1. Introduction
Since the Fukushima accident, many countries have been carrying out tasks to secure countermeasure systems against severe accidents so that protection of public health and environment from radiation hazards can be assured [1]. In Korea, a special safety inspection was conducted in March of 2011 by experts from regulatory organizations, industry, university, and research institutes. Based on the results, 56 items, including six measures taken by the operator itself, were identified as countermeasures against postulated severe accidents triggered by natural disasters. Many of these countermeasures, including installation of passive autocatalytic recombiners in the operating plants, have already been implemented for all domestic nuclear power plants (NPPs) [2], [3].
Meanwhile, international efforts are underway to limit the release of radioactive material from severe accidents at NPPs by strengthening international convention. The International Atomic Energy Agency (IAEA) issued the Vienna Declaration on Nuclear Safety in February 2015, calling for dedicated efforts to limit off-site releases that could result in long-term contamination, as well as early or large releases. In addition, the IAEA conducted an Integrated Regulatory Review Service mission for the Korean regulatory body in July 2011 and provided follow-up actions in December of 2014. As a result, it was recommended that off-site dose criteria for severe accidents be established. Subsequently, rulemaking for severe accident requirements was finalized in June 2016. Severe accident management guidelines (SAMGs) must be submitted as an operating licensing requirement for new plants, while they must be submitted within 3 years for all operating NPPs. Therefore, the Nuclear Safety Act was revised to require the submission of an accident management program that covers not only the scope of design basis accidents, but also that of severe accidents when applying for an operating license. In order to implement the additional requirements, several subordinate decrees and laws have been established, including new design criteria for acceptable quantity and frequency of a large release and acceptable off-site dose due to a severe accident.
Under such circumstances, examination of the phenomena related to radioactive material release during a severe accident and development of mitigation measures were considered urgent tasks for the Korean nuclear community. Following discussions among domestic researchers in October 2014, a special committee on “Development of a Research Roadmap for Examination of Severe Accident Phenomena and Establishment of Countermeasure System” was set up in early 2015 by the Korean Nuclear Society (KNS). Accordingly, three subcommittees were organized to develop a comprehensive roadmap for the following areas: in-vessel phenomena, ex-vessel phenomena, and fission product (FP) behavior. In particular, the subcommittee on FP behavior operated effectively, holding nine meetings during the designated term between the kickoff meeting on January 22, 2015 and August 31, 2016.
In the meantime, similar activities had already been done in Europe [4], [5] and Japan [6]. In Europe, “European expert network for the reduction of uncertainties in severe accident safety issues” established phenomena identification and ranking tables (PIRTs) for all aspects of severe accidents [4]. Then, its priority ranking was reassessed within the domain of the severe accident research network of excellence work program “Severe Accident Research Priorities” to harmonize and reorient research programs, to define new ones, and to close resolved issues on a common basis [5]. Meanwhile, based on findings from the Fukushima Daiichi NPP accident, Japanese experts developed PIRTs for thermal hydraulics and source term (ST) and summarized the highly ranked phenomena [6]. These previous studies have provided good examples for our subcommittees in developing domestic roadmaps. This paper describes the dedicated activities of the FP behavior group and elucidates recommendations for future research in Korea. It solely covers the FP release from an NPP, mainly focusing on pressurized water reactors.
2. Approaches to developing the roadmap
During a severe accident involving reactor core degradation, radioactive materials can be released to the environment if the reactor containment fails, is vented or is bypassed. The ST is normally defined as the magnitude and composition at the time of release, as well as the chemical and physical forms of released material [1], [7], [8]. Among radioactive materials, such as fission and activated products and actinides, FPs are the source of activation and may comprise the major portion of radioactivity during an accident. The magnitude of their release depends on the accident sequence, which includes the initiating event, subsequent plant response, and mitigating actions taken by the plant personnel. The accident progression paths can be characterized by three release stages: the release of radionuclides from the degraded fuel, their behavior during transport in the reactor coolant system (RCS), and their behavior in the containment. Furthermore, the amount of release is largely affected by the volatility of radionuclides, which governs the physical forms of their release and transport, i.e., gas/vapor or condensed material, namely, aerosol. Since they rely on chemical speciation, FP chemistry over a wide range of temperatures is very important in determining an accurate ST. For example, chemical reactions of FPs with the structural materials released from the damaged core or coolant material in the RCS play important roles in determining their airborne concentration in the containment atmosphere [9]. Aerosol physics also governs the radionuclide behavior in the RCS and in the containment because these materials are condensed during transfer from the damaged core to the containment, except for noble gases and gaseous iodine and ruthenium [1].
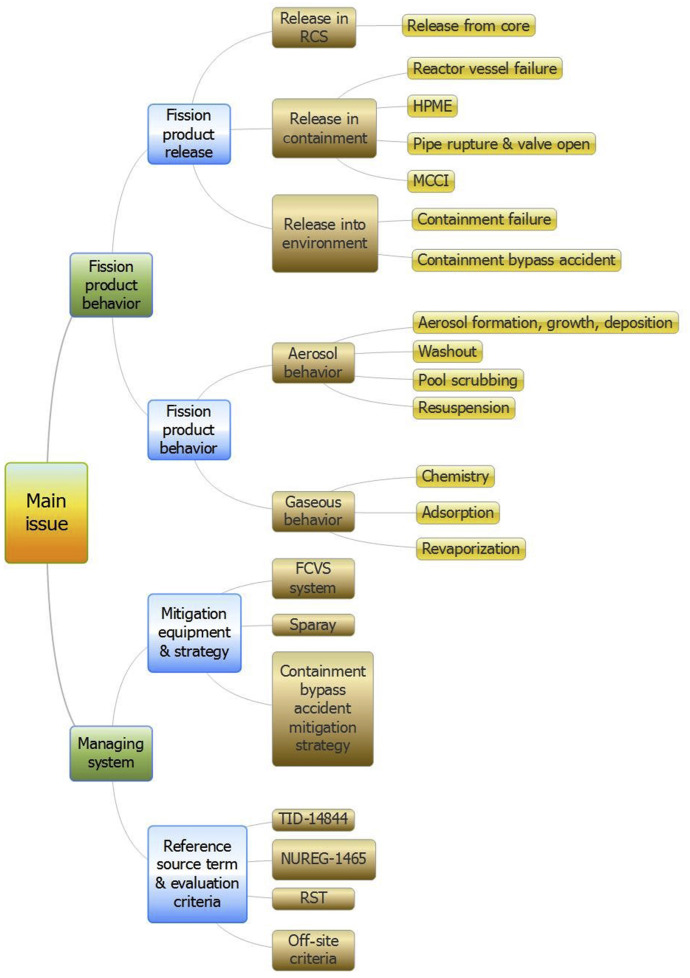
The subcommittee members discussed the release characteristics and agreed to examine the technical issues relevant to the three release stages and physical forms of radionuclides released and transported as described above. Thus, through internal brainstorming and an overview of the EU PIRT, they developed a tree for FP release phenomena by tracing failures of the defense-in-depth barriers. Fig. 1 shows a tree that describes the major elements in this task. In addition, elements for the establishment of countermeasures were added to the tree. In order to examine technical issues, they took into account the phenomena, accident management actions, and regulatory aspects relevant to mitigation features for containment and mitigation strategies against containment bypass accidents. Regulatory requirements for STs including the reference accident ST [7], [10], [11] and the acceptance criteria [12] were also included in the tree. Then, the group identified further research needs for important technical issues based on the degree of the current knowledge level in Korea and in foreign countries, the significance and urgency of those issues, and the expected research period required to reach the international level of knowledge. While the activities of each subcommittee were ongoing, the steering committee convened regularly to review the activities and to give feedback to the subcommittees. A report prepared by the subcommittee on FP behavior was reviewed by independent external experts who provided feedback. After collecting the relevant feedback, the report was finalized [13].
 Fig. 1. Elements of the roadmap for examination of fission product release phenomena and establishment of countermeasures. FCVS, filtered containment venting system; MCCI, molten core concrete interaction; RCS, reactor coolant system; HPME, High pressure met ejection; RST, Reference source term.
Fig. 1. Elements of the roadmap for examination of fission product release phenomena and establishment of countermeasures. FCVS, filtered containment venting system; MCCI, molten core concrete interaction; RCS, reactor coolant system; HPME, High pressure met ejection; RST, Reference source term.3. Identification of technical issues on FP release phenomena and the countermeasure system
With regard to the elements of the three release stages, the physical forms of radionuclides, and the countermeasure system described in section 2, the following aspects were examined: the relevant phenomena, experimental programs, computer modeling, the current knowledge level in Korea and in foreign countries, the significance and urgency of issues, and the expected research period required to reach the international level of knowledge. A summary of each element and the identified technical issues relevant to FP release and countermeasures is provided in the following sections.
3.1. FP release phenomena
Fig. 2 shows the general behavior of FPs from severe accidents in an NPP. Various kinds of elements, such as Xe, Kr, Cs, I, Ba, Sr, Te, Sb, Mo, Ru, Zr, Sn, and La are generated by fissioning uranium in fuel rods. The inventories of the FPs depend on thermal power, uranium enrichment, refueling cycle, and burn-up of uranium. Some volatile and semivolatile FPs diffuse to the grain boundaries and move to the open fuel porosities by vaporization and mass transfer processes, and then accumulate in the gap between the fuel rod and cladding during normal operation. If the fuel cladding fails, the FPs in the gap are released to the RCS. As fuel heat-up occurs during a severe accident, FPs including low-volatile FPs can be released outside of the fuel matrix, and the release of low-volatiles is considered to be governed by the volatilization of UO2 [14]. In addition to this, a large amount of species other than the FPs might also be released from the heated cladding and structural materials, such as stainless steel and Inconelalloys.
 Fig. 2. Fission product behavior during a severe accident in a nuclear power plant. FCVS, filtered containment venting system; FP, fission product; MCCI, molten core concrete interaction; IRWST, In-containment refueling water storage tank.
Fig. 2. Fission product behavior during a severe accident in a nuclear power plant. FCVS, filtered containment venting system; FP, fission product; MCCI, molten core concrete interaction; IRWST, In-containment refueling water storage tank.Such species are released into the RCS in the form of gases or vapors and are then swept by a steam-hydrogen gas mixture toward the break point in the RCS, experiencing a number of physicochemical processes. As the vapors cool down in the RCS, they take the form of aerosol particles when released into the containment, with the exception of the noble gases, iodine, and ruthenium, which can remain in partly gaseous form in certain circumstances [8]. The amount of gaseous iodine released into the containment depends on the reaction kinetics of the I-O-H system for the RCS, which is under the influence of FPs (Cs, Mo), boron (B) and control rod material (Ag, In, Cd, or B4C) [15].
FPs experience a significant retention in the containment according to various mechanisms, including natural removal processes or plant features. Major natural removal mechanisms are aerosol deposition, adsorption of vapors on structural surfaces, and scrubbing through water, for instance, that overlying the core debris. Major engineered safety features for FP removal are the containment spray system and the filtration systems. The airborne FPs remaining in the containment atmosphere can escape to the environment via damaged areas or penetration leakage from the containment. Studies of the Fukushima Daiichi accident show that most of the airborne dose was caused by Cs, I, and Te species, and that the releases into the environment were driven mainly by chemical volatility at a given temperature and reduction potential within the containment [16]. In addition, FPs can also leach from the fuel and release to the environment through water pathways, which appeared to be the case for the Fukushima Daiichi accident.
3.1.1. Aerosol behavior
3.1.1.1. Aerosol formation and growth
Aerosols are solid particles suspended in the gas phase or in liquid droplets, ranging in size from 0.01 μm to 20 μm. Behavior of most aerosols that appear during severe accidents show very complicated aspects ranging from continuum mechanics to free molecular physics. The physical aspects affecting the characteristics of the aerosols can be enumerated according to the formation, growth, and shape of the aerosol particles. In addition, deposition of particles on the surfaces and resuspension of aerosol particles are also deemed important aspects.
Formation of aerosol: aerosol particles can be formed during various interactions between materials and by steam condensation. The major processes that form aerosol particles during an accident include the following: entrainment of solids or liquid droplets in a high velocity gas flow, expulsion of droplets by gases bubbling through liquids, shock waves produced in energetic interactions of molten materials with coolants, and high pressure melt ejection from the RCS [17]. The sizes of aerosols formed by mechanical processes and suspended in the gas phase are not relatively large and typically do not exceed 1–2 μm. Thus, these types of aerosol tend to be ignored during the accident analysis. However, the formation of aerosol particles from supersaturated steam is deemed the most important source and is treated very critically in the accident analysis.
Growth of aerosol: the major mechanisms that explain the growth of aerosols are growth by coagulation, by condensation, and by hygroscopicity. Aerosols can grow through continuous steam condensation or coagulation of particles. Of these processes, coagulation is considered the dominant mechanism responsible for the growth of aerosols following nucleation. Coagulation of aerosols affects the size and mobility of aerosols. In addition to the condensation and coagulation, hygroscopicity of aerosols is considered an important growth mechanism. For example, CsI and CsOH, which show strong solubility, can grow by hygroscopicity until equilibrium pressure in the solution is achieved.
3.1.1.2. Aerosol deposition and resuspension
Deposition of aerosol: The major processes that result in the deposition of aerosols include gravitational settling, diffusion to surfaces, turbulent deposition, inertial deposition, and phoretic processes, but this is not a complete list. Gravitational settling and diffusion significantly affect the behavior of aerosols. Gas viscosity and particle density are the major parameters that govern gravitational settling. Deposition velocity according to diffusion is greatly affected by the structural geometry and flow conditions. It decreases when particle diameter increases, while the deposition velocity according to gravitational settling shows the opposite trend. Inertial deposition occurs when particles are captured or accelerated in their motion toward obstacles. In such circumstances, if the aerosol particles are sufficiently large, they collide with the obstacle surfaces and remain deposited thereafter. Another important mechanism in aerosol deposition is phoretic deposition. Thermophoretic and diffusiophoretic depositions are detailed mechanisms; the former is caused when the temperature gradient produces a movement of the particles towards the lower temperature zone and the latter appears in the direction of flux of a condensed vapor such as steam. The thermophoretic process can be significant when high temperature aerosols and gases confront a large area of cold structures, for instance containment walls or floors.
Resuspension: Aerosols deposited in a pool or on the surface of a structure can be physically entrained or evaporated with changes of gas flow, temperature, or concentration. A resuspension may occur in the core, the RCS, or the containment. Aerosols deposited on the surface of the primary system can be resuspended by turbulent flow. A sudden increase in steam flow due to core cooling or relocation of core debris is the main source of potential resuspension of aerosols. Aerosols transported to the containment can be deposited on the surface of the containment, and on structures and water pools in the containment. The gas turbulent flow due to hydrogen deflagration or steam explosion can be the main source that induces aerosol resuspension. In addition, aerosols deposited in the containment can also be resuspended if there is a sudden change of containment pressure due to the failure of the containment pressure boundary or containment discharge. Modeling of aerosol resuspension is based on adhesion and removal forces. For the dry aerosols, the van der Waals force, electrostatic force, and chemical cohesive force are the important adhesion forces to be taken into account. In the meantime, the lift force perpendicular to the surface and drag force parallel to the surface should be considered as important removal forces. The deposited aerosols are removed layer by layer by agitation on the surface and moved with a rolling behavior [17], [18].
3.1.1.3. Pool scrubbing
When gases including radioactive materials are ejected into the pool, some radioactive aerosols and gases are captured in the pool by pool scrubbing. This phenomenon is taken into account by defining the decontamination factor (DF), which is the ratio of the initial mass of the specific radioactive material to the final mass after it passes through the water pool, in a wet-type filtered containment venting system (FCVS), as well as in other gas sparging systems. When the carrier gases including radioactive aerosols and gases enter the pool through a vent, the carrier gases leaving the vent form large globules that break up into a swarm of small bubbles. Several physical processes are involved in transporting aerosols to the liquid-gas interface (equal bubble surface) when steam/gas mixtures are bubbled through a water pool [19]. The DF of aerosols can be calculated in three regions, that is, gas injection, bubble rising, and pool surface regions [20]. The total DF is obtained by the product of the values calculated in those regions. In the injection zone, aerosol removal occurs through several mechanisms such as: Stefan flow from steam condensation during gas equilibration to pool conditions; inertial impaction of aerosols in a rapid gas velocity decrease; and centrifugal, diffusional, and gravitational aerosol deposition during gas injection through small orifice or multihole vents. In the rising zone, aerosol removal occurs from centrifugal, diffusional, and gravitational aerosol deposition within a bubble. The pool scrubbing phenomenon has been modeled and embedded in several computer codes, such as SPARC (Suppression Pool Aerosol Removal Code), BUSCA (BUbble SCrubbing Algorithm), and SUPRA (SUppression Pool Retention Analysis) [19]. These codes aim at simulating the pool scrubbing process and estimating the DFs of the radioactive aerosols and of iodine gas in the water pool.
3.1.1.4. Leaching
During the Fukushima accident, radionuclides from the damaged fuel were continuously dissolved in the coolant and then accumulated in the containment and turbine building in the form of contaminated water. Thus, there could be leakage paths for these radionuclides that leached out from the turbine building to the sea. In particular, compared to the case of Cs-137, Sr and Ba releases into water were much higher than those into the atmosphere. However, knowledge of the leaching mechanism and accurate models for the prediction of release rates are limited [16].
3.1.2. Gaseous behavior
3.1.2.1. Chemistry
Cs, I, and Te species caused most of the airborne dose during past severe accidents at NPPs due to their high volatilities in the containment under severe accident conditions. It was observed from PHÉBUS-FP tests that a large amount of gaseous iodine was released from the RCS to the containment when nuclear fuel was degraded. The existence of Cs in the absence of other elements allows the quick production of CsI, because there is no obstacle in the reaction of CsOH + HI ↔ CsI + H2O. CsI can be produced even without the existence of gaseous I2 because a much larger amount of Cs exists compared to the I species. However, when Mo is added to the Cs-I system, the reaction changes completely due to the high affinity of Mo for Cs; as a result, partial cesium molybdatesmake a complex to CsxMoOy, which is the most stable gaseous molybdate. Nevertheless, CsxMoOy cannot be produced in a hydrogen atmosphere, and this result is very similar to the case of the nonexistence of Mo, in which CsI aerosol production is dominant. In experiments with a steam atmosphere, a maximum of 80% of the iodine transferred from the primary circuit, under a steep temperature gradient and with a small fraction, was in the form of aerosols. Gaseous iodine is mostly composed of molecular iodine; the remaining portion is HI or HOI. When boron (B) is added to the Cs-I system, gaseous iodine is activated due to the production of CsBO2, but the degree is smaller than it is in the case of Mo. With control-rod materials such as Ag and Cd, it is surmised that Ag and Cd react with iodine and prevent the production of gaseous iodine [9], [11], [14], [21].
Iodine aerosols released from the RCS to the containment may sediment and settle on the walls in the containment. If they are soluble, they can be dissolved in the sump or become plated out on wet surfaces as ions in the form of I–. Then, the behavior of I– changes according to time and the pH of the aqueous solution. Several FPs dissolved in the solution cause water radiolysis and decrease the pH in the pool. At this time, a large amount of the dissolved I–changes to elemental iodine and moves to the containment atmosphere. Organic iodide is also produced by the reactions of I2 and I– with paints on the containment surfaces, and with organic materials decomposed radiolytically in solution. Gas-phase radiolysis is an important decomposition route, as well as solution phase radiolysis. Thus, radiolytic products of air, such as NO2, O3, or HNO3, oxidize molecular and organic iodine and produce IxOy in the containment atmosphere. However, the IxOy aerosols are deposited on the containment surface, and are then partially decomposed to molecular iodine under irradiation. Decomposition of IxOy by CO produced from degradation of B4C (control rod) may also affect the amounts of gaseous iodine [9], [11], [14], [22], [23].
3.1.2.2. Adsorption and desorption
Volatile iodine can be adsorbed by surfaces in the containment over time to form organic iodides. Painted surfaces act as sinks for I2 and as a source for volatile organic iodine. The I2 adsorption on surfaces depends on the gas and water temperatures and the gas flow velocity along the walls. Under wet conditions, the wall condensation rate drives I2 diffusion towards the surfaces. Radiation induces fast radiochemical reactions between iodine and the paint [22]. Models for the adsorption of I2 on paint, steel, and aerosols have been developed, but uncertainties remain concerning the effect of paint aging on the iodine volatility, deposition of iodine oxides on the surface and the subsequent decomposition and volatilization of iodine species, iodine adsorption kineticson representative multicomponent aerosols, and temperature and loading effects from H2 phenomena on FPs deposited on the wall [14].
3.1.2.3. Revaporization
After condensed species are deposited in the RCS, they can be revaporized by the displacement of the thermodynamic equilibrium in case of changes in temperature or carrier gas composition, or decreases in partial pressures in the gas phase. The reasons for the equilibrium vapor pressures exceeding the ambient partial pressures of the vapor species may include decay heating of deposited materials and changes in the chemical environment within the RCS. For instance, in a high pressure scenario, natural circulation with high temperature can cause a creep rupture of the RCS and revaporization of volatile material such as species formed from cesium and iodine. Furthermore, rupture of the RCS can radically change the chemical environment of deposits and raise the oxygen potential of the atmosphere, which can produce various volatile species of FP elements. Therefore, revaporization may be a source of delayed release to the containment. The revaporized materials may be redeposited in cooler regions of the RCS or be swept out of the system [9], [24]. PHÉBUS FP tests demonstrated this type of revaporization of several elements, with other evidence such as reaction of the deposited species with the substrate, stratification of deposits in the upper plenum samples, different stages of release, and effects of changes in the atmosphere. Recently, revolatilization and distribution of ruthenium deposited on the surfaces under irradiation and ozone action have been studied in several international programs [9].
3.2. Issues relevant to FP behavior
In this section, three stages of FP release, from the core, the RCS, and the containment, are considered. Another type of containment breach, i.e., containment bypass, is also considered, while filtered containment venting is dealt with as a countermeasure against FP release. The identified technical issues concerning FP behavior at each stage are described in Table 1, Table 2, Table 3. They are divided into aerosol release (Table 1) and gaseous release (Table 2). Since iodine and ruthenium behave in aerosol and gaseous forms, issues specific to them were dealt with separately, as shown in Table 3.
Table 1. Knowledge level, significance, and research period for aerosol behavior issues.
| Technical issues | Knowledge level | Significance (urgency) | Research period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High | Mid | Low | High | Mid | Low | Short (2–3 yr) | Mid (3–5 y) | Long (5–10 y) | N/A | |||
| Aerosol release | Release from core | FP release in accordance with fuel type and burnup | W,D | O | O | |||||||
| Improvement of the model for degradation of core structure (mainly control rods) and release of material including aerosols | W | D | O | O | ||||||||
| Effect of reflooding and hydrogen generation at high burnup(≥60 MWd/kgU)/MOX fuel | W | D | O | O | ||||||||
| Release from RCS | Aerosol resuspension in the RCS (mechanical resuspension) | W | D | O | O | |||||||
| Aerosol deposition in singularities and complex structures | W | D | O | O | ||||||||
| Particle break-up in highly turbulent flows | W | D | O | O | ||||||||
| Influence of chemistry on aerosol release | W | D | O | O | ||||||||
| Containment issue | Phenomena related to formation, growth, and deposition of aerosols including growth through coagulation and condensation, condensation on the containment surface | W | D | O | O | |||||||
| Charge effects | W | D | O | O | ||||||||
| Mixed aerosols in condensing atmospheric conditions | W | D | O | O | ||||||||
| Re-entrainment from pools (including resuspension with pool scrubbing in the sump) | W | D | O | O | ||||||||
| Influence of recombiners | W | D | O | O | ||||||||
| Hydrogen-burn effects on suspended aerosols | W | D | O | O | ||||||||
| Release from MCCI pool | W | D | O | O | ||||||||
| Fire aerosols | W | D | O | O | ||||||||
| FP (Sr, Cs, Ba, Sb, Ce/Pr, Eu and actinides) releases to cooling water | W,D | O | O | |||||||||
| Resuspension | Model improvement and additional validation for aerosol resuspension in the containment (mechanical resuspension) | W | D | O | O | |||||||
| Transport and deposition of aerosols through turbulence effect when corium is ejected | W | D | O | O | ||||||||
| Washout | Model improvement for the aerosol removal by spray | W | D | O | O | |||||||
| Pool scrubbing | Experiments with higher gas temperature, steam flow rate and hydrogen concentration in the carrier gas than those of the current tests | W | D | O | O | |||||||
| Pool tests enlarged to the saturated condition and comparison of the decontamination capability with that of the subcooled pool | W | D | O | O | ||||||||
| Effect of the high pressure condition on the pool surface, and the effect of water pH on the retention of aerosols and gaseous iodine | W | D | O | O | ||||||||
| Integrated effect tests using the representative aerosol materials with well-defined severe accident conditions to examine the resuspension phenomena | W | D | O | O | ||||||||
| Establishment of the systematic experimental database to validate stand-alone or integral code models | W | D | O | O | ||||||||
| MCCI | Re-evaluation of the current experimental results and additional validation of the codes related to MCCI, characterization of aerosols in terms of the concrete type | W | D | O | O | |||||||
| Quantity of FPs and non-radioactive aerosols carried by the gas products from MCCI | W | D | O | O | ||||||||
| Containment bypass accident | Aerosol retention in the SG | W | D | O | O | |||||||
| Development of the measures and strategies for mitigation of the radiological consequences from ISLOCAs and SGTRs | W,D | O | O | |||||||||
| Leaching | Experimental study (separate, integral) on the scenarios and phenomena for the development and validation of models concerning the FP transport along the leak paths | W | D | O | O | |||||||
| Confirmation of available models and experimental study on the reaction between corium and water beneath the basemat | W | D | O | O | ||||||||
“O” is an indication of the corresponding column for each item.
D, domestic; FP, fission product; ISLOCA, interfacing system loss of coolant accident; MCCI, molten core concrete interaction; N/A, not applicable; RCS, reactor coolant system; SG, steam generator; SGTR, steam generator tube rupture; W, worldwide.
Table 2. Knowledge level, significance, and research period for gaseous release behavior.
| Technical issues | Knowledge level | Significance (urgency) | Research period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High | Mid | Low | High | Mid | Low | Short (2–3 yr) | Mid (3–5 yr) | Long (5–10 yr) | N/A | |||
| Gaseous release | Release from core | Analysis and evaluation with consideration for the plant conditions based on the existing experimental results | W | D | O | O | ||||||
| Experimental study of the gaseous nuclides produced in the core and RCS | W | D | O | O | ||||||||
| Modeling of critical nuclides and relevant reactions | W | D | O | O | ||||||||
| Release from RCS | ||||||||||||
| Release from containment | ||||||||||||
| Leak from containment boundary | Quantification of FPs leaked through the containment penetrations with cracks formed and released into the environment, based on the dynamic behavior of the containment | W | D | O | O | |||||||
| Washout | Review and evaluation of the existing experimental and theoretical research results, and further study using experiments and modeling | W | D | O | O | |||||||
“O” means an indication of the corresponding column for the issue.
D, domestic; FP, fission product; N/A, not applicable; RCS, reactor coolant system; W, worldwide.
Table 3. Knowledge level, significance, and research period for iodine and ruthenium behavior issues.