1. Introduction
Cement being the most important binder of all construction materials has immense demand in civil engineering application. The highly reactive nature of cement with water makes it the best suitable option as a binder. The production process of cement involves chemical conversion of limestone into lime which emits CO2 as byproduct (Gibbs et al., 2000). This CO2 produced in cement industry is considered as major greenhouse gas (GHG), which is responsible for global warming (Zhang et al., 2012). The role of CO2 in global warming is well accounted in latest Intergovernmental Panel on Climate Change (IPCC), special report on global warming of 1.5 °C (Masson-Delmotte et al., 2018). Benhelal et al. (2013) and Andres et al. (2012) reported that 1 ton production of cement contains 900 kg of CO2 which accounts for 5–7% of global carbon emission, which is directly responsible for global warming. Therefore, in order to reduce the carbon emission, the world has come together and signed the Bali agreement and the Kyoto protocol under the United Nation Framework Convention on Climate Change (UNFCCC) (Skutsch and Trines, 2008) and (Heitmann and Khalilian, 2011). In this agreement, member countries are intended to develop substitute mechanism of production in response to counter carbon emission. Therefore, in construction industry, to limit carbon emission, many materials having binding properties are used as a replacement to cement.
The selection of material while replacing cement requires fundamental understanding of cement hydration. Mehta and Monteiro, (2017) and Neville, (1995), discussed the basic hydration chemistry of cement which states that cement compounds which reacts with water to produce strength contributing compounds are tri Calcium silicate (C3S), di Calcium silicate (C2S), tri Calcium aluminate (C3A), and tetra Calcium alumino-ferrite (C4AF). The initial hydration occurs in C3A and C4AF to produce calcium trisulphoaluminate hydrate (ettringite) in presence of sulphate (primarily in gypsum), to provide initial setting to mixture; however, once the sulphate content depletes, ettringite converts into calcium monosulphoaluminate hydrate. On later C3S and C2S hydrates to form calcium silicate hydrate (C–S–H) gel, which provides later stage strength. Therefore, the key ingredients in cement are oxides of calcium, silica, and alumina with gypsum. Hence, in same literatures, authors suggested the use of natural pozzolans and cementitious materials as replacement to cement. These pozzolans are natural occurring materials that have binding properties, and cementitious materials are generally by-products of different industries, having rich content of aluminosilicate.
The most widely recommended industrial by-product having cementitious materials property is blast furnace slag due to its high content of aluminosilicate (Ozbay et al., 2016) and (Shi and Qian, 2000). Ogawa et al. (2012) and Ulubeyli and Artir, (2015), performed several experiments to investigate the optimum replacement percentage of furnace slag with cement which vary from 20% to 40%. The optimum replacement percentage is that at which considerable amount of strength is obtained. Meanwhile, Ofuyatan et al. (2020) and Dinakar et al. (2013) preferred using furnace slag with other mineral admixtures like they developed self-compacting concrete and high-performance concrete using blast furnace slag in conjunction with different mineral admixtures like eggshell and metakaolin. They observed that utilization of blast furnace slag can be incorporated in the production concrete with improved durability properties like lesser shrinkage, cracking resistance, less water penetration depth, and freezing resistance. Nicula et al. (2020) investigated the resistance of concrete prepared using slag powder as a replacement to cement and artificial crushed blast furnace slag replacement to natural aggregate against freezing and thawing action. They observed that mixture containing 15% slag powder and 20% artificial crushed blast furnace slag performed better compared to specimens prepared using other conventional materials. Further, Sadawy and Nooman, (2020) used nano blast furnace slag as replacement to cement in different percentages and noticed that very dense microstructure was observed and significant amount of resistance was offered against corrosion. Palod et al. (2020) used blast furnace slag with steel slag to produce ternary cement in which comparable compressive strength was observed in later days. Even blast furnace slag has been used as a replacement to cement in the production of sprayed concrete/shotcrete, leading to improved mechanical strength (Salvador et al., 2019).
Siddique, (2011) studied the use of industrial by-product silica fume as replacement to cement due to its higher content of silica. Suda and Rao, (2020) recommended GGBS, Madani et al. (2018) recommended pumice, Meddah et al. (2018) recommended metakaolin and Mustapha et al. (2020) recommended fly ash as a mineral admixture with silica fume to enhance the strength. Tripathi et al. (2020) assessed the durability of concrete prepared using silica fume in acidic environment of nitric acid. They observed that resistance against aggressive environment was significantly improved. However, Tam and Tam, (2008) adopted the strategic use of silica fumes in strengthening the properties of recycled coarse aggregate concrete by coating the gaps and pores of adhered mortar on surface of recycled aggregate. Okoye et al. (2017) and Daniel et al. (2017) observed significant improve in engineering properties of geopolymer concrete prepared in conjunction with silica fume against sulphuric acid, chloride solution and torsional load. Some researchers assessed the durability properties of mortar prepared using silica fumes like water absorption (Nayana and Rakesh, 2018), sorptivity test (Zhang et al., 2016) and sulphate attack test (Chakraborty and Dutta, 2001), where it performed remarkably well.
Scrivener et al. (2018) proposed a revolutionary method in cement production field in which they recommended the use of clinker, limestone, clacined clay, and gypsum in specified proportion to produce new cement referred as Limestone calcined clay cement (LC3). The basic ingredients of cements like calcium are obtained from limestone and aluminasilicates from calcined clay. Clay chosen for calcination is natural mineral kaolinite, since it requires lesser temperature for calcination compared to Portland cement hence emitting lesser carbon. Dhandapani et al. (2018) observed the improved mechanical and durability properties of concrete prepared using LC3 cement attributing to its dense and strong microstructure. Even, Pillai et al. (2019) performed the life cycle assessment of reinforced concrete using LC3 and its equivalent reduced carbon footprint compared to specimen prepared using OPC.
Jia et al. (2019) and Li et al. (2014), recommended the use of chemically bonded phosphate magnesium phosphate cement (MPC) for rapid construction and repair work which got its tremendous demand in cold surroundings temperature. It requires Magnesium oxide which can be obtained by calcination of natural mineral magnesite (Arora et al., 2019). Researchers observed that because of its high chemical reactivity with silica fume, it delays the initial setting of magnesium phosphate cement and improves the strength as well (Ahmad and Chen, 2018) and (Ahmad and Chen, 2020). Ma and Chen, (2017) and Li et al. (2019), recommended the production of foamed concrete using magnesium phosphate cement. They utilized the unique advantage of rapid hardening potential of MPC for quick setting and early high strength by using foaming agent as sodium bicarbonate and hydrogen peroxide. Feng et al. (2020) and Fang et al. (2018), also assessed the novel use of MPC by using different fibers like steel fiber and glass fiber. Feng et al. (2020) observed that using 10% of silica fume in MPC greatly improved the bond and interfacial transition zone (ITZ) between steel fiber and MPC resulting into improved strength. Fang et al. (2018) established the use of glass fiber in MPC mortar which significantly improved the resistance against water penetration. Similarly, Ahmad and Chen, (2020) proposed the use of basalt fiber in MPC mortar which established that using basalt fiber in conjunction with silica fumes in MPC could be used against water resistance and high temperature resistance in mortar.
Similarly in reducing the carbon emission from cement production industry, researchers also recommended Calcium sulphoaluminate (CSA) cement which is also adopted in many researches because of its early strength attainment quality (Pera and Ambroise, 2004). It has explicit use in cold environment against frost attack because of its rapid initial strength (Li et al., 2020). Huang et al. (2019) cured cement mortar prepared using CSA in frozen sand to mimic the influence of permafrost. They observed that specimen responded well in frozen environment and attained rapid strength in between −5 °C to −10 °C. Ingaglio et al. (2019) and Chen et al. (2020) used CSA cement with sand as a binder in producing 3D printed samples having adequate mechanical strength with desired geometries. Winnefeld et al. (2017) and Wu et al. (2020), investigated the role of gypsum obtained from different sources in CSA cement and observed that by increasing the content of gypsum in a specific proportion, the initial strength of specimen gained significantly.
With the aim of reducing carbon emission during cement production, many agricultural wastes like baggase ash, rice husk ash, palm oil fuel ash serve as prime constituent in replacing cement because of its rich silica content (Loganayagan et al., 2020) and (Hu et al., 2020). Utilization of sugar cane bagasse ash in production of sustainable concrete is most appreciated technique in country like India and Brazil, due to its largest production of sugarcane (Yogitha et al., 2020). Bagasse ash use in construction industry is not only limited to cement replacement but also to sand replacement (Almeida et al., 2015). The silica content in bagasse ash is obtained by higher degree calcination of sugarcane waste which is done during its extraction process. Though initially, it is not cementitious in nature but once it comes in contact with alkaline solution like calcium hydroxide and water, it can be used as cementitious compounds (Yadav et al., 2020). Rajasekar et al. (2018) and Gar et al. (2017) investigated the durability properties of concrete prepared using bagasse ash and it was observed that its performance in resistance against chloride penetration, water absorption, and elevated atmosphere temperature improved significantly. Le et al. (2018) and Pereira et al. (2015) stated that potential of bagasse ash as binder is increased when it is used with furnace slag in the mixture. Their studies ensured the improved mechanical strength in longer days of curing and also better resistance against sulphate attack. Larissa et al. (2020) recommended the use of bagasse ash in conjunction with metakaolin as replacement to cement. They observed that using metakaolin with bagasse ash improved the deterioration of concrete at high temperature curing without affecting its workability.
This paper attempts to summarize and review the available literature on the sustainable application of different industrial by-products like blast furnace slag and silica fume. Moreover, the detailed study and micro-analysis is done for the natural minerals like kaolinite, magnesite, ye'elimite, gehlenite and belite for the production of sustainable cements like Calcined clay limestone cements, Magnesium phosphate cement and Calcium sulphoaluminate cement. Further, the production of sustainable cement from agricultural waste sugarcane ash is discussed and reviewed. Also, the detailed comparative analyses of their engineering properties are performed. Besides, this to understand better the micro-structure development of sustainable concrete, a detailed explanation of cement hydration established from published results is presented.
2. Cement hydration reaction at microstructural level
2.1. Ettringite formation
Quennoz and Scrivener, (2012) showed the initial stage of hydration of OPC in presence of sulphate (present in cement in the form of gypsum (CaSO4·2H2O) or in the locally available soil or in water) reacts with C3A to form a needle shaped prismatic crystals of calcium trisulfoaluminate hydrate (Aft phase), called Ettringite shown in Fig. 1(a).
 Fig. 1
Fig. 1In normal OPC, the gypsum content is 5–6%, which helps in formation of Ettringite which leads to stiffening, setting, and early strength development in concrete. Later, depending on the alumina/sulphate ratio of the OPC, once when the sulphate content starts depleting, the aluminate ion in the solution increases due to further hydration of C3A and C4AF, which makes ettringite unstable and it decompose to form calcium monosulfoaluminate hydrate (AFm phase) C4AH18 (Mehta and Monteiro, 2017).
Calcium monosulfoaluminate hydrate is having the hexagonal plate morphology which is the characteristics of strength imparting compound Calcium aluminosilicate hydrate (C-A-S-H) (Mehta and Monteiro, 2017). Scrivener, (2004) noticed that formation of Ettringite is possible in old paste also but its individual crystals are difficult to identify since it is densely intermixed with C–S–H gel. This similar property is observed in elevated temperature curing leading to delayed formation of ettringite.
2.2. C–S–H
(Mehta and Monteiro, 2017) highlighted that Calcium Silicate Hydrate (C–S–H) is principal binding compound formed during hydration of tri Calcium Silicate (C3S) and di Calcium Silicate (C2S) by giving Calcium Hydroxide (CH) as by product. It consists of 50–60% of the total volume of hydrated cement paste responsible for long term strength of concrete. Sometimes it is also referred as tobermorite gel because of its resemblance with natural mineral tobermorite. Richardson, (2004) showed that C–S–H product is classified into two types: the outer product (Op) and inner product (Ip) shown in Fig. 2. The unhydrated cement clinkers form Ip, whereas original water filled space form Op. The Op formed in paste is fibrillar in nature whose density depends on no. of curing days. The pore space is also responsible for finer and coarser formation of fibrils. Minimal pore space forms finer fibrils, whereas larger pore space forms coarse fibrils.2C3S + 6H → C3S2H3 + 3CH2C2S + 4H → C3S2H3 + CH (Mehta and Monteiro, 2017).
 Fig. 2
Fig. 23. Blast furnace slag blended cement
The water quenched slag obtained from iron industry is referred as ground granulated blast furnace slag (GGBFS) which contain oxides of Calcium, Aluminum, and Silica (Ozbay et al., 2016). The use of furnace slag in construction enhances the mechanical and durability strength of concrete/mortar and reduced the demand of cement (Palod et al., 2020) and (Song and Saraswathy et al., 2006). The detailed chemical composition of GGBFS compared to cement is given in Table 1 (Oner and Akyuz, 2007).
Table 1. Chemical composition of Cement and GGBS (Oner and Akyuz, 2007).
| Binders | Chemical composition (%) | LOIa | IMb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | Cl− | ||||
|
Cement GGBS |
20.72 39.18 |
4.88 10.18 |
2.95 2.02 |
61.83 32.82 |
1.39 8.52 |
2.33 – |
0.19 1.14 |
0.67 0.30 |
0.0060 – |
3.17 1.0 |
0.63 0.88 |
|
- a
-
Loss on ignition.
- b
-
Insoluble material.
In Cho et al. (2017) research, blast furnace slag blended cement is prepared with OPC and GGBFS in 1:1 ratio. Further, GGBFS content is varied by different percentages of Aluminum rich slag (ARS) and gypsum to investigate its microstructural change. Aluminum rich slag is obtained as a byproduct from steel manufacturing industry which primarily contains oxides of Calcium and Aluminum. The compressive strength of the mortar obtained is highest in case of GGBFS blended OPC (Plain O50B50) i.e., blast furnace slag blended cement, next mortar containing 67.5% ARS and 22.5% gypsum (O50B30AS15G5), and after that 45% ARS and 45% gypsum (O50B30AS10G10). However, they conducted Scattered Electron Microscopic (SEM) test for two samples only, containing 90% gypsum (O50B3020), and 45% of ARS and gypsum each (O50B30AS10G10). In Fig. 3 (a), sample O50B3020 is observed under SEM. The blending of OPC with GGBFS provides sufficient amount of aluminosilicates in mixture, and an additional amount of gypsum present reacts to produce ettringite in hydration process which is responsible for initial setting and strength. But once the content of alumina depletes, ettringite becomes weak and small. It can be observed in picture that ettringites are small and rare. But in Fig. 3 (b), sample O50B30AS10G10, ARS, and gypsum are present in equal proportion; hence, the formation of needle shaped prismatic crystal of calcium trisulfoaluminate hydrate (Aft phase) are dense and it fills the gap between C–S–H and C-A-H hydrates. However, slower decomposition of sulphate in gypsum results into formation of calcium monosulphoaluminate (Afm phase) which provides strength to the mortar.
 Fig. 3
Fig. 33.1. Engineering properties
Özbay et al. (2016) highlighted that mechanical strength of GGBFS blended cement mortar or concrete depends on multiple factors like, percentage content of GGBFS in OPC, water-binder ratio, testing age, and curing type. Oner and Akyuz, (2007) prepared concrete by fractionally replacing GGBS by 0%, 15%, 30%, 50%, 70%, 90% and 110% of cement content. It was observed that 7 days strength attained by blended cement concrete was little less than control mix, but the 365 days strength increased significantly up to 50% replacement. The further increase in replacement percentage showed a decrease in compressive strength. Since, hydration of OPC resulted into formation of C–S–H which imparts strength to concrete and the obtained CH during hydration process reacts with silica and alumina present in GGBS to further produce C–S–H and C-A-H gel (Mehta and Monteiro, 2017). But once the production of CH starts depleting, the increasing content of silica in GGBS remains unreacted and hence leads to decrease in later strength of concrete. Similarly, Ganesh and Murthy, (2019) fractionally replaced GGBS with cement by weight in 0, 20, 40, 60 and 80%. They observed that compressive strength of concrete increased significantly up to 60% after which it decreased. Mohamed and Najm (2016) replaced OPC with GGBS at 10, 35, 45, 50, 60, 70 and 80% where the compressive strength was found to be highest at 35% and tensile strength at 45%. However, the pattern of the compressive and tensile strength was observed to be same. Further, Barnett et al. (2006) and Hwang and Lin, (1986), highlighted that curing at elevated temperature enhances the early strength of cement replaced by GGBS. Babu and Kumar, (2000) studied the efficiency of GGBS by fractionally replacing the cement with GGBS from 10 to 80% and it was observed that an additional of 8.5% and 19.5% is required in total cementitious materials at 50% and 65% of cement replacement. Suda and Rao, (2020) adopted several replacement percentage in ternary mix containing micro silica and GGBS, but the maximum compressive strength was obtained in TC9 sample having 60% OPC, 10% micro silica, and 30% GGBS. The use of micro silica provides extra silica content to the mix and strengthens the production of strength imparting compound C–S–H. Kumar et al. (2018) recommended new chemical admixture, calcium nitrate to be used with ultrafine GGBS and OPC. Ultrafine GGBS, increased silica content in the mixture which increased the strength up to 18%. Moreover, after using Calcium nitrate as chemical admixture, the compressive strength was increased by 32%.
In the recent production of geopolymer concrete, Kumar et al. (2018) and Jithendra and Elavenil, (2019) recommended the use of GGBS with other mineral admixtures to completely subdue the demand of OPC, in which the artificial polymer used is prepared using a source of aluminosilicate mineral, which is activated by alkaline hydroxide and silicate solution. Kumar et al. (2018) used a mixture of GGBS, metakaolin, and sodium silicate as an alkaline activator. They observed an increase of 35.3% in compressive strength by specimen having 80% GGBS and 20% metakaolin. Jithendra and Elavenil (2019) used 2%, 3%, 4%, 5%, and 6% percentage dosage of superplasticizer with 100% GGBS. They observed that with the increasing percentage of superplasticizer, the compressive strength of concrete was decreased.
Similarly, Hadi et al. (2017) followed Taguchi method (Taguchi et al., 2005) to prepare geopolymer concrete in which fly ash, silica fume, and metakaolin are used with GGBS to increase the setting time of concrete. Guneyisi and Gesoglu, (2008) and Hooton, (2000), investigated the durability properties of GGBS induced concrete, which showed resistance against sulphate attack, alkali-silica reaction, chloride ingress and reduced heat of hydration as well. The observations from different research are summarized in Table 2.
Table 2. Results and remarks obtained from different literature.
| References | Specific gravity (g/cm3) | Slump range (mm) | Percentages of replacement of cement with furnace slag (%) | Best percentage of replacement (%) | Remark |
|---|---|---|---|---|---|
| Oner and Akyuz, (2007) | 2.87 | 115–120 | 0, 15, 30, 50, 70, 90,110 | 50 | Compressive strength found to be decreasing after 50% |
| Ganesh and Murthy, (2019) | – | – | 0, 20, 40, 60, 80 | 60 | Hardened properties are increased up to 60% under temperature curing |
| Güneyisi and Gesoğlu, (2008) | 2.82 | - | 50, 60, 70, 80 | 50 and 60 | Better strength at 50% for initial days of curing and 60% at later days of curing |
| Boukendakdji et al. (2012) | 2.95 | 235-378 (Self Compacting Concrete) | 10, 15, 20, 25 | 20 | Strength decreases in initial days of curing but increases in later days of curing |
| Mohamed, (2018) | – | – | 10, 25, 35, 45, 50, 60, 70, 80 | 35 | Concrete shows higher resistance to chloride penetration |
| Mohamed, (2019) | – | – | 10, 20, 30, 40 | 30 | High strength can be attained by using GGBS in conjunction with fly ash and silica fume |
4. Silica fume blended cement
Silica fume is the by-product from the induction arc furnace in silicon metal and ferrosilicon alloy industries. This is produced during reduction of quartz into silicon at temperature around 2000 °C resulting into formation of SiO2 vapours which oxidizes in low temperature to condense into tiny particles to form nano-crytalline silica (Siddique, 2011). The detailed chemical composition of OPC and silica fume is shown in Table 3 (Karein et al., 2017). The rich content of silica in silica fume is highly responsible for accelerating and strengthening the growth of strength compounds in the mixtures like C–S–H. But since, the formation of C–S–H requires reaction of silica with portlandite (CH) which is a product obtained during hydration of portland cement. Hence, it is recommended to use silica fume in a fractional replacement with cement which eventually decreases the demand of cement (Mehta and Monteiro, 2017).
Table 3. Chemical composition of OPC and silica fume (Karein et al., 2017).
| Oxides | Chemical Analysis (wt%) | |
|---|---|---|
| OPC Silica fume | ||
| SiO2 | 20.8 | 87.5 |
| CaO | 65.3 | 1.27 |
| MgO | 2.17 | 1.01 |
| Al2O3 | 4.3 | 0.5 |
| Fe2O3 | 2.2 | 1.53 |
| K2O | 0.63 | 1.14 |
| Loss on ignition | 0.91 | 5.92 |
Rossen et al. (2015) adopted 10, 20, 25, and 45 percentage of silica fume in OPC to observe change in C–S–H growth. CH formed during hydration of C3S and C2S precipitated around the cement clinker as CH rim and gets consumed during pozzolanic reaction with silica fume shown in Fig. 4. They also observed that cement hydration kinetics was accelerated at elevated temperature resulting into speedy formation of C–S–H. In Fig. 4 (a) and (b) samples shows hydrated product of OPC in the form of C–S–H and CH. Since there wasn't enough silica present, to react with CH, to produce more C–S–H, hence unreacted CH presence is observed in 4 (a) and a rim of CH over unhydrated clinker is seen in 4 (b). In Fig. 4 (c), 25% of OPC is replaced by silica fume, which provided extra silica content to mix. But the replaced amount of silica is not sufficient enough to completely dissolve the obtained CH, hence in SEM images, very clearly CH and a rim of CH around unhydrated clinker are visible. Lastly, in Fig. 4 (d), 45% replaced sample have enough silica for complete reaction of CH, hence a strong and dense C–S–H is observed in the sample with no clear presence of CH.
 Fig. 4
Fig. 44.1. Engineering properties
Koksal et al. (2008) used different percentage of steel fibers in conjunction with silica fumes by replacing cement in the mixture. Moreover, before adding steel fibers, they investigated the effect of silica fumes by varying its percentage from 0, 5, 10, and 15%. They noticed that specimen with 15% of silica fumes achieved highest strength. Verma et al. (2020) replaced the fine aggregate with stone dust, and cement with silica fume. The fractionally replaced cement with silica fume was 0, 5, 10, and 15%. They observed that sample containing 10% silica fumes and 30% stone dust, achieved highest compressive strength after 28 days of curing. The strength of specimen containing 30% stone dust gained compressive strength by 2.32%, but when silica fume with 10% replacement was used in conjunction with stone dust, the gain in compressive strength recorded was 6.83%. The gain in strength is attributed to stone dust which provided a well graded denser matrix paste and high content of silica provided denser formation of C–S–H gel in the paste. Wong et al. (2005) investigated the strength of concrete prepared using calcined kaolin (metakaolin) and silica fume at different water binder ratio individually. The replacement percentage was 0, 5, 10, and 15% for both the admixtures. In the mixture, water binder ratio adopted was 0.27, 0.30, and 0.33. Initially it was observed that early stage strength of silica fume induced concrete was slow as compared to metakaolin specimen. This is because of lesser content of aluminate in silica fumes. But after 28 days of curing, it was observed that strength of silica fumes specimen was higher than metakaolin specimen, and it was highest at 15% replacement level among total fraction of silica fume. This strength development is in agreement with the fact that silica fume contains higher silica content which produces C–S–H gel, which provides later strength to the concrete. Roastami and Behfarnia, (2017) examined the response of silica fumes in alkali activated slag with different percentages like 0, 5, 10, and 15%. The slag used in the investigation was activated using sodium hydroxide (NaOH) and sodium silicate (Na2SiO3). It was observed that with the increasing percentage replacement with silica fume, the compressive strength of the concrete was increased after 28 days of curing. Moreover, the use of silica fume also prevented the flow of charge in rapid chloride penetration test, hence ensuring its better durability properties. The total development in strength was 28% higher in 15% replaced sample. This enhanced mechanical and durability property is in agreement with the formation of denser C–S–H gel, which induced strength in the mixture. The comparative compressive strength from different literature is shown in Fig. 5.
 Fig. 5
Fig. 55. Calcined clay limestone cements (LC3)
Scriviner et al. (2018) and Scriviner et al. (2015), proposed the use of LC3 by recommending the use of calcination of clay containing kaolinite with clinker, limestone and gypsum in specified proportion. The calcination process of clay requires lesser temperature around 700–850 °C compared to temperature required for calcination of clinker for producing OPC. The reactivity of calcined clay is highly dependent on natural mineral kaolinite content, hence Scrivener et al. (2018) recommended to use 40% or above content of kaolinite in clay. LC3-50 designates 50% clinker, 30% calcined clay, 15% limestone and 5% gypsum. So altogether, the demand of clinker is reduced by 50% which is a key factor in reducing carbon emission (Scrivener and Favier, 2015). Basically the main product obtained in calcination of kaolinite clay is metakaolin which is aluminosilicates amorphous and plays major role in enhancing the strength (Scrivener and Favier, 2015). In Table 4 detailed chemical composition of kaolinite clay and metakaolin is shown. Antoni et al. (2012) showed the metakaolin reacts with calcium hydroxide produced during hydration of clinker to give C-A-S-H and aluminate hydrate which is a strength imparting compound.
Table 4. Chemical composition of Kaolin and Metakaolin (Scrivener and Favier, 2015) and (Antoni et al., 2012).
| Binders | Chemical composition (%) | LOIa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | TiO2 | |||
|
Kaolinite Metakaolin |
53.5 50.62 |
32.1 46.91 |
0.3 0.38 |
0.1 0.02 |
0.2 0.09 |
– 0.08 |
0.28 1.14 |
0.18 0.30 |
0.12 1.29 |
14.17 0 |
|
- a
-
Loss on ignition.
Moreover, its Alumina content further reacts with limestone in presence of gypsum, to form calcium trisulfoaulminate hydrate/ettringite (Aft phase) and later converts into monosulphoaluminate hydrate (Afm phase), to strengthen the mechanical and durability properties. In Avet et al. (2019) C-A-S-H morphology is compared at microstructural level between Portland cement (PC) and LC3. In LC3 sample, content of kaolinite in calcined clay is varied at 17.0%, 50.3%, and 95.0%. In Fig. 6, fibrillar C-A-S-H morphology is shown in Bright Field (BF) mode and in High Angle Annular Dark Field (HAADF) mode. It is observed that C-A-S-H is clearly visible in PC as well as in LC3-50 (17%).
 Fig. 6
Fig. 6This clearly highlights that formation of strength compound like C-A-S-H is not diminished using calcined clay containing lesser content of kaolinite at 17%. Moreover, needle shaped crystal of calcium trisulfoaluminate hydrate/ettringite (Aft) is also visible in LC3 sample. Presence of Aft phase at 28 days of curing denotes that alumina and gypsum content is still not depleted. Similarly, in Fig. 7, both the samples of LC3-50 (50.3%) and LC3 -50 (95.0%) are shown in which clear fibrillar C-A-S-H morphology is visible, which validates the reaction of calcined clay and the different content of kaolinite in calcined clay doesn't affect the C-A-S-H morphology, and at high content of kaolinite in calcined clay, also show unreacted metakaolin. This shows the presence of aluminosilicates in mixture which is responsible for the further production of C-A-S-H and C-A-H. Hence, the presences of strength compounds are ensured in LC3 cement.
 Fig. 7
Fig. 75.1. Engineering properties
Bishnoi et al. (2014) investigated the mechanical property of LC3 by comparing the compressive strength test results of different samples. LC3 specimens were prepared from two different types of clay in which Clay1 was containing 70–80% kaolinite content, and Clay2 was containing 20–30% kaolinite content. The overall composition of LC3 was 50% clinker, 5% gypsum, 15% limestone, and 30% calcined clay. Further, the limestone used in LC3 was of two types, having slight higher content of silica and carbonate in the later one. Finally the samples were prepared, designated as LC3A, LC3B, LC3C, and LC3D in which clay 1 was mixed with both the types of limestone to produce LC3A and LC3B, and clay 2 was mixed with both the types of limestone to produce LC3C and LC3D. For control mix, Portland cement (OPC) was used, prepared using the same clinker used in production of LC3 cement. Moreover, one more sample was prepared using same clinker with locally available fly ash cement as Portland pozzolanic cement (PPC). In Fig. 8, the compressive strength results of all the samples are shown together.
 Fig. 8
Fig. 8It is observed in Fig. 8 (a), that higher strength is achieved by LC3B mortar sample after 28 days of curing compared to OPC specimen and in, Fig. 8 (b), LC3A concrete sample attained highest strength after 28 days of curing. The point to be noted here is that both the samples contain Clay 1 which contains higher content of kaolinite. Hence, the kaolinite content in clay plays major role in gaining strength. The developments of LC3 cement is also getting attention in developing countries like Cuba and India where it is being running as pilot project (Berriel et al., 2016) and (Emmanuel et al., 2016).
6. Magnesium phosphate cement (MPC)
MPC is used very frequently for its niche applications like cold weather repair material because of its rapid hardening property (Le Rouzic et al., 2017). It composes of Dead Burnt Magnesite (DBM) MgCO3 which is calcined at 1500 °C to obtain Magnesia (MgO), commercially available crystalline Mono-Ammonium Phosphate (NH4H2PO4), and retarders like Sodium Tri-Polyphosphate (Na5P3OI0), and Di-Sodium-Tetraborate (Borax) (Na2B407.10H20) (Seehra et al., 1993). The following reaction occurs during its hydration (Miyaji et al., 1982)MgO + 2NH4H2PO4 + 3H2O → Mg(NH4)2(HPO4)2. 4H20Mg(NH4)2(HPO4)2. 4H2O + MgO + 7H20 → 2MgNH4PO4. 6H20
The product obtained in above reaction 2MgNH4PO4. 6H20 is referred as mineral Struvite which is main binding material in MPC (Allan and Asgar, 1966). In order to address the issue of chemical stability of Struvite (2MgNH4PO4. 6H20), Rouzic et al. (2017) used Potassium di hydrogen phosphate in place of Ammonium di hydrogen phosphate leading to formation of k-struvite (MgKPO4. 6H2O) which acts as main binding material in cement.MgO + KH2PO4 + 5H2O → MgKPO4. 6H2O
Boisettle et al. (1983) paid emphasis on pH of solution, at lower pH, struvite precipitate to form newberyite (MgHPO4·3H2O) which may tend to reduce the strength. Hence, Rouzic et al. (2017) recommended magnesia to phosphate molar ratio (Mg/P) of 1 (lesser than 5) and water to binder weight ratio of 0.2 to avoid the precipitation from struvite into newberyite shown in Fig. 9.
 Fig. 9
Fig. 9Liu et al. (2020) fractionally replaced the Iron oxide (Fe2O3) with 0%, 5%, 10%, 20%, and 30% of MgO and named it magnesium-iron phosphate cement. A detailed microstructure analysis was conducted in SEM which is shown in Fig. 10. Specimen containing 20% of iron oxide showed the highest strength among other specimens because of lesser micro-cracks and micropores. Moreover, presence of iron oxide powder accelerated the formation of strength imparting compound struvite, leading to development of denser microstructure.
 Fig. 10
Fig. 10Qin et al. (2020) used silica fumes in magnesium phosphate cement referred as Magnesium silicon phosphate cement (MSPC). They used silica fumes in different percentages to investigate the mechanical strength of the MSPC. They observed that specimen with 15% replacement achieved highest strength as compared to other specimens. Xu et al. (2017) proposed the use of fly ash with Magnesium Potassium Phosphate Cement (MKPC) and deeply analyzed its microstructural changes. In Fig. 11, two specimen's SEM images are shown and discussed. In Fig. 11 (a), control specimen is shown referred as M8 is containing M/P molar ratio as 8. The formation of strength imparting compound k-struvite crystals is clearly visible with unreacted magnesia and sand. Meanwhile, lots of cracks and pore are visible in the image
 Fig. 11
Fig. 11with heterogeneously distributed constituents; whereas, in Fig. 11 (b), use of fly ash in the mixture referred as M8 FA-C is clearly noticeable because very dense microstructure is observed and the traces of unreacted fly ash is also seen in between sand and unreacted magnesia. Overall homogenously distributed constituents are observed which attribute to its better strength.
6.1. Engineering properties
Many researchers have adopted different SCMs like fly ash (B. Xu et al., 2017), cement kiln dust (Baghriche et al., 2020), wollastonite (B. Xu et al., 2020), dolomite (Yu et al., 2020) and red mud (Liu et al., 2020a, Liu et al., 2020b), in conjunction with MPC to improve its mechanical strength, durability, and reduce the heat of hydration, to make it overall a cheaper and best substitute over OPC. Qin et al. (2020) suggested the use of metakaolin in MPC, which decreases the porosity. This improves pore structure which attribute to enhanced mechanical strength. Yu et al. (2020) replaced calcination of magnesite to calcination of dolomite, for extraction of magnesium, for producing MPC. This calcination process of dolomite requires lesser temperature compared to magnesite calcination hence, ensuring cheaper rate and less energy consumption as well. Moreover, the compressive strength obtained using dolomite as raw material was also improved. Similarly, Baghriche et al. (2020) used dolomite as raw material but, in conjunction with cement kiln dust (CKD). CKD utilization in the mixture helped in promoting the growth of strength compound struvite in MPC. The tensile and compressive strength of prepared specimens was considerably high. Xu et al. (2020) produced magnesium potassium phosphate (MKP) cement and observed its hydration reaction with wallastonite. It was noticed that wallastonite prevented the formation of efflorescence. This significant change in hydration process increased the mechanical strength. Liu et al. (2020) recommended the use of red mud in production of MPC, where red mud was used fractionally. They observed that specimen containing 20% red mud achieved highest compressive strength. In SEM images, it showed that red mud specimen microstructure was densely packed and hence reduced porosity was noticed. The observations from different research are summarized in Table 5.
Table 5. Observation and remarks obtained from different literature.
| References | MPC cement production source | Other major raw materials | Clinkering temperature (0C) | Remark |
|---|---|---|---|---|
| Yu et al. (2020) | Dolomite | Bauxite, Gypsum | 1300 | High early compressive strength with good stability |
| Zhang et al. (2020) | Red mud | Dead-burnt magnesia | – | Mechanical strength and fluidity are increased |
| Viani and Gualtieri, (2014). | Asbestos containing wastes | Magnesium carbonate | 1100–1300 | Compact microstructure is observed |
| Baghriche et al. (2020) | Dolomite | Cement kiln dust | 720 | Higher mechanical and tensile strength is observed |
7. Calcium sulphoaluminate cement
Calcium sulphoaluminate (CSA) cement is in recent demand for its use in rapid repair work primarily because of its high potential in gaining early high strength in concrete (Li et al., 2020). Moreover, Sharp et al. (1999) and Garcia-Mate et al. (2013) stated that CSA cement emits less carbon compared to conventional Portland cement production. The detailed mineralogical and chemical composition of CSA cement is shown in Table 6 and Table 7 (Li et al., 2018).
Table 6. Mineralogical composition of CSA (Li et al., 2018).
| Phase | Ye'elimite | Anhydrite | Belite | Gehlenite | Calcite | Dolomite |
|---|---|---|---|---|---|---|
| Content/wt % | 40.57 | 34.78 | 9.96 | 8.42 | 3.12. | 2.32 |
Table 7. Chemical composition of CSA (Li et al., 2018).
| Components | CaO | Al2O3 | SO3 | SiO2 | Fe2O3 | MgO | Na2O | K2O | Loss |
|---|---|---|---|---|---|---|---|---|---|
| Content/wt % | 40.57 | 34.78 | 9.96 | 8.42 | 3.12. | 2.32 | 0.22 | 0.05 | 0.56 |
The construction issues in subzero temperatures like delay in setting time and lesser workability is successfully overcomed by using CSA cement (Li et al., 2018) and (Zhang et al., 2020). Li et al. (2018) recommended the use of admixture likes lithium carbonatealuminum sulphate (LC-AS), which significantly improved the formation of ettringite and accelerated the process of belite hydration. Moreover, Zhang et al. (2020) used CAS as mineral accelerator in Portland cement and observed that freezing of water and inorganic salts are successfully avoided. The hydration process of CAS cement is discussed in detail (Kasselouri et al., 1995) and (Pelletier et al., 2010). Winnefeld and Lothenbach, (2010) performed thermodynamic modeling for two different cement pastes specimens which were prepared by varying the water/cement ratio. In the initial hour hydration of CSA's main element ye'elimite occurs in presence of gypsum to produces ettringite (Aft phase) and Al(OH)3
Once the sulphate presence is exhausted, Aft phase converts into Afm phase, means formation of calcium monosulphoaluminate hydrate. Further, the hydration of C3S starts and C–S–H and CH is formed. Now AH3 produced in reaction (8), reacts with C3S and CH also to produce C-A-S-H and C-A-H.C3S + AH3 + 6H → C2ASH8 + CHC3S + 3:7H → CSH1.7 + 2CHAH3 + 3CH + CC + 5H → C4ACH11 (Pelletier et al., 2010).
Now the formation of CAH is continuous through reaction of CH with C3A and AH3 till it gets exhausted.(12)AH3 + 4CH + 6H → C4AH13C3A + CH + 12H → C4AH13 (Pelletier et al., 2010).
Finally the remaining Aft, reacts with newly formed C-A-H to produce monosulphoaluminate hydrate. In Fig. 12, thermodynamic modeling development of individual phase volume is shown (Winnefeld and Lothnbach, 2010). They observed that with the passage of time, the volume of liquid phase decreased and volume of solid phase got increased. Overall, in the solid phase it is observed that there is a major contribution of ettringite. Zhao et al. (2014) prepared two samples with same mix proportions, one with portland cement, another with CAS cement. Both the samples were tested against the chloride ion penetration resistance in which CAS sample performed better in resisting the penetration; hence, ensuring the lower permeability. In Fig. 13, SEM images of OPC and CAS specimen is shown. The end products of CAS cement are Aft, C–S-A-H, and Fe/Al gel. Aft is responsible for early strength attainment and also acts as filler in microcracks and pores. It also seals the gap between aggregate and mortar, and restricts the transport of chloride ions. This makes it less permeable which is clearly seen in Fig. 13(b). Meanwhile, the morphology of monosulphoaluminate obtained has the characteristics of C–S-A-H which is important element in determining the transport properties of concrete. Hence, altogether it resulted into denser and stronger microstructure of hydrated CAS.
 Fig. 12
Fig. 12 Fig. 13
Fig. 137.1. Engineering properties
Zhang et al. (2020) suggested use of pre wetted lightweight aggregate in production of calcium sulphoaluminate cement concrete to reduce autogenous shrinkage. This reduced shrinkage in concrete is directly attributed to its high strength. Meanwhile, Jin et al. (2020) recommended the use of cementitious materials like Beta-hemihydrate phosphogypsum (β-HPG), obtained from dehydration of phosphogypsum in conjunction with calcium sulphoaluminate cement, to produce high mechanical strength and good waterproof properties. The waterproof property is associated with the formation of ettringite and aluminate hydroxide in the mixture. Similar efforts have been made by researchers (Shen et al., 2014), to use and investigate the role of phosphogypsum with CSA cement. Ma et al. (2019) developed binary system for accelerated hydration mechanism in early age. It was achieved by using limestone powder with CSA cement together. The strategic use of limestone prevented porosity and improved the pore structure as well. Similarly, an efficient increase of strength in mortar, prepared from CSA containing limestone as filler is also observed in Pelletier-Chaignat et al. (2012). Even Zhang et al. (2020) recommended the use of CSA in OPC as mineral accelerator to overcome the negative effect of sub-zero temperature. They observed that compressive strength of OPC can be enhanced by 300% by using optimum dosage of CSA in which dense microstructure was observed. The observations from different research are summarized in Table 8.
Table 8. Observation and remarks obtained from different literature.
| References | CSA cement production source | Other major raw materials | Clinkering temperature (0C) | Remark |
|---|---|---|---|---|
| Singh et al. (2008) | Phosphochalks from Fertilizer plant wastes | Iron ore slimes, Bauxite, Gypsum | 1230 | Tolerant to impurities present in raw material |
| Wu et al. (2019) | Flue gas desulfurization (FGD) gypsum | Aluminum slag, Fly ash, Red mud, | 1270–1310 | Replaces the use of limestone and natural gypsum in production of cement |
| Mao et al. (2020) | Municipal solid waste incineration fly ash | Flue-gas desulfurization gypsum, Aluminum ash | 1270 | Effectively solidifies heavy metals present in the raw materials |
| Shen et al. (2014) | Industrial waste Phosphogypsum | Low-grade bauxite, Coal gangue, Limestone, Natural gypsum | 1250–1300 | Mechanical performance is improved |
8. Bagasse ash blended cement
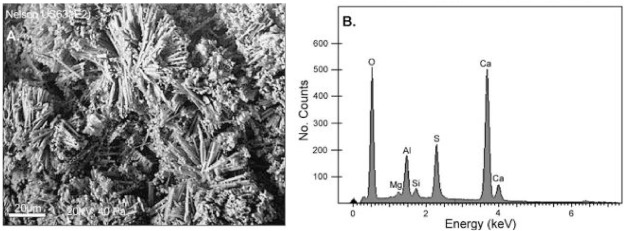
Solid waste material obtained after juice extracted sugar cane is referred as bagasse and burning this bagasse in controlled environment produced amorphous silica having pozzolanic properties (Singh et al., 2000). Cordeiroet et al. (2009) highlighted that pozzolanic nature of bagasse ash is directly related to its degree of fineness. Bagasse ash potential as pozzolans could be achieved by ultrafine grinding through regress milling. The detailed chemical composition of bagasse ash is shown in Table 9, and the flowchart in Fig. 14 (Praveenkumar et al., 2020), which represents the full processing from wet sugarcane bagasse to treated bagasse ash.
Table 9. Chemical composition of Bagasse ash (Praveenkumar et al., 2020).