1. Introduction
Cereals constitute a prime global human food source. Among them, wheat (Triticum aestivum L.) ranks as the second most important food after rice, and is the most widely cultivated cereal in the world. It is one of the central pillars of food security, supplying 20% of total calories and a similar portion of total protein to the world’s population [1]. The average global wheat yield is 3.3 t·hm−2; however, it varies widely, with regional averages ranging from 1.7 t·hm−2 in Australia to a potential of up to 9 t·hm−2 in other parts of the world (data from FAOSTAT database 2015 [2]). The yield penalty is usually due to different environmental stresses that reduce yield potential by 69.1% [3]. In most developed countries, wheat is mainly grown in rain-fed marginal land, where inadequate and erratic rainfall limits the yield (Table 1) [4]. Drought is a key stress that constrains wheat production on about 6.5 × 107 hm2 of land worldwide [5] and reduces yield by up to 50% [6]. Modeling exercises have revealed that water stress in marginal wheat-growing environments reduces 50%–90% of their yield potential under irrigated conditions [7]. In 2012, the overall global wheat production decreased by 1.4%, mainly due to severe drought in the United States, Europe, and central Asia (data from FAOSTAT database 2013 [2]). The Australian wheat yield dropped by 46% in 2006 compared with the yield trend of the previous 50 years, resulting in billion dollar losses for the wheat industry (data from FAOSTAT database 2012 [2]).
| Country/Region | Production (t) | Area harvested (107 m2) | Area irrigated (107 m2) |
|---|---|---|---|
| Australia | 24.51 | 13 650 | — |
| Canada | 26.22 | 9 200 | — |
| China (mainland) | 116.15 | 24 110 | — |
| France | 38.37 | 5 540 | 30.24 |
| India | 84.36 | 28 640 | — |
| Pakistan | 23.40 | 8 860 | 7335.00 |
| Russian | 52.19 | 24 090 | — |
| Turkey | 19.99 | 7 980 | — |
| Ukraine | 20.34 | 6 480 | 46.90 |
| USA | 60.91 | 20 060 | 1662.00 |
| World | 678.02 | 220 400 | 13 241.50 |
The impact of future drought episodes on wheat production is expected to increase due to the effects of climate change on temperature and precipitation. It is estimated that the 1 °C increase of temperature that has occurred during the last 29 years has resulted in a 6% reduction of wheat yield compared with the expected yield without global warming effects [8]. According to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC), the global mean temperature will increase by 3.7 °C by the end of this century, with incidents of hottest days and coolest nights occurring 50% more frequently than at present [9]. Changes in the precipitation pattern coupled with increasing temperature would affect the major crop production of the world, and wheat production could decline by 23.2%–27.2% by 2050, unless protective measures for limiting global warming or appropriate cultivars and crop management practices are adopted [10]. Wheat production in low-latitude sites would be more vulnerable with the rise of a 3–5 °C temperature scenario, compared with production in high latitude regions, and yields could decline by up to 40% with an increase of 2 °C in temperature [11]. In Australia, wheat belts are typical of those in a Mediterranean climate: most precipitation occurs in winter, followed by less-frequent rain in spring and hot dry summer. Thus, water stress in spring is the major factor limiting yield improvement in these regions and often coincides with stem elongation, flowering, and grain filling [12]. In these environments, terminal heat often combines with drought during the grain-filling period and further limits grain yield [13]. In their Fifth Assessment Report, the IPCC predicted that an increase in mean annual temperature by 2.2–5 °C with +5% to −30% change in precipitation patterns will result in the expansion of drought-affected areas by 5.4%, 4.6%, and 3.8% by 2030, 2050, and 2070, respectively [9]. In this situation, plant breeders must be well-prepared to embrace the challenges of climate change and to feed the world by developing varieties that are better adapted to water-limited environments. Better utilization of the available genetic resources of wheat is essential in order to maintain and maximize wheat yield potential in water-limited environments, and the optimization of phenology is one of the most effective ways to achieve this goal.
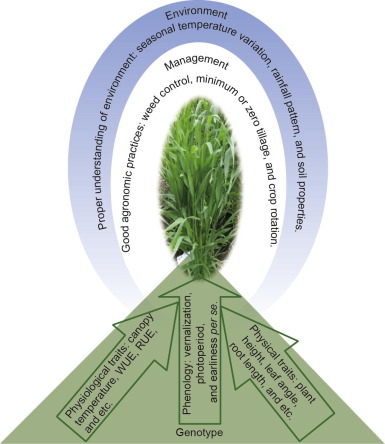
Phenology is the key factor for crop adaptation to a particular environment. A proper understanding of the genetic control of phenological traits will enable breeders to develop crops that are better adapted to a specific environment. It is well documented that yield loss due to drought depends on the growth stage at drought occurrence, as well as the duration and intensity of the stress [14], [15]. Spike development, from terminal spikelet initiation to anthesis, is the most important phase in determining grain yield, as it has been observed that a heavier spike at anthesis is positively correlated with grain yield [16] and can be manipulated without affecting other phases [17]. Therefore, adverse effects of drought could be minimized by ensuring that the most sensitive developmental stages do not occur during stress periods [18]. Hence, fine-tuning of flowering and the duration of developmental phases are advocated for better adaptation of wheat in water-limited environments or to escape from these constraints [19], [20], [21], [22]. Phenology genes also regulate the physiological development of wheat [23], and some morpho-physiological traits have been identified as effective in breeding drought-adaptive varieties [24], [25]. Taking account of many important traits and their interactions in stress environments, a sound understanding of the genetic control and physiological basis of drought tolerance would facilitate the improvement of yield in water-limited environments. We acknowledge the importance of good agronomic practices, that is, management, and several other traits involved in physiological mechanisms to reduce adverse effects of drought; however, this review focuses on the phenology genes as one of the most important measures to avoid drought stress (Fig. 1). Therefore, an effort has been made to summarize the progress achieved to date on key phenology genes and the integration of this knowledge in breeding new varieties that are adapted to future climate change. Moreover, an attempt has been taken to compile information on molecular markers for the identified alleles of vernalization (Vrn) and photoperiod (Ppd) genes that will help in evaluating the cultivars that are adapted to target environments as well as marker-assisted breeding.
 Fig. 1. Drought shield: complementary approaches to sustain wheat yield in water-limited environments. WUE: water-use efficiency; RUE: radiation use efficiency.
Fig. 1. Drought shield: complementary approaches to sustain wheat yield in water-limited environments. WUE: water-use efficiency; RUE: radiation use efficiency.2. Phenology genes
Wheat is adapted to a wide range of agricultural environments [26]. The synchrony of flowering to a wider range of climatic conditions is largely controlled by ① Vrn genes (exposure to cold temperature requirement), ② Ppdgenes (photoperiod sensitivity), and ③ autonomous earliness per se (Eps) genes [27]. Hence, the adaptation of a genotype to a particular environment depends on the interaction of these three groups of genes.
2.1. Vernalization genes
Vernalization promotes the switching of the plant vegetative phase to the reproductive phase by inducing floral primordia from leaf primordia in the shoot apical meristem [28], [29]. In wheat, three genes determine the vernalization requirement: Vrn1, Vrn2, and Vrn3 [30], [31], [32]. The three orthologous Vrn1 genes—Vrn-A1, Vrn-B1, and Vrn-D1—are located on the long arms of the homoeologous chromosomes 5A, 5B, and 5D, respectively, in common wheat, and mainly control the vernalization requirement [30], [31], [33], [34]. The Vrn2 gene is also located on the long arm of 5A; and Vrn3 is located on the short arm of chromosome 7B [32], [35], [36], [37]. Winter wheatvarieties require a certain period of cold to induce flowering, whereas varieties that flower without vernalization are referred to as spring types. The dominant alleles of Vrn-A1, Vrn-B1, Vrn-D1, and Vrn3 are responsible for the spring growth habit; thus, a dominant allele at any of the three Vrn1 loci confers a spring type. On the other hand, Vrn2 is dominant for the winter type and is epistatic to dominant alleles of Vrn1 [31], [37], [38], [39]. Vrn2 is a floral repressor that delays flowering, but vernalization under long days suppresses the expression of Vrn2 and enhances the expression of Vrn1 [40]. Multiple alleles of Vrn1 with different levels of responses to vernalization and effects on flowering have been identified (Table 2) [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], and have an adaptive value [56], [57], [58], [59], [60], [61]. The extent of flowering depends on the basal level of Vrn1 expression [62]; some alleles of Vrn1 are expressed without prior cold treatment, thus allowing flowering without vernalization [36], [62], [63]. Mutations in the promoter or deletion in the first intron of the Vrn1 gene cause expression of Vrn1 without vernalization, and the alleles lacking the larger section are more active during earlier flowering without vernalization [36], [44], [45], [64], [65]. On the other hand, varieties of wheat and barley flower early without vernalization when they lack a functional copy of the Vrn2 gene [31], [37]. The Vrn3 gene also expresses at a high level when Vrn2 is absent, and active alleles of Vrn3accelerate flowering irrespective of day length or vernalization [32]. Thus, five loci of Vrn genes influence flowering by controlling the vernalization requirement of wheat cultivars in different parts of the world [30], [32], [66], [67].
Table 2. Current status of the identified alleles for Vrn1 and Ppd1 loci.
| Gene | Allele | Sequence variation from wild type | Response to light/temperature | Ref. |
|---|---|---|---|---|
| Vrn-A1 | Vrn-A1a | 231-bp and 140-bp insertions in the promoter region of common wheat | Insensitive | [44] |
| Vrn-A1b | 20-bp deletion in the promoter region of common wheat | Insensitive | [44] | |
| Vrn-A1c | 7222 bp deletion in intron 1 | Insensitive | [45] | |
| Vrn-A1d | 32-bp deletion in the promoter region of tetraploid wheat | Insensitive | [44] | |
| Vrn-A1e | 54-bp deletion in the promoter region of tetraploid wheat | Insensitive | [44] | |
| vrn-A1 | Wild type | Sensitive | [44] | |
| Vrn-B1 | Vrn-B1a | 6850 bp deletion in intron 1 | Insensitive | [45] |
| Vrn-B1b | 6850 bp and 36 bp deletion in intron 1 | Insensitive | [46] | |
| Vrn-B1c | 817 bp deletion and 432 bp duplication in intron 1 | Insensitive | [47] | |
| vrn-B1 | Wild type | Sensitive | [45] | |
| Vrn-D1 | Vrn-D1a | 4235 bp deletion in intron 1 | Insensitive | [45] |
| Vrn-D1b | C replaced by A at translation site in CArG-box of wild type | Facultative | [48] | |
| vrn-D1 | Wild type | Sensitive | [45] | |
| Ppd-A1 | Ppd-A1a1 | 1085 bp deletion in the promoter region | Insensitive | [49] |
| Ppd-A1a2 | 1027 bp deletion in the promoter region | Insensitive | [20] | |
| Ppd-A1a3 | 1117 bp deletion in the promoter region | Insensitive | [50] | |
| Ppd-A1a4 | 684 bp deletion in the promoter region | Insensitive | [51] | |
| ppd-A1b | Wild type | Sensitive | [50] | |
| Ppd-B1 | Ppd-B1a.1 | 308 bp insertion in the promoter region | Insensitive | [49] |
| Ppd-B1a.2 | Four copies of Ppd-B1 | Insensitive | [52] | |
| Ppd-B1a.3 | Three copies of Ppd-B1 | Insensitive | [52] | |
| Ppd-B1a.4 | Two copies of Ppd-B1 | Insensitive | [52] | |
| Ppd-B1e | — | — | [53] | |
| ppd-B1b | Wild type | Sensitive | [52] | |
| Ppd-D1 | Ppd-D1a.1 | 2089 bp deletion in the promoter region | Insensitive | [54] |
| Ppd-D1a.2 | 5 bp deletion in exon 7 | Intermediate | [55] | |
| ppd-D1b.1 | Wild type | Sensitive | [54] | |
| ppd-D1b.2 | Insertion of transposable element in the intron 1 | Sensitive | [55] | |
2.2. Photoperiod genes
Wheat is a long day plant, requiring exposure to long days (> 14 h light) for flowering, whereas photoperiod-insensitive varieties flower early in short days (10 h or less light) [54], [68], [69]. This photoperiod sensitivity is controlled by the semi-dominant homoeologous Ppd1 gene on the short arm of chromosome group 2; as is the case with Vrn1, the dominant allele confers photoperiod insensitivity [70], [71], [72], [73], [74]. The effects of the photoperiod-insensitive allele Ppd were studied thoroughly by Worland [22] over a 14 year period in different wheat-growing regions; their work revealed that insensitive Ppd1advances flowering time by 9–15 days, and that this earliness can be utilized to obtain yield advantages in water-limited environments by drought avoidance. The early Ppd gene also has some pleiotropic effects including reduced plant height and number of tillers, and fewer spikelets per ear [73]. However, an increase in spikelet fertility can compensate for the yield penalty [74]. It is clear that Ppd insensitivity brings forward the time of terminal spikelet formation, thus advancing the flowering time by reducing the number of spikelets in the ear. However, it does not influence the rates of leaf and flower primordial production. There is also variation among the potency of three Ppd1a loci, where plants with Ppd-A1a and Ppd-D1a are earlier in flowering than plants with Ppd-B1a [52]. In the same way that a number of Vrn1 alleles have been identified, a number of alleles and their haplotypes have also been identified recently for all three homoeologous loci of the Ppd gene (Table 2) in both bread and durum wheat [49], [51], [53], [54], [55], [75]. These findings have a great agronomic importance for deployment in breeding programs.
2.3. Earliness per se genes
The Eps genes control flowering time independent of temperature and photoperiod. To date, very few Eps genes have been identified in wheat, but several quantitative trait locus (QTL) studies revealed that most of the chromosome groups carry such genes and that they are present as QTL effects rather than as major genes in the Ppd and Vrn pathways [74], [76], [77], [78], [79], [80], [81]. The Eps genes are involved in the fine-tuning of flowering time [82], and hence can be utilized for adaptation to specific climatic conditions.
3. Molecular intervention of phenology genes
In concordance with studies on Arabidopsis, important progress has been made at the molecular level to elucidate the flowering pathway in wheat. Molecular and sequence analysis revealed that Vrn1 encodes a MADS-box transcription factor similar to the Arabidopsis meristem identity genes APETALA1 (AP1), CAULIFLOWER (CAL), and FRUITFULL (FRU), which regulate the shoot apical meristem to determine the transition from vegetative to reproductive development [36]. Insertions, deletions, and mutations in the promoter regionare associated with allelic variation of Vrn1 [44]. Following this finding, a series of molecular markers have been developed (Table 3) [32], [44], [45], [48], [52], [54], [55], [83], [84] and successfully utilized to identify allele frequency of the local wheat cultivars as well as these from the International Maize and Wheat Improvement Center (CIMMYT) collection [45], [83], [85], [86]. The Vrn2encodes a zinc finger-CCT domain transcription factor and is a floral repressor, down-regulated by both vernalization treatment and short day length [37]. Vrn2plays a very similar role to that of FLOWERING LOCUS C (FLC) in Arabidopsis but actually has no orthologs, suggesting an independent evolution of the vernalization pathways [87]. Vernalization gene Vrn3 is similar to Arabidopsis FLOWERING LOCUS T (FT), and the dominant allele is associated with a retro element insertion in the Triticum aestivum L. (TaFT) promoter, results in early flowering [32]. Recent screening of a set of Chinese wheat cultivars led to the discovery of two more dominant alleles of Vrn3, and 80 days variation in heading has been observed due to their action [83]. Allelic variations of these Vrn1 genes quantify the vernalization effects, and determine flowering time by interacting with photoperiod gene Ppd1. The latter is a member of a pseudo response regulator (PRR) gene family in which insensitivity is associated with deletion or transposon insertion within the promoter region, as well as with copy number variation [52], [54]. In wheat, Ppd1 directly regulates the FLOWERING LOCUS T1 (FT1); mutants with promoter deletions result in the overexpression of FT1, causing early flowering [51]. Markers have been developed to identify the Ppd mutants with different promoter deletions (Table 2) that will facilitate their effects on the flowering time of wheat [49], [51], [53], [54].
Table 3. Polymerase chain reaction (PCR) markers for the different vernalization and photoperiod response alleles.
| Allele | Primer | Primer sequence (5′–3′) | Annealing temperature (°C) | Product size | Ref. |
|---|---|---|---|---|---|
| Vrn-A1a | VRNA1F | GAAAGGAAAAATTCTGCTCG | 50 | 965 and 876 | [44] |
| Vrn-A1b | 714 | ||||
| Vrn-A1c | VRN1-INT1R | TGCACCTTCCC(C/G)CGCCCCAT | 734 | ||
| vrn-A1 | 734 | ||||
| Vrn-A1 | BT706 | CATTGTTCCTTCCTGTCCCACCC | 63 | 1431 | [84] |
| BT750 | ATTACTCGTACAGCCATCTCAGCC | ||||
| Vrn-A1c(Langdon) | Ex1/C/F | GTTTCTCCACCGAGTCATGGT | 55.6 | 522 | [45] |
| Intr1/A/R3 | AAGTAAGACAACACGAATGTGAGA | ||||
| Vrn-A1c (IL 369) | Intr1/A/F2 | AGCCTCCACGGTTTGAAAGTAA | 58.9 | 1170 | |
| Intr1/A/R3 | AAGTAAGACAACACGAATGTGAGA | ||||
| vrn-A1 | Intr1/C/F | GCACTCCTAACCCACTAACC | 56 | 1068 | |
| Intr1/AB/R | TCATCCATCATCAAGGCAAA | ||||
| Vrn-B1a | Intr1/B/F | CAAGTGGAACGGTTAGGACA | 58 | 709 | |
| Intr1/B/R3 | CTCATGCCAAAAATTGAAGATGA | ||||
| vrn-B1 | Intr1/B/F | CAAGTGGAACGGTTAGGACA | 56.4 | 1149 | |
| Intr1/B/R4 | CAAATGAAAAGGAATGAGAGCA | ||||
| Vrn-D1 | Intr1/D/F | GTTGTCTGCCTCATCAAATCC | 61 | 1671 | |
| Intr1/D/R3 | GGTCACTGGTGGTCTGTGC | ||||
| vrn-D1 | Intr1/D/F | GTTGTCTGCCTCATCAAATCC | 61 | 997 | |
| Intr1/D/R4 | AAATGAAAAGGAACGGAGCG | ||||
| Vrn-D1a | VRN1DF | CGACCCGGGCGGCACGAGTG | 65 | 612 | [48] |
| VRN1-SNP161CR | AGGATGGCCAGGCCAAAACG | ||||
| Vrn-D1b | VRN1DF | CGACCCGGGCGGCACGAGTG | |||
| VRN1-SNP161AR | AGGATGGCCAGGCCAAAACT | ||||
| Vrn-3 | FT-B-INS-F | CATAATGCCAAGCCGGTGAGTAC | 63 | 1200 | [32] |
| FT-B-INS-R | ATGTCTGCCAATTAGCTAGC | ||||
| vrn-3 | FT-B-NOINS-F or FT-B-NOINS-F2 | ATGCTTTCGCTTGCCATCC or GCTGTGTGATCTTGCTCTCC | 57 | 1140 or 691 | |
| FT-B-NOINS-R | CTATCCCTACCGGCCATTAG | ||||
| Ppd-D1a | Ppd-D1_F | ACGCCTCCCACTACACTG | 54 | 288 | [54] |
| Ppd-D1_R2 | AND CACTGGTGGTAGCTGAGATT | ||||
| ppd-D1b | Ppd-D1_F | ACGCCTCCCACTACACTG | 54 | 414 | |
| Ppd-D1_R1 and | GTTGGTTCAAACAGAGAGC | ||||
| 16 bp deletion in exon 8 | Ppd-D1exon8_F1 | GATGAACATGAAACGGG | 52 | 320 or 326 and 22, 257, 69, and 22 | |
| Ppd-D1exon8_R1 | GTCTAAATAGTAGGTACTAGG | ||||
| Ppd-B1 | Ppd-B1exon3SNP_F1 | AGACGATTCATTCCGCTCC | 55 | 471, 328, and 155 | |
| Ppd-B1exon3SNP_R1 | TCTGAATGATGATACACCATG | ||||
| Ppd-B1_2ndcopy_ F1 | TAACTGCTCGTCACAAGTGC | 55 | 425 and 475 | ||
| Ppd-B1_2ndcopy_R1 | CCGGAACCTGAGGATCATC | ||||
| 5 bp deletion in exon 7 | D5-1F | GAATGGCTTCTCCTGGTC | 50 | 1032 or 1027 | [55] |
| D5-1R | GATGGGCGAAACCTTATT | ||||
| D5-2F | GTGTCCTTTGCGAATCCTT | 53 | 184 or 179 | ||
| D5-2R | TTGGAGCCTTGCTTCATCT | ||||
| A TE insertion in intron 1 | D520F | AGGTCCTTACTCATACTCAATCTCA | 50 | 2612 | |
| D520R | CTCCCATTGTTGGTGTTGTTA | ||||
| D78F | CCATTCGAGGAGACGATTCAT | 55 | 1005 | ||
| D78R | CTGAGAAAGAACAGAGTCAA | ||||
| Truncated Ppd-B1 gene in the “Chinese Spring” allele | 219H05F2 | TAACTGCTCCTCACAAGTGC | 56 | 425 | [52] |
| 97J10R2 | CCGGAACCTGAGGATCATC | ||||
| Intact Ppd-B1 copies in the “Chinese Spring” allele | Ppd-B1_F25 | AAAACATTATGCATATAGCTTGTGTC | 58 | 994 | |
| Ppd-B1_R70 | CAGACATGGACTCGGAACAC | ||||
| Intact Ppd-B1 copies in the “Sonora64”/”Timstein” allele | Ppd-B1_F31 | CCAGGCGAGTGATTTACACA | 58 | 223 | |
| Ppd-B1_R36 | GGGCACGTTAACACACCTTT | ||||
| Vrn-A1b | Vrn-P2 | CCTGCCGGAATCCTCGTTTT | 63 | 147 or 167 | [83] |
| CTACGCCCCTACCCTCCAACA | |||||
| Vrn-B1b | Vrn-P7 | CCAATCTCACATGCCTCCAA | 59 | 215 or 252 | |
| ATGCGCCATGAACAACAAAG | |||||
| Vrn-B3c | Vrn-P14 | GCTTTGAACTCCAAGGAGAA | 52 | 1401 | |
| ATAATCAGCAGGTGAACCAG | |||||
| vrn-B3/Vrn-B3 | Vrn-P15 | ACTCATCATCACCACTTCCT | 51 | 1499 | |
| TAATGCTTAATTCGTGGCTG | |||||
| Vrn-B3promoter | Vrn-P16 | GTCCATACAAATCATGCCAC | 51 | 491 | |
| TTCTGACAGTTTTAGTTGCG | |||||
| Vrn-B3promoter | Vrn-P17 | GCTTTCGCTTGCCATCCCAT | 62 | 898 | |
| GCGGGAACGCTAATCTCCTG | |||||
| Vrn-B3promoter | — | TTTGAGACAGGAGATTAGCG | 53 | 1131 | |
| ACCATCATGAGGCACCATTA | |||||
| GCTTTGAACTCCAAGGAGAA | 52 | 1425 | |||
| ATAATCAGCAGGTGAACCAG | |||||
| CCGTTCACCATCTATTGCTC | 55 | 1259 | |||
| CACCCAAATCCTTCATCTCA | |||||
| Vrn-B3-RT | — | GGAGGTGATGTGCTACGAGA | 55 | 147 | |
| TTGTAGAGCTCGGCGAAGTC | |||||
The complicated interaction of these phenology genes has resulted in two conflicting models of the flowering regulatory network: The first model, designated as Vrn2 to FT [88], recommends that Vrn2 represses FT expression but that vernalization during winter slightly up-regulates Vrn1, causing down-regulation of Vrn2 and the release of FT expression. This FT then interacts with Ppd1 and again up-regulates the Vrn1 beyond the threshold to initiate flowering under long day length [89]. By contrast, the second model, known as FT to Vrn2, was proposed by Shimada et al. [90], who suggested that Vrn1 promotes FTtranscription, which down-regulates Vrn2 to initiate flowering based on the fact that the maintained vegetative phase (mvp) mutants lacking Vrn1 fail to up-regulate FT. Subsequent detailed experimentation by Distelfeld and Dubcovsky [88] with the mvp mutants segregating for Vrn1 and Vrn2 deletions resulted in evidence to contradict both of the previously proposed models; we therefore suggest that more investigation should be conducted to elucidate the flowering network of wheat, and that doing so may lead to the identification of more genes that interact in the flowering pathway.
4. Dwarfing genes
The introduction of dwarfing (Rht) genes into cereals, including wheat, was a key driver of the green revolution. Since then, Rht-B1b and Rht-D1b (previously known as Rht1 and Rht2, respectively) are the most commonly adopted Rhtgenes in wheat-breeding programs throughout the world [91]. Together, these two semi-dwarfing genes produce the dwarf phenotype, whereas alone in combination with their counterpart Rht-B1a or Rht-D1a, they produce semi-dwarf plants in nature. The plants with these genes are less prone to lodging and are more effective in partitioning assimilates to the grain. Some researchers have suggested that the improved yield potential of such varieties is only limited to a favorable growth environment [92], [93]. However, these specific Rht genes are insensitive to endogenous gibberellins, and produce shorter plants with smaller cells [94]. These smaller size cells are consequently responsible for the shorter coleoptile length, less early vigor, smaller leaf area, lower water-use efficiencies, and poor seedling establishment, especially in water-limited environments [95], [96], [97], [98], [99]. The insensitivity to gibberellins of both the Rht-B1b and Rht-D1b alleles is due to single nucleotide substitutions that create a translational stop codon, TGA, reducing the plant’s ability to respond to gibberellins [100].
Most of the world’s wheat is grown without irrigation; because of the dependence on seasonal rainfall, the potential yield is often hampered by water scarcity. About 50% of rainwater can be lost directly through soil evaporation, whereas early vigor can increase water-use efficiency by 25% and thus improve yield [101], [102], [103], [104]. Again, deep sowing of longer season varieties is often recommended to obtain yield benefits in dry areas, such as those occurring in southern Australia, but seedling establishment is impaired when dwarf/semi-dwarf varieties are sown more than 5 cm deep [96]. Consequently, farmers wait until the first rains before sowing, resulting in between 140 and 330 kg yield loss per week per hectare being reported in Australian wheat crops [105], [106]. Wheat varieties with longer coleoptiles are able to emerge sooner when sown deep, and have greater early vigor [107], [108]. Moreover, early vigor and longer coleoptiles help plants to avoid the phytotoxic effects of residual herbicides, compete against weeds, and reduce evaporative water lossby shading. Hence, breeding for vigorous seedling growth and breeding for longer coleoptiles are the prime objectives for the better adaptation of wheat in water-limited environments [109], [110], [111]. A project with these objectives is currently underway at the CIMMYT in Mexico†.
On the other hand, a number of Rht genes such as Rht 7, Rht 8, Rht 9, Rht 13, and Rht 14 have been reported, which have potential in reducing plant height without affecting seedling vigor and tissue response to gibberellins [112], [113], [114]. Under stress conditions, taller varieties store assimilates in the stem and do not depend entirely on current assimilation for grain filling [115]. Several studies across many favorable and unfavorable environments demonstrated that plants with heights of 70–100 cm are better yielders than those that are taller or shorter than this range [97], [116]. Therefore, the accumulation of minor Rht genes or combination with one of the gibberellins insensitive genes for shorter plant height are desirable [116], [117], as shown by different studies that used Rht8 and/or Rht13 alleles with Rht1 and/or Rht2 to maximize yields compared with other dwarf/semi-dwarf varieties [91]. Markers linked to these Rht alleles make it easier to select both alleles simultaneously across a large population [118], [119].
5. Physiological aspects of phenology and dwarfing genes
Grain yield is strongly influenced by the timing of developmental stages in a particular environment, making crop phenology a critical component for yield physiology [120]. Moisture stress at the reproductive stage, especially that period from a few weeks before anthesis to a few days after anthesis, has the most critical effect on crop yields in water-limited environments [25], [121]. Passioura [122] emphasized the importance of water use, water-use efficiency, and harvest index (HI) for crop yields in dry areas. In dry environments, an important portion of soil moisture that could be available for transpiration is evaporated from a barren soil surface, thus indirectly affecting dry matter accumulation by limiting water availability to roots, and modifying canopy temperature [123]. In this situation, faster early seedling growth is beneficial to prevent evaporation by shading. Moreover, late-flowering cultivars continue to produce tillers until they receive the signal for reproductive development, and many of them cannot produce fertile spikes, but put pressure on the available soil moisture through normal transpiration. In this regard, heading date and effective tiller number should be additional considerations for improving water-use efficiency in varieties being developed for drought environments. Moreover, water requirement varies throughout the growth period and is higher during seed setting and development stages. Hence, there is an opportunity to improve yield through changes in crop development. The synchronization of crop developmental stages by phenological adjustment with seasonal moisture availability should be the most important target for new wheat varieties being developed for water-limited environments such as those that occur in Mediterranean climate regions.
HI and ultimately final grain yield largely depend on pre- and post-anthesis biomass production, mobilization of assimilates to florets, and the pattern of water supply during the life cycle [111], [124]. One strategy for raising the HI may be increasing the assimilate movement to developing florets, which will prevent floret abortion before anthesis. This can be done by increasing the duration of spike growth with a reduction in the earlier period for larger ear development [16]. Moreover, this larger ear will also contribute more photosynthate during grain filling along with the flag leaf, thereby increasing the HI. Studies on two alternative spring alleles of Vrn-A1 have shown their significant influence on the variation in root and vegetative morphology such as rosette growth habit, plant height, and leaf length [125]. A significant relation has recently been observed between the duration of pre-anthesis growth phases and the tillering and dry matter accumulation [126]. A detailed study of Australian wheat cultivars over several years and a wide range of locations has revealed that cultivars with one spring allele in any of the three Vrn1 loci are the earliest in heading when compared with cultivars having two spring alleles [127]. Again, spring alleles in all three Vrn1 loci have very small effects in forwarding the heading date, which suggests the presence of epistatic or overdose effects. This study also showed that Vrn-B1 has a weaker effect on the reduction of heading time compared with Vrn-A1 or Vrn-D1. Recently, however, it has been shown that Vrn-B1 has the greatest effect on grain yield [128].
Semi-dwarf varieties with Rht-D1b are advantageous over Rht-B1b in environments with high maximum temperatures and lower rainfall during the flowering and grain-filling periods, as Rht-D1b is associated with less leaf porosity in plants relative to Rht-B1b, leading to slow transpiration before heading and leaving more soil moisture for later use [128], [129]. Plants reduce their water use during drought stress by means of accelerated leaf desiccation and death, which causes a reduction of current photosynthate [130], [131], [132], [133]. As a result, stem reserves become an important source of carbohydrate for grain filling [134], [135], [136]. However, Rht-B1b and Rht-D1bgenes reduce stem reserves by 35% and 39%, respectively [137]; hence, taller varieties often perform better in stress environments when there is a shortage of assimilates, compared with the modern dwarf cultivars.
6. Future strategies
Difficulties in the identification and precise measurement of key physiological determinants of yield is the bottleneck in the improvement of drought tolerance in plants, and its complex genetic control makes progress more difficult [138], [139], [140]. Hence, the improvement of plant traits at both physiological and molecular levels is vital in order to address this complex issue.
The current use of automated high-throughput plant-phenotyping facilities greatly assists researchers in phenotyping plants more precisely and accurately. An in-depth understanding of plant physiology will help dissecting the genetic components of drought tolerance, while molecular and genomic tools will help to identify candidate genes and QTLs for drought-tolerance traits. The integration of physiology with molecular tools will provide new insights into gene function. To optimize output in drought research, a detailed knowledge of the growing environment and of genotype-environment interactions is essential. Fine-tuning a genotype to a specific environment is possible by combining the best-suited alleles of phenology genes to adapt better in the existing environment.
Recent studies have revealed that copy number variation of phenology genes also plays a vital role in crop adaptation. An increased copy number of Ppd-B1confers earlier flowering, and an increased copy number of Vrn-A1 requires a longer vernalization period and is thus associated with late flowering [52]. A similar investigation for the copy number variation of Ppd-B1 in Australian wheat cultivars resulted in the identification of five alleles with one to four copies as well as a null copy of Ppd-B, where plants with an allele with a lower copy number were the latest in heading relative to plants with an allele having more copies [53]. In addition, the haplotypes variation identified in other studies for these genes was found to affect several yield-contributing parameters, and thus adaptation to different environments [51], [55]. As a result, several attempts have been made to determine the value of the alleles of Vrn1 and Ppd1 genes over the past few years in the local environments of different countries [52], [55], [68], [73], [83], [84], [86], [127], [128], [141]. In most cases, the plant material did not cover all the available alleles present in nature, or even the same allele in different genetic backgrounds. Thus, obtaining the true effect of an allele in breeding a variety will warrant the development of appropriate near-isogenic line (NIL) populations of the locally adapted cultivars with different alleles, which will require significant effort. An earlier example of a successful attempt is Triple Dirk, which was developed by Pugsley [30] to study the alleles of the Vrn1 gene; at present, the John Innes Center in the UK is performing substantial research in developing this type of population for different Ppd1 alleles. Such efforts will certainly advance research into phenology genes in optimizing plant development and productivity in local water-limited environments. Therefore, intensive and thorough research to optimize the effect of each allele/haplotype of the phenology genes would enable plant breeders to determine the basic genetic architecture of wheat in each key growing environment, for better yields under stress conditions. Thus, once molecular and physiological tools are used to identify and prove the efficiency of different traits and their regulating genes for drought tolerance, these various useful traits could be aggregated in the base population through a marker-assisted gene pyramiding scheme, as demonstrated by Servin et al. [142]. In summary, success against the adverse effects of climate change relies on the consequences of proper characterization of target environments (i.e., soil properties, precipitation pattern, drought severity, and etc.); and then on designing an appropriate crop ideotype that combines useful phenology with other drought-tolerance-attributing genes, along with good management practices.
7. Conclusion
Drought is a major threat to world agriculture, and is predicted to worsen in the near future due to climate change. Wheat is the most widely grown cereal cropin the world and is vital for global food security. Altering the developmental stages and maturity of wheat is one of the best ways to combat drought without compromising yield. However, current knowledge about the number of genes that control flowering and maturity in wheat is limited. Based on knowledge obtained from the model plant species Arabidopsis, in which more than 80 genes have been reported to control flowering, it is logical to conclude that many new genes and genetic pathways for wheat flowering and maturity are yet to be discovered. However, the fact is that different genetic pathways finally converge, interact, and ultimately lead to the activation of floral identity genes in the floral primordia [143], and these interacting networks that promote flowering are yet to be unveiled. As significant research efforts are currently underway, knowledge on wheat-flowering genes and pathways will increase over time and will require advances in computational biology in order to integrate and interpret this information. In addition, future international collaboration will help to combine the cumulative efforts of the research underway in different research groups and disciplines; the challenge for the breeders will be to integrate this work into new genetic combinations.
Compliance with ethics guidelines
M.A.N. Nazim Ud Dowla, Ian Edwards, Graham O’Hara, Shahidul Islam, and Wujun Ma declare that they have no conflict of interest or financial conflicts to disclose.