. The basic structural units for this type of zeolite are sodalite cages linked with double 6-rings resulting in a super cage with 12 rings pore opening (Wilson et al., 1982) with a free diameter of 7.4 Å (Bhatia, 2020). These structural features of Zeolite Y make it a highly versatile and efficient adsorbent material with a wide range of applications in various fields, including catalysis, separation, and environmental remediation.
It has been widely applied as one of the most efficient catalysts in several petrochemical processes including catalytic cracking of heavy petroleum molecules into gasoline-range hydrocarbons (Yang et al., 2017), isomerization, aromatization due to its large micropores (0.73 nm), high surface area, active acid sites and high selectivity (Choo et al., 2022) and CO2 adsorption (Djeffal et al., 2017). Some of the most frequently utilized precursors for Zeolite Y synthesis are: diatomite (Garcia et al., 2016), bentonite (Hashemi et al., 2019), kaolin (Ajayi et al., 2018, Bahgaat et al., 2020), fly ash (Ren et al., 2020), and construction and demolition industry wastes (Hernández-Palomares and Espejel-Ayala, 2022).
Diatomite is a desirable raw material for zeolite synthesis because of its high silica concentration and availability in bulk quantities at a moderate cost ( El Ouardi et al., 2020). In addition, it does not require any extra heat treatment or silica source for the synthesis of FAU-type zeolites due to its amorphous structure (Zhang et al., 2016). However, an extra alumina source is usually required to achieve the requisite Si/Al ratio for the synthesis. An extensive study investigated zeolite Y synthesis from diatomite using a chemical pretreatmentwhere the mineral was mixed with H2SO4 solution for contaminant removal (Garcia et al., 2016). The acid-treated diatomite was combined with Al2(SO4)3 to modify the SiO2/Al2O3 molar ratio, and the mixture was aged for 24 h in NaOH solutions of varying concentrations. The synthesis solutions had the following molar ratios: Na2O/SiO2 = 2, SiO2/Al2O3 = 11, and H2O/Na2O = 40. After ageing, the mixture was transported to a stainless-steel autoclave at 373 K for various time durations. The final solid product was filtered and washed with distilled water until pH 9, then dried overnight at 100 °C. As a result, highly pure zeolite Y with a SiO2/Al2O3 ratio of 5.3, a Na2O/SiO2 ratio of 0.6 was synthesized, despite the low reaction yield. The study found that diatomite facilitated the synthesis of high silica zeolite Y, behaving similarly to colloidal silica in classical synthesis. This is likely due to high degree of polymerization in both sources of silica. Interestingly, the presence of minerals and clays in the raw diatomite had little impact on the final material. Despite the low cost and high purity of zeolite product, the use of H2SO4 could pose environmental concerns, while the long aging and heating steps might hamper the commercialization of the method.
Another study used a template-free synthesis method for the preparation of zeolite Y using diatomite as precursor (Zhang et al., 2016). The initial stage was to dissolve NaOH in distilled water, followed by the addition of sodium meta-aluminate and diatomite under continuous stirring. The final mixture had a molar composition of 7.01 SiO2, 1.00 Al2O3, 10.05 Na2O, and 397.81 H2O. After 30 min of stirring and 16 h of aging at 40 °C, the mixture was subjected to hydrothermal treatment. The solids were washed using deionized water and dried overnight at 100 °C. The obtained zeolite was modified by dimethylglyoxime (DMG) and used for the removal of Ni(II) from aqueous solution. According to the characterization investigations, DMG molecules were adsorbed on the exterior and interior surfaces of zeolite Y, and the channels and cavities of the zeolite should be occupied by DMG throughout the modification process. Ni(II) is removed from the zeolite Y modified by DMG (Y-DMG) sample by ion exchange and complexation reactions. Adsorption studies revealed that Y-DMG had a high capacity for Ni(II) adsorption in aqueous solutions, with a maximum capacity of 71.33 mg/g at 25 °C. The template-free method presents a simple and eco-friendly approach for the synthesis of modified zeolite Y with excellent adsorption properties, but the resulting material may not be suitable for catalytic applications due to impurities. In contrast, the method presented by Garcia et al. resulted in highly pure zeolite Y with a desirable SiO2/Al2O3ratio, and the material was used for catalytic applications. However, the use of strong acid for pretreatment could in that study pose environmental and safety concerns. In summary, both studies demonstrate the potential of diatomite as a precursor for the synthesis of zeolite Y with different methods and purposes. The choice of method depends on the desired application and the specific requirements of the resulting material.
Bentonite was utilized as precursor for the synthesis of Zeolite Y where it was dissolved in concentrated HCl acid (30% v/v, 4 ml acid/1 g bentonite). Then, the mixture was reacted with NaOH solution with a ratio of 1:1.2. NaAlO2 was utilized as additional aluminum source to obtain the SiO2/Al2O3 ratio of 3.0–6.0 in the reaction medium. After the activation at 300–320 °C, the mixture was dispersed in deionized water for 3 h under stirring. Finally, it went through aging for the formation of gel slurry and crystallization for 24 h. Zeolite P was reported as the competitive phase and it was present in most of the products. Pure and highly crystalline Zeolite Y had a surface area of 486.1 m2g−1, and it was obtained under optimum conditions for crystallization temperature (97 °C), NaOH concentration (3 N), ageing time (20 h) (Faghihian and Godazandeha, 2009). Hashemi et al. synthesized zeolite Y from bentonite for the first time under air pressure, without using an autoclave (Hashemi et al., 2019). The reaction began with the treatment of bentonite in an oil with HCl. The mixture was then filtered, rinsed with distilled water, and dried in a 100 °C oven. The acid-treated bentonite was subsequently homogenized with NaOH and sodium aluminate and underwent heat treatment for 1.5 h at 310 °C. The mixture was dispersed in distilled water and agitated for 1 h before ageing for 24 h in a round-bottom flask at 97 °C. The synthesized zeolite Y was modified with hexadecyltrimethyl ammonium bromide CTAB surfactant at various loading amounts to remove organic pollutants from olefin plant wastewater. The results showed that the sample adjusted by 50% of the CEC loading amount was the most effective, removing 89% of the organic material from the industrial effluent (30.87 mg.g−1). This method offers a simple process for zeolite Y synthesis that can be performed under air pressure, without an autoclave, making the process more accessible and inexpensive than traditional methods which require high-pressure and high-temperature conditions.
The alkali hydrothermal synthesis method for the synthesis of zeolite A cannot be directly applied to the crystallization of zeolite Y. This is due to its substantially greater SiO2/Al2O3 ratio than kaolinite; therefore, it requires additional SiO2 source (Wang et al., 2016). An innovative hydrothermal alkaline activation method was developed for the synthesis of zeolite Y from kaolin, where the obstacles of high temperature required for kaolin activation and acid insoluble aluminosilicates were addressed successfully (Wang et al., 2016). In this method, kaolin and quartz were applied for the preparation of hydroxycancrinite, which was easily dissolved in HCl solution. Then, the mixture was neutralized with alkali solution and the formed aluminosilicate gel went through crystallization. The three steps of the synthesis are illustrated in Fig. 6 and these steps are as following: (1) kaolin, quartz, and NaOH aqueous solutions were combined under vigorous stirring. Then, the mixture was heated for 8 h at 240 °C in a closed Teflon-lined stainless steel autoclave. Filtration was used to separate the solid materials from the liquid mixture. (2) The solid ingredients from the first stage were mixed with water and vigorously stirred to produce slurries, which were then dissolved in HCl for 10 min. After filtering out any insoluble contaminant, a clear solution was obtained. (3) The resulting transparent solution was neutralized by precipitation of a white gel with the recycled alkali liquid from the first step. The zeolite Y products were filtered out after hydrothermal crystallization, washed with deionized water, and dried at 90 °C overnight. This study paved a new path for the synthesis of various mesoporous and macroporous materials utilizing low-cost natural kaolin and quartz instead of the traditional process that requires expensive chemical reagents.
 Fig. 6. Flow chart summarizing the steps for the synthesis of zeolite Y (Wang et al., 2016).
Fig. 6. Flow chart summarizing the steps for the synthesis of zeolite Y (Wang et al., 2016).In a recent study, Egyptian kaolin was utilized for the synthesis of zeolite Y without the use of any additional source of alumina or silica (Bahgaat et al., 2020). Kaolin was initially calcined to form reactive metakaolin via the elimination of the undesirable volatile compounds. Newly generated metakaolin was then mixed with a NaOH solution with a concentration of 1.0 M (10 g/100 ml). The solution was stirred at 800 rpm for 24 h and subsequently crystalized at 90 °C for two days. Finally, the resultant zeolite Y was rinsed and centrifuged with deionized water several times and dried overnight at 100 °C. As a result, the authors reported that the crystallinity of the zeolite Y increased with increasing crystallization time at 48 h at 90 °C. The zeolite Y produced was of exceptional quality in shape and size, with a CEC of 401.09 meq/100g. Li et al. synthesized zeolite Y using an alkali solution aided activated kaolin as the primary source of aluminosilicates (Li et al., 2017). The resulting product had a high zeolite content, making it highly desirable for fluid catalytic cracking catalysts. This activation and synthesis process can also be applied to natural clays such as rectorite, sepiolite, and montmorillonite, making it a versatile method for synthesizing various types of zeolites. In another study, Chandrasekhar and Pramada synthesized the zeolite Y through a hydrothermal process using kaolin as a starting material (Chandrasekhar & Pramada, 2004). The study highlights the potential of kaolin-based zeolite Y as a low-cost and abundant precursor for the production of cordierite ceramics, with potential applications in various industrial sectors.
Zeolite Y is an important active component in the catalytic cracking, hydrocracking, and isomerization processes thanks to its homogeneous cavities, high activity, and good stability. One of the most important factors that influence zeolite synthesis, thus determining the characteristics of the zeolite product, is the raw material used in the synthesis. Several investigations have found that natural clays such as bentonite, diatomite, and kaolin are suitable sources for zeolite synthesis. Among these sources, kaolin is one of the most frequently used materials because of its availability. The use of kaolin as a raw material in zeolite synthesis represents a significant breakthrough in terms of reducing pollution caused by chemical reagents and the difficulties associated with their subsequent treatment.In general, there are several synthesis methods for preparing zeolite Y, including hydrothermal synthesis, sol–gel synthesis and template-assisted synthesis. Hydrothermal synthesis is the most widely used method for synthesizing zeolite Y. In this method, a mixture of alumina, silica, and sodium hydroxide is heated in water under high pressure and temperature. The advantages of this method include its simplicity, high yield, and low cost. However, it requires high-energy consumption, and harsh reaction conditions, which can have a negative impact on the environment. Additionally, hydrothermal synthesis of zeolites can take a long time due to the need for slow and controlled nucleation and crystal growth. Sol-gel synthesis involves the hydrolysis and condensation of metal alkoxides in the presence of water and a surfactant. This method has the advantage of producing a homogeneous and pure product with a high degree of crystallinity. However, it is more expensive than hydrothermal synthesis and requires a longer reaction time. Template-assisted synthesis involves the use of organic templates to guide the crystal growth of zeolite Y. Although this method can produce zeolite Y with a high degree of crystallinity and specific morphology, the removal of organic templates can be difficult, resulting in product contamination. The cost of this method is also higher than hydrothermal synthesis. In terms of the quality of zeolite Y produced, hydrothermal synthesis and sol–gel synthesis are both capable of producing high-quality products with a high degree of crystallinity and specific surface area. Nevertheless, template-assisted synthesis may produce zeolite Y with a more uniform morphology, which may be advantageous in certain applications. The environmental impact of each synthesis method is also an important consideration. Hydrothermal synthesis and sol–gel synthesis both require the use of high temperatures and pressure, which can have a negative impact on the environment. Template-assisted synthesis involves the use of organic templates, which can be difficult to remove and may result in contamination of the product. The cost of each method may also vary depending on the specific conditions used in the synthesis process. The choice of synthesis method ultimately depends on the specific application and the desired properties of the zeolite Y product. The different parameters used for synthesizing zeolite Y using natural clays have been tabulated in Table 2.
Table 2. Synthesis of zeolite Y from different natural clays.
|
Raw material |
Synthesis conditions |
Product |
Ref. |
||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2/ Al2O3 | Initial Treatment | Contents | Ageing | Heating | |||||
| T(°C) | Time (h) | T(°C) | Time (h) | ||||||
| Bentonite | Bentonite was reacted with HCl for 8 h at 95 °C. | The residue was mixed with NaOH solution (1:1.2) and NaAlO2 was added for a SiO2/Al2O3 molar ratio of 3–6. Activation at 300–320 °C. | 25 | 20 | 97 | 24 | Zeolite Y | (Faghihian and Godazandeha, 2009) | |
| Diatomite | 16.4 | Diatomite was crushed and treated with 6 M H2SO4 at 100 °C for 24 h. | Treated diatomite was mixed with Al2(SO4)3 and NaOH. | 25 | 24 | 100 | 48 | Zeolite Y | (Garcia et al., 2016) |
| Diatomite | – | – | Diatomite + NaOH + deionized water + sodium meta-aluminate (Na2Al2O4) | 40 | 16 | 100 | 10 | Zeolite Y | (Zhang et al., 2016) |
| Bentonite | 3.7 | Bentonite was treated with HCl with 1 g/4 ml ratio, in an oil bath, for 8 h at 95 °C. | 5 g reacted bentonite, 6 g NaOH and 0.88 g NaAlO2. Calcination for 1.5 h at 310 °C | 25 | 24 | 97 | 24 | Zeolite Y | (Hashemi et al., 2019) |
| Kaolin | 1.4 | 1.5 g kaolin + 0.6 g quartz + 16 ml of 8 M NaOH. Heating at 240 °C for 8 h. | Resultant material was mixed with water (10 ml) and 5 ml HCl (37%). | – | – | 90 | 6 | Zeolite Y | (Wang et al., 2016) |
| Kaolin | 2.43 | Calcination of kaolin at 800 °C for 6 h. | Metakaolin + NaOH 1.0 M (10 g/100 ml). | 25 | 24 | 90 | 48 | Zeolite Y | (Bahgaat et al., 2020) |
|
Kaolin And Diatomite |
– |
Quasi-Solid-Phase activation 50 g of kaolin + 75 g of NaOH + 10.2 ml of H2O at 100 °C for 30 min. |
5 g activated kaolin added to 6.8 g of the thermally treated diatomite, 48 g H2O, 6.3 g of seed solution (NaOH, Al2(SO4)3, water glass (27.6 wt% SiO2) and distilled water). | 60 | 12 | 100 | 24 | Zeolite Na-Y | (Yang et al., 2017) |
| Kaolin | – | Calcination of kaolin at 600 °C for 6 h. | Metakaolin + NaOH to obtain Na2O:SiO2 ratio 0.7. | 25 | 96 | 95 | 72 | Zeolite Na-Y | (Ajayi et al., 2018) |
| Kaolin | 1.16 |
Calcination of kaolin at 600 °C for 3 h. Calcined kaolin + 50% wt Na2CO3was heated at 800 °C for 2 h. |
Dissolution of metakaolin in NaOH and an amount of ludox (40 wt% SiO2). |
25 | 24 | 75 | 96 |
Zeolite Na-Y |
(Djeffal et al., 2017) |
| Kaolin | – |
Activation of kaolin at 850 °C for 6 h. Dealumination of metakaolin with 0.1 M H2SO4. |
Treated metakaolin was mixed with water and NaOH. | 25 | 0.5 | 95 | 48 | Zeolite Y | (Akinruli et al., 2021) |
3.3. Zeolite X synthesis from natural clays
Zeolite X is a synthetic aluminosilicate material classed as faujasite (FAU) with a SiO2/Al2O3 ratio of 2–3. Zeolite X is composed of 8 cubo-octahedrons joined by 12 cuboids to a cavity known as the cage, the porosity of which has a diameter of 0.42 nm (Srilai et al., 2020b). The octahedral morphology of Zeolite X can be seen in the SEM image presented in Fig. 7. Zeolite X is an appealing material for both technical and environmental purposes. Large micropores of zeolite X make it suitable for gas purification and separation (C. Chen et al., 2014, Garshasbi et al., 2017) and catalyst (Srilai et al., 2020b), while its high exchange capacity allows for the adsorption of heavy metals (Derkowski et al., 2007; Jinlong et al., 2011).