1. Introduction
Bone functions in human body range from shielding and supporting other vital organs to producing blood, storing minerals, housing several progenitor cells, and many others. Treatments of many bone complications including osteogenesis imperfecta, osteomalacia, osteoporosis, osteosarcoma and traumatic injuries may require bone sectioning inducing cavities, imperfections and non-unions. Considering that the natural healing capability of bone tissue is reduced by age and injuries, the gold standard in such cases has been autogenous bone grafting. It consists in removing bone segments from other parts of the same patient's body to fill the gap in the damaged area. Bone grafting can lead to donor site morbidity, excessive inflammation, disease transmission, extensive hospitalization and rehabilitation, infection and limited availability besides patient pain and suffering [1]. In order to address the high rate of the complications associated with cancellous bone grafting, scientists developed various synthetic materials as bone implants and scaffolds. Therefore, synthetic orthopedic implants, including prosthetic joints and fixation plates, became a routine practice with worldwide growing number of procedures annually. However, scientists and clinicians are still dealing with many challenging issues of the currently used synthetic strategies imposed by the bioinert nature of commonly used materials, inadequate mechanical strength, stress shielding, fibrous encapsulation around the implant and insufficient expression of extrinsic factors required for the regeneration process. One possible strategy to address these challenges is to functionalize the synthetic material. So far, a wide variety of surface modifying approaches ranging from mechanical and physical to biological and chemical functionalization have been applied to provide material surface with specific microstructure, topography, porosity as well as chemical and biological characteristics to optimize mechanical functionality and modulate the implant-tissue interaction [2], [3], [4]. Surface nanocrystallization, multiscale surface roughening, surface patterning through lithography, photoetching, anodization, etc., and various coating techniques have been employed to modulate protein absorption, guiding cellular contact and growth, enhancing extracellular matrix deposition, as well as limiting bacterial adhesion and proliferation [5]. Rapid development of such approaches have led to advanced and refined solutions that provide mechanically stable and reliable implant solutions. However, still considerable re-operation rates are reported at later follow-ups involving persistent symptoms, progression of degenerative changes, non-healing damages, implant loosening, infection and antimicrobial resistance associated problems; the latter has been recognized among the top three issues affecting human health [6], [7], [8]. Moreover, current bone fracture treatments largely fail to address open fractures and critical size bone defects when the natural healing process is impaired and external intervention is required for large defect healing and union.
The aforementioned challenges and complications have pushed new trend of solutions towards developing multifunctional and bioactive bone scaffolds acting as local drug delivery platforms, which are intended to dynamically contribute to the regeneration process interfering with the host body response, promoting integration, oteoconduction and angiogenesis, impeding bacterial infection and/or offering many other desired functions to promote faster and secure healing. Controlled and regulated administration of a series of biologically active molecules inducing signals to direct bone regeneration are found to effectively increase bone healing and regeneration process [8], [9], avoiding systematic toxicity that is commonly caused by traditional drug administration methods and promoting their effectivity due to higher concentration of drug in the implantation site [10], [11]. Locally delivered molecules can range from therapeutic agents including antibiotic and anti-inflammatory substances to various growth factors, proteins, enzymes, and non-viral genes (DNAs, RNAs) that can be used to address musculoskeletal syndromes in different ways.
Sustained local delivery of therapeutic agents via functionalized implant material has the potential to address the common intrinsic challenges of systematic drug delivery such as inadequate physiological stability. High doses of drug administered to achieve sufficient therapeutic effects, due to the lack of targeting specificity and solubility of some common drugs, can regularly lead to adverse drug reactions including increased antibiotic resistance, as well as renal and liver complications [12]. Local delivery can also reduce the risk of potential toxicity and increase cost and time efficacy.
The bone scaffold used as potential drug career can contain the active substance on its surface or within the bulk structure [13], [14], [15], either through physical incorporation or chemical bonds and immobilization; it should preserve the stability of the active molecules over time and ensure precise control over the substance's release rate [16]. The drug loaded in the bulk material can be released in the physiological environment through diffusion, matrix degradation over time or drug discharge by osmotic pressure [17]. Surface grafting approach can be either dipping and soaking, chemical bonding or incorporation of the drug in surface coatings. Most surface grafting approaches are prone to burst release and provide less control on the release kinetics. Many parameters including size, composition and the microstructure of the drug career as well as its molecular weight, drug solubility, drug loading method and its efficiency can directly affect the release kinetics over time [18]. For example, in case of porous templates, the drug loading step is commonly based on capillary system through immersion in concentrated aqueous drug solution or fluid impregnation of the surface material. In these cases, delivery is mainly through diffusion and thus controlled by the pore size, particularly in nanoporous scaffolds/coatings where the pore size becomes comparable with the drug molecule size [19].
Surface coatings to add desired functions through incorporating specific drugs to facilitate local drug delivery have been widely exercised to functionalize the surface of biomedical implants. FDA approved biodegradable polymer films have been broadly used to deliver active therapeutic cargo to modulate the tissue response and healing rate [20]. Among polymer materials, poly(lactic acid-co-glycolic acid) (PLGA) is unquestionably the most widely used drug career in bone tissue engineering [21]. Nevertheless, the inadequate mechanical strength of polymer materials has limited the clinical application and has motivated the fabrication of polymer based composite materials [166], [167]. On the other hand, deposited polymeric coating are reported to cause complications including detachment, compromised chemical stability in biological environment and probable adverse reactions from the side products; thus studies have focused on application of inorganic coatings as drug career [11]. Calcium phosphates (CaPs) are among the most common choices in hard tissue engineering thanks to the high similarity of their composition to bone mineral, outstanding bioactivity and cost effectiveness. Their physical and chemical characteristics have made them a suitable drug career and thus they have been widely studied as cements/bone scaffolds/coatings on implants as delivery vehicles for various growth factors and drugs for bone regeneration [22]. However, CaP based coatings, although offering additional biomimetic and osteogenic characteristics, have limited mechanical strength due to their intrinsic brittleness. This problem can also be resolved by adopting composite coatings as enhanced compressive strength and elastic modulus are reported for hydroxyapatite (HaP)-poly(epsilon-caprolactone) and HaP-chitosan-gelatin composite coatings/scaffolds [23], [24].
More recently, bioactive glasses particularly, silica-based mesoporous materialshave attracted more attention for drug delivery purposes in bone tissue engineering thanks to the high surface area and large pore volume, which facilitate incorporation of larger amounts of drug [25], [26]. Besides efficient drug loading and release characteristics, such ordered macro/meso porous platforms have high potential for promoting cell penetration, bone ingrowth as well as vascularization and bone oxygenation thanks to their biomimetic hierarchical and interconnected porous architecture [27]. Drug loading and sustained release can be also precisely modulated through chemical modification of pore walls via electrostatic interactions, controlling hydrophilic characteristics via hydrophilic-hydrophobic forces or electronic interactions [26], [28]. Another widely used approach to obtain more control on the release kinetics is to use nano/micron size drug careers which have been dispersed in the homogeneous or composite scaffolds and coatings [8].
Despite all the advancements, there are still challenges to be addressed in the field of local bone drug delivery including effective and sustained release control, prolonged drug stability and activity, toxicity issues as well as immune inflammatory response [29]. In this review paper, multiple recent concepts of implantable devices for sustained bone drug delivery and their limitations are discussed. The choices to enhance the therapeutic effects of the bioactive agent loaded bone scaffolds are highlighted.
2. Advanced drug loaded bone implants
Wide varieties of scientific disciplines are involved to advance the bone tissue engineering field and respond to the high demand for hard tissue replacements imposed by the aging population, recognized as one of the leading reasons of patients visiting doctors by The American Academy of Orthopedic Surgeons (AAOS) [22]. In this section, we review and discuss the main current approaches of drug functionalizing of synthetic bone implants to overcome clinical limitations addressing the most common challenges.
2.1. Anti-bacterial agents delivery
Post-surgical implant-associated infections are among the major causes substantially increasing the likelihood of revision surgeries and accounting for more than half of the hospital acquired infections [22], [29], [30]. Total hip replacement surgeries are reported to have an infection rate ranging between 0.5 and 3% [31] with a re-infection rate of up to 14% even after revision surgery [32]. Open fractures are also reported to have a significantly high infection rate of 20% [33]. Therefore, local antibiotic delivery at the implantation site is among the most common drug delivery applications for synthetic orthopedic implants [34].
Infection is normally initiated through adhesion and colonization of micro-organisms in the implantation site coming from the outside (skin, surgical instruments or the environment), by bacteria on the patient's skin or those present in the body which can become pathogenic [35], [36]. Such infections are typically managed by extensive surgical debridement and irrigation, implant removal and extended anti-biotic treatments that have in turn additional adverse effects including patient trauma, prolonged hospitalization with serious health and social costs [37], [38]. Thus developing implants with intrinsic antibacterial properties can reduce the risk of late complications and potentially decrease the associated enormous financial and social burden. Integration of antibacterial agents in the implants has been used to develop bacteriostatic and bactericidal effects as well as antibiofouling properties achieved through controlled surface topography or surface chemistry [39]. Current implant based strategies include coatings, bone cement or composite material releasing active antibacterial agents or inclusion of antibacterial loaded polymethylmethacrylate (PMMA) beads [40], [41], [42]. Such approaches have been relatively successful in reducing infection rates; however, their initial burst release and poorly controlled release profiles are still far from optimized [8]. Inadequate bonding of the coating to the substrate and the necessity of a retrieval surgery to remove the not biodegradable PMMA beads are among other downsides of the current procedures. Composite coatings of osteoconductive mineralized biomaterials and various antibacterial agents have been widely used to move towards sustained release of the active agent at the contaminated site over time.
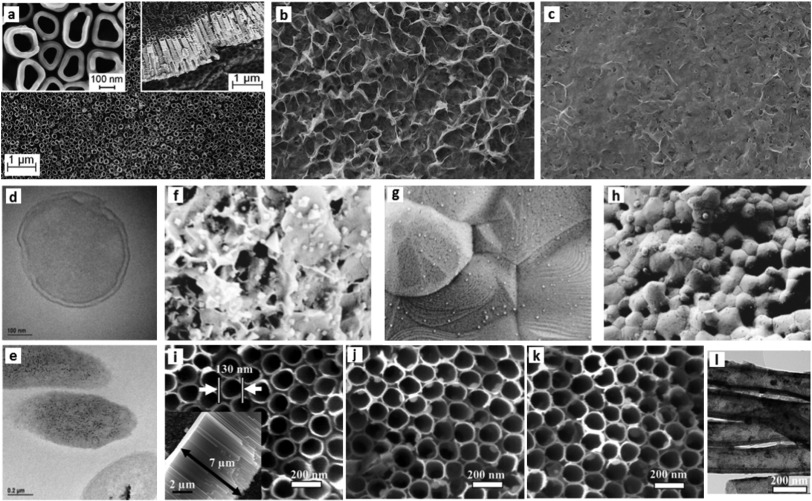
The choice of proper antibiotic agent among the many possible options, is of utmost importance as few antibiotic drugs have demonstrated adverse effects on osteogenic cells' activities at bactericidal dosages [43]. Vancomycin was reported to have less side effects among possible common choices [44]. Vancomycin loaded CaP coating on Ti rods showed reduced infection through degradation of the drug eluted coating in an in vivo rat model [45]. HaP has been used to deliver multiple antibiotics as amoxicillin, clavulanic acid and erythromycin [46]. Multi-layered TiO2 nanotubes-calcium phosphate and a phospholipid coating (Fig. 1(a)–(c)) loaded with an antimicrobial peptide applied on the surface of Ti implants was reported efficient in controlling release kinetics resulting in reduced infection caused by Gram-positive (Staphylococcus aureus) and Gram-negative (Pseudomonas aeruginosa) bacteria with no toxicity to osteoblast cells and negligible red blood cell lysis [47].
 Fig. 1. SEM observation of three thin layers of multilayered coating impregnated with antimicrobial peptide (a) bottom titania nanotube layer obtained by anodization technique with the inserts showing top and lateral views (b) middle layer of CaP crystal flakes formed by drop and dry covering nanotubes (c) topmost phospholipid that provides sustained release [47].TEM images of P. aeruginosa before (d) and after (e) silver treatment showing the silver nanoparticles (black) granules mounted up inside the bacteria [48]; SEM micrograph of silver particles synthesized on (f) CaP (g) HaP (h) β-tricalcium phosphate coatings [49]; SEM images of silver impregnated titania nanotubes (NTs): (i) TiO2-NTs, (j) NT-Ag1.0, and (k) NT-Ag2.0. In the inset in (i) the NT length is measured around 7 μm, (l) TEM image of NT-Ag1.0 [50].
Fig. 1. SEM observation of three thin layers of multilayered coating impregnated with antimicrobial peptide (a) bottom titania nanotube layer obtained by anodization technique with the inserts showing top and lateral views (b) middle layer of CaP crystal flakes formed by drop and dry covering nanotubes (c) topmost phospholipid that provides sustained release [47].TEM images of P. aeruginosa before (d) and after (e) silver treatment showing the silver nanoparticles (black) granules mounted up inside the bacteria [48]; SEM micrograph of silver particles synthesized on (f) CaP (g) HaP (h) β-tricalcium phosphate coatings [49]; SEM images of silver impregnated titania nanotubes (NTs): (i) TiO2-NTs, (j) NT-Ag1.0, and (k) NT-Ag2.0. In the inset in (i) the NT length is measured around 7 μm, (l) TEM image of NT-Ag1.0 [50].Contrary to many antibiotic drugs, biocidal metals present high efficiency and both bacteriostatic and bactericidal characteristics against a broad spectrum of pathogens. Moreover, their extraordinary advantage is that the bacteria cannot develop resistance against them, as it is the case for most common antibiotic drugs [51], [52], [53]. These agents such as silver, zinc and copper have attracted a great deal of attention for developing contact killing surfaces, considering their wide application in wound healing solutions since ancient times and no toxicity effects in controlled concentrations in human body [54]. They have been widely used to suppress/prevent infection in wounds [55], [56], many biomedical devices such as urinary and peritoneal catheters, cardiovascular grafts, heart valve sewing rings, surgical sutures, fixation devices [57], [58], [59]and textiles [60]. Their mechanism against bacteria is postulated to include inactivating respiratory enzymes, meddling with DNA replication and unsettling the cell membrane [49], [61], [62].
Silver is undoubtedly the most widely used metal agent for antibacterial purposes [63]. A recent study reveals the even more prolonged bactericidal effect of silver ions, transmitted through the bacteria already killed by silver to viable populations of the same strain. In this so called “Zombie effect”, the bacteria act as silver cations reservoirs even after death [48]. Fig. 1 shows the TEM images of cross sections of P. aeruginosa before (Fig. 1(d)) and after (Fig. 1(e)) being treated by silver nitrate, illustrating the silver nanoparticles visibly accumulated throughout the dead bacteria.
Metallic bactericidal agents have been used also as composite coatings to functionalize inorganic components of bone such as CaP. Roy et al. studied the antibacterial effect of electrodeposited Ag on tricalcium phosphate (TCP) coated titanium substrates against Pseudomonas aeruginosa and Pseudomonas aureus to assess the optimal Ag concentration for desired bactericidal effect with no toxicity [40]. Various spray-coating techniques have been also used for fabrication of Ag based antibacterial composite coatings. Cold spray coating, as an emerging spray based coating process with growing applications in many industrial fields, was used to prepare samples using a composite of PEEK (polyether-ether-ketone) and silver-doped HaP powders as well as chitosan-copper composite [41], [64] and Novaron VZ 600, a commercially available inorganic antimicrobial material functionalized with ZnO [65]. The results confirmed the bacteriostatic characteristics of the composite coatings against various pathogenic bacteria species. The antibacterial effect was postulated to be due to both the presence of antibacterial agents and the surface topography on the coating that could have contributed to decrease the bacterial adhesion.
With the advancement of nanotechnologies, various medical applications have been examined for silver nanoparticles ranging from nanosilver-incorporated textile and wound dressings to nanosilver coated medicinal implants [66], [67], [68]. The attention is due to the notably increased surface area to volume ratio of nanoparticles as well as their superior chemical and physical characteristics [68]. Silver nanoparticles have been incorporated either in polymer or ceramic matrixes and evaluated in terms of mechanical stability and antibacterial effects [66], [69]. Alt et al. [70] loaded PMMA bone cement with silver nanoparticles of 5–50 nm. In vitro toxicity and antibacterial tests against multi-resistant bacteria strains showed the higher efficiency of nanoparticles in inhibiting bacteria proliferation with no toxicity compared to commercial silver powder of 500–1000 μm. Lee and Murphy incubated CaP in silver nitrate solution to grow silver micro- and nanoparticles on CaP coatings and present a sustained release within the clinically acceptable range of total release. The fabricated coating showed minimized burst release and was validated against gram-positive Staphylococcus aureus and gram-negative Escherichia coli [49]. Fig. 1(f)–(h) show SEM micrographs of silver nanoparticles on various Cap minerals including also HaP and β-tricalcium phosphate as typical examples of CaP minerals showing the versatility of the silver releasing coating methods [49]. Feng et al. [71]deposited silver nanoparticles on HaP coated Alumina substrates, reporting strong antibacterial effects against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Staphylococcus epidermidis. Similar results are reported for magnetron co-sputtered and plasma sprayed silver-doped HaP coating [72], [73], spin coated HaP coatings incorporated with Ag or Zn ions on Ti alloy substrate [74], silver substituted HaP synthesized by microwave processing [67], and silicon elastomers impregnated with Ag nanoparticles [66]. In another study, silver nanoparticles were adhered to the inner walls of titania nanotubes along the entire length through immersion in AgNO3 and UV irradiation. The impregnated nanotubes were then coated on titanium implants (Fig. 1(i)–(l)). The coated implants showed effective antibacterial properties against planktonic bacteria in the first few days as well as reduced bacterial adhesion up to 30 days [50]. Similar antibacterial results were reported by Das et al. [75] who electrodeposited silver on titania nanotube surface that exhibited excellent antibacterial activity against Pseudomonas aeruginosawithout interfering with the human osteoblast bone cell activities.
Implant associated infections can substantially hinder the regeneration process and remain as a leading post-surgical issue. Moreover, with the antibiotic resistance on the rise, future trends should minimize the misuse of antimicrobials and provide alternative safe solutions to manage bacterial infections.
2.2. Growth factor delivery
The role of growth factors (GF), as large group of polypeptides with the ability to locally regulate cell activities, in simulating osteointegration, bone formation and remodeling and tackling impaired healing is indisputable. GFs are known to contribute to stimulating the intricate biological cascade of bone regeneration and thus have the ability to enhance bone healing rate by promoting cell proliferation and differentiation as well as accelerating osteoclastic resorption [76], [77]. Osteogenic GFs as bone morphogenetic proteins (BMP's), recombinant human bone morphogenetic protein (rhBMP), vascular endothelial GF (VEGF), platelet derived GF (PDGF), insulin-like GF-I (IGF-I) and transforming GF β1 (TGFβ1), are among numerous GFs which help recruiting progenitor cells (MSCs) to induce chondrogenesis, osteogenesis and angiogenesis at bone injury site [8], [78]. Being osteocunductive, the subfamily of BMPs have the distinctive capability of inducing bone formation also beyond bone tissue through activating differentiation of pluripotent cells into bone forming ones [18]. Such osteocunductive factors are particularly needed in case of critical size defects. TGF- β, on the other hand is known to mediate osteoprogenitor cells metabolism, modulate inflammatory response and promote angiogenesis [79]. IGF is also reported to affect osteoprogenitor cells proliferation, mineralization and migration [80]. A detailed review on GF's contribution to bone tissue healing process can be found in [81]. Despite their notable potential and the development of techniques that enable large quantity production of high purity GFs, their wide and efficacious application in bone healing is still partially restricted considering the proteins' fast diffusion from administration sites and lack of adapt methods that provide the possibility of their precisely controlled release [18].
Various approaches have been used to locally incorporate GFs in the injured bone cite. Collagen-based scaffolds impregnated with GF [83] and GF loaded CaP coatings on orthopedic and craniofacial implants have been widely used [22]. Probably the most studied GF is the rhBMP-2 that is one of the few members of transforming BMPs superfamily identified to be certainly efficient in simulating the whole process of stem cell differentiation into mature bone forming cells [84]. It has been used in pre-clinical and clinical studies within multiple careers ranging from natural polymers including hyaluronic acid [85], [86], alginate [87], chitosan [88] and silk fibroin [89] to biodegradable synthetic polymers as poly(lactic acid) (PLA) [90] and poly(lactic-co-glycolic acid) (PLGA) [91]. Fast and sustained release of rhBMP-2 is reported to promote fast cell infiltration in the scaffold and new bone formation respectively [82], [92]. A detailed study confirmed the role of delivery scheme and dose dependency of bone regeneration to rhBMP-2 delivery. In this study, a hybrid poly-(3-caprolactone) (PCL) nanofiber mesh-alginate template was used to fill a 8 mm defect in an animal model with different rhBMP-2 dosages (0.1–5 μg) and compared the results with a collagen template incorporating the same drug dosages [82]. The results indicated higher efficiency of the hybrid template with a notable dose dependency of bone formation and its quality in terms of volume, density and mechanical characteristics (Fig. 2). The bridging happened much earlier using the hybrid template compared to the collagen one, highlighting the importance of the drug career in the delivery process.
 Fig. 2. Digital radiographs (left) and microCT reconstructions (right) of the critical size rat bone defect treated with collagen sponge and nanofibermesh/alginate scaffolds incorporated with rhBMP-2 after 12 weeks. Saggital cross sections depict mineral density maps as an indication of maturity of the newly formed bone [82].
Fig. 2. Digital radiographs (left) and microCT reconstructions (right) of the critical size rat bone defect treated with collagen sponge and nanofibermesh/alginate scaffolds incorporated with rhBMP-2 after 12 weeks. Saggital cross sections depict mineral density maps as an indication of maturity of the newly formed bone [82].There are also indications of stimulating effect of BMP-2 in vasculogenesis [93], [94] that will largely increase bone healing rate [95]. Two examples of clinical application of BMP-2 delivery are the US Food and Drug Administration (FDA) approved Infuse® Bone Graft (Medtronic, Memphis, TN USA) loaded in collagen sponge and OP-1 (Olympus Biotech) rhBMP-2 loaded collagen powder used in degenerative spinal diseases and fusion cages, tibial fractures as well as sinus and alveolar ridge augmentations [96]. In vivo continuous release and improved ossification was reported upon BMP-2 delivery using a biomimetically coprecipitated CaP and BMP-2 coatings on Ti disks implanted in rat model for five weeks. The control groups, in which BMP-2 was superficially absorbed to the coatings, showed a much lower efficiency mainly due to the burst release [97]. A study on osteointegration of HaP-coated Ti implants soaked in nonglycosylated BMP-2 in aged sheep model confirmed the positive role of the released drug even in aged-compromised individuals. The in vitro results also emphasized the role of loading concentration on the release profile [98]. Another example of obtaining desirable osteocunductive properties through incorporation of GFs in the bone implants is using the combination of a porous Ti fiber mesh and a CaP coating loaded with recombinant human Transforming GF β1 (rhTGF-β1) in a series of in vivo experiments on a rabbit non-critical size cranial defect model up to eight weeks [24], [99].
Soaking and absorbing drugs in the coatings have turned out to be effective in promoting osteointergarion, new bone formation and filling the gaps; however, the results of other studies point out that incorporation of the GFs in the coating rather than simple absorption can be much more effective in osteoinductivity. In case of a CaP coated implant the sustained delivery was achieved through osteoclast-assisted degradation of the coating compared to the notable initial burst release characterizing coatings with superficially absorbed osteogenic agents; this observation highlights the role of delivery mode in controlling the efficacy of the GF [100], [101]. Another common delivery approach is to cover the implant with a drug loaded degradable sleeve providing fine control on the type and dosage of the drug. Henslee et al. [102]used a porous polypropylene fumarate (PPF) sleeve to encase a solid PPF intra-medullary rod, where rhBMP-2 loaded poly(dl-lactic-co-glycolic acid) (PLGA) microparticles were incorporated into the porous sleeve.
An important aspect that cannot be ignored is the contribution of endothelial cells in the bone fracture healing. Vasculogenesis is an extremely important process in bone repair as new bone formation, the regenerative capabilities of macrophages, fibroblasts, and endothelial cells and the antibacterial characteristics of granulocytes are all affected by perfusion rate of oxygen, metabolites and cytokines [77], [96], [103]. Pro-angiogenic VEGF delivery has increasingly shown potential in bone tissue engineering enhancing osteoblast differentiation and vascularized new bone formation using various forms of delivery ranging from CaP coatings on Ti implants [104] to collagen nano-HaP scaffolds [105] and injectable hydrogel systems [106].
In spite of the positive results reported in case of local GF delivery, the problems of finding the desired doses and optimized release profile are still to be resolved. High doses of rhBMP-2 have been reported to increase the risks of ectopic bone formation, erythema, oedema, and bone resorption in treatments of tibial fractures [107]. More recently safety concerns were raised caused by reports of adverse side effects including ectopic bone formation, osteolysis and renal problems, etc. associated with rhBMP-2 used in spinal fusion surgeries [108]. Another issue of concern in case of GFs delivery is the extremely limited half-lives of the soluble recombinant GFs in vivo [109], which has limited wide application of this solution.
2.3. Anti-inflammatory and immunosuppressing drug delivery
Implantable medical devices are commonly known to induce inflammatory cascades and adverse immune reactions as foreign bodies. Such reactions can vary from pain, dramatic inflammation to even rejection of the implant all directly affecting the patient life quality and functionality of the implanted material. With inflammation ever more acknowledged as a vital factor affecting the regeneration process, different approaches for delivery of anti-inflammatory and immunosuppressing drugs have been suggested. Multiple soluble anti-inflammatory drugs including dexamethasone, and superoxide dismutase mimic have been delivered from the implant surface or matrix material to successfully reduce foreign body reaction and fibrous capsule formation around the implanted device [110], [111]. Recent advancements in the field of nanomedicine have provided the possibility of sustained delivery of these drugs while preserving the drug's bioactivity over time. Dexamethasone loaded nanoparticles embedded in coatings were reported to notably reduce the tissue reaction to silicon and platinum-polyimide neural probes up to three weeks [111], [112]. Another successful strategy for modulating the inflammatory response to implants is cytokine and GF delivery as they play a significant role in controlling immune cells' phenotype. Poly(ethylene glycol) (PEG) hydrogel with surface immobilized immunosuppressive cytokines (TGF-β1 and IL-10) were reported to significantly decrease bone marrow-derived dendritic cells maturation [113].
A main challenge regarding cytokines direct delivery is the biological stability of clinically appropriate dosages over time. Chemical conjugation of cytokines and gene delivery systems have been used to address this issue maintaining the desired cytokines' concentration at the required levels over time [114].
2.4. Gene therapy and nucleic acid delivery
Gene therapy genetically manipulates the cells to control specific protein expression. Gene-activated scaffolds containing physically entrapped nucleic acid have shown high potential as an alternative method of GF delivery, reducing considerably the toxicity effects at much lower cost of higher pure quantity production. Meddling with the host cells, gene activated scaffolds stimulate the patients' own cells to express the factors that accelerate the regeneration process. Such scaffolds using safe non-viral vectors can offer the possibility of controlling the expression of specific genes mediating corresponding protein expression and can result in bridging even critical size defects in animal models over few weeks [115], [116]. These scaffolds can also be used to differentially control more than one gene expression and subsequent proteins release at slower rates compared to the ones obtained from GF incorporated templates. When encapsulated in different scaffolds, genes seem to be more efficient in transfecting the host cells compared to naked gene delivery.
The first evidences of potential effect of gene delivery in bone tissue engineering was verified in 1999 through incorporation of plasmid DNA (pDNA) in a bovine collagen scaffold used to fill a tibial defect in a canine model. The results indicated extensive time and dosage dependent new bone formation after 6 weeks [117]. Since then many groups have been trying to incorporate nucleic acids in different careers as collagen sponge, PLGA scaffolds to encode VEGF, BMPs and PDGF onto the scaffold and promote angiogenesis and osteogenesis in bone defects in in vivo models [115], [118], [119].
The present data indicate substantial potential of gene-activated bone scaffolds with higher shelf lives compared to the GF loaded ones, to enhance the therapeutic effectiveness of bone implants. An important issue in nucleic acid delivery is to design the appropriate delivery vector that ensures efficient release and entry of the nucleic acid into the appropriate intracellular compartment, since naked nucleic acid lose their efficacy due to the negative charge of their backbone that limits cell attachment and uptake [109].
2.5. Antiresorptive drug delivery
Bisphosphonates (BPs), identified as antiresorptive drivers, are a set of synthetic drugs used in case of musculoskeletal disorders such as osteoporosis, osteolysis and hypercalcemia [22]. The structural backbone of the BPs has the ability to impede osteoclast's activity, reduce the risk of osteoporosis and thus induce osteogenesis. Adverse secondary effects of oral administration or intravenous injection of BPs as well as their low bioavailability, have brought more attention to BP's local delivery [120]. BPs were loaded within plasma sprayed HaP coatings on Ti implants and used in an in vivo study, the results of which revealed enhancement in mechanical fixation and higher density of the peri-implant bone [121].
Alendronate (monosodium 4-amino-1-hydroxybutylidene-1, 1-diphosphonate trihydrate) is a BP drug reported to be effective for osteoporosis and osteogenesis imperfecta perturbing osteoclasts and promoting osteoblast activities. Garbuz et al. [122] used a rabbit femur in vivo model to investigate the role of CaP-coated microporous tantalum (Ta) soaked in alendronate-phosphate buffer (PBS) solution on new bone formation at the presence of a gap between the implant and host bone tissue (Fig. 3(a) and (b)). The results showed notable increase in new bone formation and bone growth into the 400–500 μm pores (Fig. 3(a) and (b)) compared to the control groups after four weeks.
 Fig. 3. Backscattered electron micrographs of the alendronate soaked CaP coated porous Ta implants highlighting the gap filling and bone ingrowth in (a) antiresorptive drug loaded coated porous implant compared to (b) uncoated porous tantalum implant [122]; microCT observation and H&E stained histology sectional images of critical size bone defect filled with collagen-nHaP scaffolds representing the extent of new bone formation 4 weeks post-implantation (c) empty defect (d) gene-free scaffold (e) dual gene activated scaffold (f) Representative image depicting blood vessels ingrowth in the defect area; Blue, green, yellow and white arrows denote respectively fibrotic tissue, acellular regions, new bone formation/nucleation and formed blood vessels [105]. H&E staining (g, h, and i) and Taylor modified Brown and Brenn gram stain (j, k, and l) S. aureus contaminated rat femoral segmental defects with different concentrations of BMP-2 (30 μg/mL) doped nanosilver-PLGA composite bone scaffold 12 weeks after implantation. Trivial bone regeneration is observed at lower concentration of nanosliver ((g) and (h)) with apparent bacterial contamination as shown with red arrows ((j) and (k)), whereas the highest nanosilver concentration represents much higher bone formation (i) and no sign of bacterial contamination (l) [136].
Fig. 3. Backscattered electron micrographs of the alendronate soaked CaP coated porous Ta implants highlighting the gap filling and bone ingrowth in (a) antiresorptive drug loaded coated porous implant compared to (b) uncoated porous tantalum implant [122]; microCT observation and H&E stained histology sectional images of critical size bone defect filled with collagen-nHaP scaffolds representing the extent of new bone formation 4 weeks post-implantation (c) empty defect (d) gene-free scaffold (e) dual gene activated scaffold (f) Representative image depicting blood vessels ingrowth in the defect area; Blue, green, yellow and white arrows denote respectively fibrotic tissue, acellular regions, new bone formation/nucleation and formed blood vessels [105]. H&E staining (g, h, and i) and Taylor modified Brown and Brenn gram stain (j, k, and l) S. aureus contaminated rat femoral segmental defects with different concentrations of BMP-2 (30 μg/mL) doped nanosilver-PLGA composite bone scaffold 12 weeks after implantation. Trivial bone regeneration is observed at lower concentration of nanosliver ((g) and (h)) with apparent bacterial contamination as shown with red arrows ((j) and (k)), whereas the highest nanosilver concentration represents much higher bone formation (i) and no sign of bacterial contamination (l) [136].2.6. Anticancer drug delivery
Considering the frequent toxic effects of chemotherapeutic agents, precise control over the doses and release profile of these drugs is of utmost importance. The undesired and adverse side effects of common chemotherapy and radiation therapy treatments in order to inhibit cancer recurring after removing tumors, have brought more attention to approaches that locally treat cancer cells at tumor site with a high potential of reducing recurrence risk without inducing systemic toxicity [34]. Magnetic hyperthermia has been suggested to be effective in killing remaining cancerous cells in the tumor site using ferromagnetic implants [123] or embolization using ferromagnetic particles [124]; however, their application on bone tumors are not always plausible due to the limited access and thermal inhomogeneity of the bone tissue [125].
Studies performed on the release kinetics of chemotherapeutic agents methotrexate (MTX) and cis-platinum embedded in porous apatite ceramic, as strut grafts in bone defects, revealed sustained release profile in vitro [126], confirming higher efficiency of local implantation in preventing tumor growth compared to intraperitoneal injection [127]. More recently, selenium based nanoclusters with simultaneous anti-cancer, antibacterial and osteogenic characteristics have been developed. Selenium nanoclusters of 20–80 nm grown on surface of stainless steel and Ti implants selectively suppressed bone cancer cells proliferation, while promoting osteoblast proliferation in vitro [128], [129].
In another report, poly-l-lysine based polymer films incorporating risedronate, as an antitumor BP agent, were coated on bone implants to inhibit cancer cells invasion in vitro, presenting a high potential to be used for preventing metastasis after cancerous tissue removal [130].
3. Dual and multiple drug delivery
Bone regeneration and healing process is known to be influenced by a multifaceted cascade involving several GFs and cytokines. Many studies have revealed vital contribution and sequential expression of multiple regulatory factors during the course of bone fracture repair [131], [132], highlighting synergetic interaction of various factors in stimulating bone formation [133], [134].
Considering the prevalence of infection regardless exhaustive surgical care, sustained local antibiotic delivery has become an apparent need after bone surgeries. At the same time, slow and impaired vascularization and regeneration process encourage GFs delivery that has turned out notably efficient in addressing biological challenges of bone regeneration. Therefore, dual or multiple purpose delivery can have a high potential to promote various aspects of the healing process, preserving the wounded area from contamination, while providing the adapt condition for vascularization, bone regeneration or reducing the foreign body effect simultaneously. There are many reports of dual delivery of antibiotics and various GFs, including gentamicin [135] silver nanoparticles [136] and vancomycin [137] delivery combined with rhBMP-2. The results of a composite graft of BMP-2/nanosilver/PLGA, showed simultaneous dose dependent antibacterial and osteoconductive characteristics without any undesirable interference between the effects of different factors (Fig. 3(g)–(l)).
Sequential release of different osteotropic GFs on later stage activities of bone forming cells including alkaline phosphatase (ALP) activity and mineralization is reported to be more effective compared to single GF delivery. The favorable effect of temporal GF release was observed in various studies for instance after early delivery of BMP-2 followed by increased release of both BMP-2 and IGF-I from a two-layered gelatin based coating [138] and also after sequential delivery of BMP-2 and BMP-7 from biodegradable microcapsules incorporated in PLGA scaffolds [139].
A collagen-nanoHaP bone scaffold with optimized structural characteristics was also used for combinative gene delivery to induce expression of both BMP-2 and VEGF in vitro and in vivo, resulting in expressively higher new bone formation and vascularization compared to gene-free scaffolds (Fig. 3(c)–(f)) [105]. The full bridging of the critical size cranial defect in four weeks and the high extent of vascularization compared to control groups, demonstrated the high potential of dual gene delivery in bone regeneration.
Multiple delivery strategies have been also implemented to control the inflammatory response. Combined delivery of immunosuppressive glucocorticoids together with anti-inflammatory IL-6-IL-4/IL-10 cytokines have turned out to effectively induce inflammatory resolution [140]. Considering the risk of impaired angiogenesis as a side effect of glucocorticoids, another study was performed incorporating dexamethasone and VEGF in a composite coating of PLGA micro careers embedded in PVA hydrogel to concurrently suppress inflammation and promote angiogenesis [141].
A very interesting approach was developed by Vorndran et al. [142] who demonstrated the efficient deposition of multiple bioactive molecules with a controlled spatial gradient into a bone scaffold. They used a commercial multijet 3D printer to locally tailor concentrations of rhBMP-2, heparin and vancomycin throughout a porous TCP bone scaffold. The obtained data revealed the possibility of obtaining fine control on the release kinetics from zero order to 0.68%–0.96% per hour using local and gradient dispersion configurations compared to the homogeneous drug loading [142]. This approach showed high potential for increasing efficiency of multiple drugs local delivery with minimal loss of drugs stability through production phase. In a similar approach, Wu et al. [143] demonstrated the application of additive manufacturing technique for construction of a multi-layered poly(dl-lactic acid) (PDLLA) bone construct with different drug models incorporated discretely into multiple layers in a specific sequence. Their results also confirmed the programmed orderly release of different drugs in vitro and in vivo.
Dual and multiple delivery approach to simultaneously modulate different aspects of repair process can be promising methods for the challenging case of critical size defects, where natural bone healing is impaired and addressing various aspects of bone repair is the requisite to achieve proper healing. Substantial effort has been made to expand the assortment of the drugs that can be efficiently delivered simultaneously or sequentially for promoting bone healing; nevertheless, one main issue remaining to be addressed is how to obtain precise control on the release profile of each drug individually in combined therapies. Application of environmentally triggered release, discussed in the next section, seems to be a promising solution.
4. Smart drug delivery
Idealistically, the release of the therapeutic agents should be precisely controlled to correspond with the physiological requirements of the tissue in a dynamic manner throughout the regeneration phase. That can be interpreted as going towards smart and on-demand (aka on/off) delivery of the bioactive molecules.
Technological achievements in fabrication of micro/nanoscale stimuli responsive careers, able to withstand aggressive biological environment over time while maintaining their function and providing precise temporal, spatial and dose control on the release profile, are the key contributor to the fast pace of smart delivery development. To date, proof of concept studies of some of these delivery approaches, as shown in Fig. 4((a)–(j)), have been carried out for different applications to demonstrate the high potential for achieving exogenous (temperature, magnetic field, ultrasound intensity and electric pulses) or endogenous (pH, enzyme quantity) stimulus-driven targeted delivery of therapeutics, responding to slight changes in the surrounding environment. Triggering elements including pH [144], temperature [145], [146], magnetic field [147], [148], ultrasounds [149], [150], [151], oxidation-reduction reactions, enzymes [27] and more recently mechanical strain [152] have been suggested for on demand delivery for various applications. For instance, temperature can rise as a function of infection rate in the surrounding tissue; thus, it can be a suitable triggering factor for on demand release of antibiotics. The main challenge of this approach is to design thermoresponsive careers that are safe and sensitive to slight variations of temperature in the physiological range. Liposomes, polymers or nanoparticles that exhibit a sharp response in their properties with variations in temperature have been used for this purpose [153]. Thermoresponsive liposomes loaded with pro-osteogenic peptides attached to the surface of a collagen-HaP bone scaffold were reported to enhance ALP activity by on demand delivery of the encapsulated drugs upon external thermal stimulus in vitro [154].
 Fig. 4. Recent examples of stimuli responsive drug delivery approaches (a) and (b) Snapshots of sharp shrinking thermo-responsive N-isopropylacrylamide (NIPAM) microparticles, (c) and (d) drug model release upon heating NIPAM microparticle [145]. (e) Schematic of a raspberry structure with a cast steel particle in the center that will trigger the burst of microcapsules containing chemicals (green) as submitted to heat generating magnetic field [147]. (f) Application of ultrasonic waves to nanoemulsions loaded with perfluorocarbon (PFC) generating microbubbles that transfer the drug to the adjacent cells [153]. (g) Liposomes with a pH sensitive PEG shell and cell-penetrating transactivating regulatory protein (TAT) moieties showing higher cell uptake at lower pH to be used for intracellular delivery [157]. (h) TEM observation of MSNs, the parallel strips and the hexagonally packed light dots depict the porous honeycomb structure of the MSNs (i) CdS capped MSN; black arrows indicate CdS aggregations on the external surface of the MSN blocking the pore openings [158]. (j) Graphical representation of various MSN based systems for stimuli responsive drug delivery platforms A) nanoparticles attached to the pores opening as gatekeepers B) Hydrophilic/hydrophobic agents trapped into the nanochannels of the porous structure C) stimuli-driven molecules that link the MSNs and gatekeepers D) polymer layer to protect the MSNs from immune system E) grafted bioimaging agents (fluophore, magnetic nanoparticles, etc.) F) Targeting ligands such as antibodies. G) Complexation with plasmid DNA I) Integrated diagnostic label K) Functional groups to interfere with the cell metabolic system [153], [159].
Fig. 4. Recent examples of stimuli responsive drug delivery approaches (a) and (b) Snapshots of sharp shrinking thermo-responsive N-isopropylacrylamide (NIPAM) microparticles, (c) and (d) drug model release upon heating NIPAM microparticle [145]. (e) Schematic of a raspberry structure with a cast steel particle in the center that will trigger the burst of microcapsules containing chemicals (green) as submitted to heat generating magnetic field [147]. (f) Application of ultrasonic waves to nanoemulsions loaded with perfluorocarbon (PFC) generating microbubbles that transfer the drug to the adjacent cells [153]. (g) Liposomes with a pH sensitive PEG shell and cell-penetrating transactivating regulatory protein (TAT) moieties showing higher cell uptake at lower pH to be used for intracellular delivery [157]. (h) TEM observation of MSNs, the parallel strips and the hexagonally packed light dots depict the porous honeycomb structure of the MSNs (i) CdS capped MSN; black arrows indicate CdS aggregations on the external surface of the MSN blocking the pore openings [158]. (j) Graphical representation of various MSN based systems for stimuli responsive drug delivery platforms A) nanoparticles attached to the pores opening as gatekeepers B) Hydrophilic/hydrophobic agents trapped into the nanochannels of the porous structure C) stimuli-driven molecules that link the MSNs and gatekeepers D) polymer layer to protect the MSNs from immune system E) grafted bioimaging agents (fluophore, magnetic nanoparticles, etc.) F) Targeting ligands such as antibodies. G) Complexation with plasmid DNA I) Integrated diagnostic label K) Functional groups to interfere with the cell metabolic system [153], [159].Magnetic field is another interesting stimulus as it can be used to trigger delivery through magnetic activation (constant magnetic field), thermal stimulation (alternating magnetic field) or combination of these two, besides proving the possibility of performing simultaneous magnetic resonance imaging (MRI) during delivery phase. However, the application of magnetically activated drug carriers is restricted to accessible sites and is not feasible in case of metastasis or distributed tumors. Non-invasive ultrasound waves have also the potential to trigger drug release via their thermal and mechanical effects induced by cavitation or radiation forces. Nanoemulsions or air pocket containing Liposomes have been used as drug carriers activated by ultrasonic waves [155], [156]. Tumors and inflammatory tissues have a fairly more acidic pH compared to the normal body tissue; bacterial infection commonly induces low pH values and there is a wide range of pH variations through gastrointestinal tract that can meddle with absorption of orally administered drugs in the intestine. In these cases, the pH responsive approaches can allow a selective release approach either through changing the solubility of the drug carrier or cleavage of pH responsive bonds upon variation of microenvironment's pH [27], [153]. Detailed discussion on the recent nanocarriers providing on demand strategies of drug delivery is available in an in-depth review in [153].
One popular drug career for on demand drug delivery are mesoporous silicabased nanoparticles (MSNs). Recent advances in fabrication of inorganic drug careers have opened up new prospects for using MSNs, with honeycomb like mesoporous architecture (Fig. 4(h)), as efficient means of smart drug delivery. MSNs have superior biocompatibility and offer the possibility of tailoring structure, morphology and drug loading capacity. Furthermore, they can be surface functionalized by adding specific organic moieties to the pore openings to act as caps modulating the release of the biological substance in response to external stimulus [27].
Fig. 4(h) represents TEM images of MSNs whose channels are physically capped with surface-derivatized cadmium sulfide (CdS) nanocrystals to retain the drug; these caps can be chemically removed for on demand delivery of the encapsulated drug [158]. Multiple factors have been reported to trigger the release of the pharmaceutical agent from the pores of MSNs. Schematic presented in Fig. 4(j) illustrates different configurations of functionalizing the pores and the surface of MSNs to incorporate active molecules [160]. Such options can offer exciting possibilities for achieving site-specific stimuli triggered release of the biological cargo from the pore reservoir. Extensive reviews on functionalized MSN for controlled drug delivery purposes are available in [159], [160].
Potential side effects, lack of degradability, inadequate biocompatibility and limited penetration depth of some externally applied stimulus are some features to be addressed not to hinder the clinical application of some of the smart delivery systems.
It is to be noted that majority of the smart delivery approaches, explained herein, are yet in early pre-clinical phases and need meticulous verification before any commercialization attempt. Moreover, most of these proposed solutions have not been suggested for or applied to orthopedic applications; one of the rare bone related applications until now, is the temperature activated drug delivery liposome based system with the trade name ThemoDox that has reached clinical trials for bone metastases. However, the development of innovative stimuli responsive drug eluting bone implant solutions are anticipated in the near future, considering the incredibly fast progress pace of these systems. It is envisioned that any progress in this area can transform orthopedic interventional care by providing maximum tempo-spatial control on the release profile through minimally invasive methods.
5. Discussion
The chief drives for the first generations of orthopedic implants have been biocompatibility and mechanical performance. Nowadays however, exploiting pharmacodynamic properties of various bioactive molecules to modulate different features of the bone implant is a rapidly expanding area. Contrary to conventional orthopedic implants, the new generation of bone and craniofacial implants are being designed to proactively contribute to the healing process and dynamically interact with the host body. Despite the valuable insight provided by the large body of knowledge produced in the last decades on bone tissue engineering, there is still a large gap between laboratory benches and clinical translation of the suggested solutions. Very recent surveys in the US, Europe and Japan show that up to date only a few of the drug activated products have managed to find their way into the market [161], [162]. The complexity of the bone regeneration process itself, major difficulties in prediction and control of release kinetics, intricate choice of the proper pre-clinical models for approval of the final product, verification of benefit to risk ratio, and lack of funding might be among the major causes of the slow translation of the developed solutions.
To achieve efficient release of bioactive molecules, the drug-impregnated template should substantially ensure achievement of desired dosage of the cargo drug, capacity to maintain agent's stability over time and precise control on the release profile including full retention of the loaded drug before implantation and controlled release rate upon stimulation. Regardless the developments in local drug delivery strategies, still burst release can be considered as the most common challenge in this field, hindering the sustained release of the drug from the implanted device overtime.
Encapsulation in micro/nano careers, using microfabrication and additive manufacturing techniques for production of porous scaffolds/coatings with finely engineered architecture and development of composite templates are the suggested methods that have proved to reduce the initial burst release and increase the chances of obtaining relative control on the temporal and spatial aspects of the release. More recently, substantial progress has been made towards on demand and controllable delivery profiles. These recent strategies open the avenue to new classes of smart implants that besides the established adequate mechanical strength and biocompatibility, possess supplementary features as stimuli responsive drug delivery and even sensing capabilities with the potential to be used for point-of-care applications. There are some reports of incorporation of wireless dental tattoo-based sensor for detection of bacterial infection in saliva [163], but examples of such built-in sensing system in bone implants are still quite rare.
Other common challenge regarding the incorporation of modified proteins and pharmaceuticals in the bone templates are their high cost and low stability. Many researchers have found bioactive inorganic ions such as magnesium, zinc and silicate to be alternatives that can enhance bioactivity and regulate cell functions without common storage and sterilization issues of general therapeutic agents [164].
The currently used trauma management strategies are commonly using all-in packages containing pre-fixed drug dosage. Some prefabricated examples to mention are coatings available in market as Expert Tibial Nail PROTect™ with an antibiotic laden biodegradable polymer coating and Biophan Technologies' nanomagnetic drug delivery system that are both coming with predefined drug type and amount [8]. Recent advancement in the field of local drug delivery are partly motivated by the need to develop personalized therapies meeting the needs of individual patients. Active biomolecule's stability and shelf time is another challenge that has limited the versatility of the developed solutions. Rapid adjustment, easy handling and application, high stability, less sensitivity to storage time, and facile sterilization are needed to obtain customized intraoperative drug delivery solutions providing the surgeon with the possibility to tailor drug type and dosage and administer it to specific zones of the implantable device based on the requirements of individual patients.
On the other hand, frequently harsh preparation techniques, for instance excessively high temperatures required for inorganic implant coatings fabrication, impede the possibility of drug incorporation in the matrix, leaving adsorption and soaking as the only scenario for loading the drug in the coating. Loosely absorbed drugs will consequently reduce the chances to achieve sustained release with precise control on release kinetics due to initial burst release [165].
The choice of the apt combination of the active molecules to be incorporated in the template, management of their synergetic effects besides controlling efficient and intelligent sequential delivery are other challenges that have be addressed to minimize any adverse effects taking full advantage of multiple drug delivery options.
6. Future strategies and conclusions
In addition to offering impeccable structural support, future generation of synthetic orthopedic and craniofacial implants are expected to astutely assist the natural healing process via stimulating new bone formation, mediating host tissue response, reducing infection risk and adding extra stimuli-responsive features based on specific scenarios.
Achieving smart multifunctional implantable devices for hard tissue engineering relies on finding proper approaches of integrating multiple functions in a single platform ensuring efficient encapsulation of the active molecules and eliciting controlled temporal and spatial sustained release of individual therapeutic agent. Future progress in this field can substantially expand the promise of efficient targeted and stimuli responsive drug delivery.
Technological developments reviewed herein, describe numerous limitations, some of which are addressed by this time, and many others are still to be dealt with to enhance the bench to bedside progress of the developed technologies. Advanced encapsulation methods based on micro/nano-technologies and application of additive manufacturing techniques to fabricate multi-layer and multi- material templates have triggered substantial progress towards targeted drug delivery and precise spatio-temporal release control, promising the opportunity to obtain more specific therapeutic actions with predictable release kinetics overtime. The proof of concepts developed for smart drug delivery for various applications together with the possibility of incorporating sensing systems to monitor the healing status, foresee upcoming of multifaceted customized orthopedic implantable devices with thriving capacity of growing supplementary functions.
This future trend is expected to pave the avenue for fabrication of resourceful orthopedic therapies, which can significantly reduce the financial and social burden of current practices, decreasing the post-surgical complications and revision surgeries rate, reducing hospitalization time, minimizing cytotoxicity and adverse side effects, providing timely customized and intelligent treatment and increasing long-standing utility of the implants.