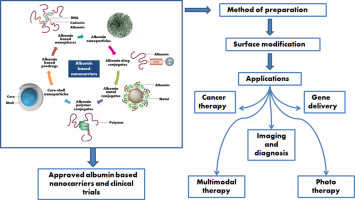
1. Introduction
Cancer is a deadly and life threatening disease with high mortality rate worldwide. According to GLOBOCAN, it caused 8.2 million deaths in 2012 [1]. The most common cancer deaths were due to lung cancer (1.6 million deaths), liver cancer (745,000 deaths), and stomach cancer (723,000 deaths) [2]. This high mortality rate may be due to several reasons like late diagnosis and detection of cancer, inability of therapeutic moiety to reach the tumor site, adverse and toxic effects towards the normal cells etc. [3]. The current strategy for the treatment of cancer lies in chemotherapy, radiotherapy, hormonal therapy and surgery. The existing conventional chemotherapy has several draw backs like less concentration of therapeutic moiety to the tumor site, less target specificity, toxicity towards normal cells and tissues, inability to cross different biological barriers like blood brain barrier, and drug and dosage form related factors which includes less solubility, less permeability, poor dissolution, less stability (photo, thermal and pH stability), degradation of drug, variable drug release from dosage form, multi drug resistance etc. [4], [5].
So there is a need to develop novel delivery system which has ability to overcome all these drawbacks of conventional chemotherapy. In past several decades, nanoparticles have gained lots of attention for treatment of cancer. Nanoparticles have potential to overcome the drawbacks of conventional cancer chemotherapy because of unique properties like small size, surface charge, variable shape, several binding sites for the attachment of target specific ligands, antibodies, peptides etc. They can also enhance the tumor targeting by both passive and active targeting mechanism. Passive targeting is possible due to enhanced permeability and retention (EPR) effect [6], [7]. Nanoparticles based delivery systems are also approved by the FDA for clinical use (Abraxane, Doxil, Genexol-PM, DepoCyt, Myocet etc.) and many more are in the clinical trials (NK105, CYt-6091, Genexol-PM, Rexin-G etc.) [6], [8]. As compared to conventional chemotherapy, nanoparticles based delivery systems have several advantages and features, including: 1) improved delivery of poorly water soluble drugs, peptides, and genes; 2) better protection of drugs, peptides or genes from harsh environments (e.g., enzymatic degradation and the highly acidic environment in the lysosomes or stomach); 3) enhanced treatment efficiency and reduced systemic side effects by cell or tissue specific targeted delivery of drugs, peptides or genes; 4) overcome multidrug resistance by co delivery of drugs, peptides, genes and/or diagnostic agents; 5) stimuli-responsive systems (pH sensitive, temperature sensitive, redox sensitive) can control release of drugs, peptides or genes over a manageable period of time at precise doses [6], [9].
Nanoparticles used as a carrier for cancer therapeutics may be of several types viz. protein based nanoparticles (albumin nanoparticles, gelatin nanoparticles etc.) [10], [11], polymer based nanoparticles (poly lactide co glycolide nanoparticles, polycaprolactone nanoparticles, polylactide nanoparticles, chitosan nanoparticles etc.) [12], [13], [14], [15], lipid based nanoparticles (solid lipid nanoparticles, nanostructured lipid carriers, liposomes etc.) [16], [17], [18], lipid polymer hybrid nanoparticles [19], metal nanoparticles [20], [21], [22], polymeric micelles (cationic micelles, unimolecular micelles, dual responsive and triple responsive micelles etc.) [23], [24], [25], [26], [27], [28], [29], [30], [31], dendrimers [32] etc. Among all these nanoparticles, protein based nanoparticles have gained much more attention in cancer therapy due to unique properties viz. relatively safe and easy to prepare, capability to deliver proteins, peptides, genes, nucleic acid, and hydrophilic as well as hydrophobic anticancer molecules, site specific targeting by surface modification, greater stability profile during storage, etc. [33]. In this review, albumin based nanocarriers and their role in cancer therapy have been discussed in detail.
2. Albumin
Albumin is a protein based macromolecule and the most abundant plasma protein (35–50 g/L human serum) of human blood which is synthesized in the liver at the rate of approximately 0.7 mg/h for every gram of liver (10–15 g daily) [34], [35]. It is nontoxic, biodegradable, biocompatible, highly water soluble, non-immunogenic, easy to purify and stable plasma protein [36].
2.1. Types of albumin
Albumins are of various type viz. Ovalbumin (OVA), bovine serum albumin (BSA), human serum albumin (HSA), rat albumin etc. Commercially, albumins are obtained from egg white, bovine serum and human serum. Apart from these, it can also obtained from soybeans, milk and grains [36].
Ovalbumin (OVA) is monomeric phosphoglycoprotein obtained from egg white and is utilized in designing food matrix as it is a food protein. It has molecular weight of 4.7 kDa, isoelectric point (pI) of 4.8 and consists of 385 amino acid residues, with each molecule having one internal disulfide bond and four free sulphydryl groups and has 3D structure with helical reactive loop arrangement. It is used as a drug delivery carrier due to its properties like low cost, easy availability, emulsion and foam stabilization ability, pH and temperature sensitive properties [36], [37].
Bovine serum albumin (BSA) is obtained from bovine serum and has a molecular weight of 6.93 kDa with pI of 4.7 in water at 25 °C. It is a water soluble monomeric protein that consists of 583 amino acid residues and contains 17 disulfide bonds resulting in nine loops formed by the bridges, one cysteine and 8 pairs of disulfide bonds. It also contains high content of aspartate (Asp), glutamic acid (Glu), alanine (Ala), luciene (Luc) and lysine (Lys). It is also used as a drug carrier because of its low cost, ease of purification, unusual ligand binding properties, biocompatibility, biodegradability, non-toxicity, lesser immunogenicity (as compared to OVA and rat albumin) and wide acceptance in pharmaceutical industry [36], [38].
Human serum albumin (HSA) is heart shaped monomeric globular protein obtained from human serum. It consists of 585 amino acid residues and contains 17 disulfide bridges and 1 sulfhydryl group which is formed by cysteinyl (Cys35) residues. It contains single tryptophan residue (Trp 214) and one free cysteine (Cys34) and high amount of glutamic acid, arginine, and lysine. HSA contains negative charge due to presence of more acidic amino residue as compared to basic amino acid. Disulfide bridges provide stability and longer biological half life (~ 19 days). It has similar properties as BSA and is also used as a versatile carrier for drugs, genes, hormones, peptides and several other molecules [36], [39].
HSA and BSA are homologous proteins and share 76% sequential identity. The major difference between the two is with respect to the number and positioning of tryptophan residues in them. HSA has only one tryptophan, located at position 214 which is equivalent to Trp-212 for BSA present buried in a hydrophobic pocket at sub domain IIA. BSA has one more additional tryptophan Trp-134, which is more exposed to solvent and found at sub domain IB. As compared to other albumins, HSA is more non immunogenic plasma protein due to which it is widely used as a safe and effective carrier protein in different delivery systems [40], [41].
2.2. Structure of albumin
The three dimensional (3D) structure of HSA (Fig.1), shown by X-ray crystallography, proposed that HSA molecule is formed from three homologous domains I, II and III which themselves contain two separate helical sub-domains A (4 α-helices) and B (6 α-helices) [35]. A heart shaped albumin with 67% α-helix and no β sheet is very stable to changes in pH, denaturating solvents and exposure to heat because it contains 17 disulfide bonds and one free thiol from an unpaired cysteine (Cyc34) in domain I [42].
 Fig. 1. Structure of human serum albumin.
Fig. 1. Structure of human serum albumin.2.3. Binding sites in albumin
HSA has two main binding sites namely, Sudlow site I (present in sub-domain IIA) and Sudlow site II (present in sub-domain IIIA). Bulky heterocyclic anions such as anticoagulant drug warfarin bind to site I and aromatic carboxylates such as diazepam bind to site II. Apart from these two binding sites, albumin also contains other binding sites like Cys34 (binding site for Au(I), Hg(II) and complexed Pt(II) in the form of cisplatin, nitric oxide) and fatty acid binding sites. Cys34 site is also used to conjugate small molecules as well as protein and peptide based drugs [42]. Different ligand binding sites are summarized in Table 1.
Table 1. Different ligand binding sites present in albumin tertiary structure (modified from Sleep et al. [42]).
| Sr. no. | Binding site | Location | Ligands | Notes |
|---|---|---|---|---|
| 1. | N-terminal site | IA | Co (II), Ni(II) and Cu(II) | Consists of the 3 N-terminal amino acids, Asp-Ala-His |
| 2. | Cys34 | IA | Au(I), Hg(II), Pt(II) and NO | Cisplatin-binding site |
| 3. | FA1 | IB | Fatty acids, haem-Fe(III), bilirubin, hemin, synthetic Fe(II) porphoryrins and Al(III) phthalocyanines (tumor localizing photosensitisers) and prostaglandins | Low-affinity FA-binding site |
| 4. | FA2 | Between IA and IIA | Fatty acids | High-affinity FA-binding site |
| 5. | MBS (also known as site A or cadmium site A) | I/II inter-domain contact region | Cu(II), Ni(II), Cd(II) and Zn(II) | Surrounded by FA1, FA2 and FA7 |
| 6. | FA7 | IIA | Fatty acids, thyroxine, bulky heterocyclic anions such as warfarin, CMPF, phenylbutazone, tolbutamide, iodipamide and indomethacin | Major drug-binding site I, or Sudlow's site I, Low-affinity FA site |
| 7. | FA6 | Between IIA and IIB | Fatty acids | Low-affinity FA-binding site |
| 8. | Met298 | Between IIA and IIB | Cisplatin (Pt(II))-binding site | – |
| 9. | FA3-FA4 | DIIIA | Fatty acids, aromatic carboxylates, ibuprofen, diazepam, diflunisal, diclofenac, iopanoic acid and thyroxine | Major drug-binding site II, or Sudlow's site II. FA3 low affinity FA binding, while FA4 high-affinity FA binding |
| 10. | FA5 | IIIB | Fatty acids and thyroxine | High-affinity FA binding |
| 11. | FA8-FA9 | Between IA-IB-IIA and IIB-IIIA-IIIB | Fatty acids | Supplementary sites only. FA8 short-chain FA and FA9 induced during FA saturation |
| 12. | Secondary MBS (site B or cadmium site B) | Currently not defined | Cd(II), Co(II), Mn(II) and Zn(II) | – |
3. Albumin based nanocarriers
Albumin is a versatile protein used as a carrier system for cancer therapeutics. As a carrier it can provide tumor specificity, reduce drug related toxicity, maintain therapeutic concentration of therapeutic moiety like drug, gene, peptide, protein etc. for long period of time and also reduce drug related toxicities. It also has the potential in the half life extension of drug. As albumin has various binding sites, ligand functionalized delivery of therapeutic moiety is also possible which can provide site specific delivery of the therapeutic moiety [11]. Two basic approaches are utilized in the development of albumin based cancer therapy system i.e. conjugation of therapeutic moiety directly to the albumin or formulation of nanoparticles incorporated with therapeutic moiety like drug, peptide, gene etc. Some of biological applications of albumin conjugates are: use as a reagent for immunoassay and immunohistochemistry, used for elucidating hormone receptor interactions and used in the treatment of various diseases like cancer, viral infection and diabetes [11], [34]. Albumin based nanoparticles are utilized for cancer treatment as they are biodegradable, non-antigenic and can be also surface modified which may help in avoiding the undesirable toxicity of drugs by modifying their body distribution and improve their cellular uptake. They also have targeting potential because proteins themselves act as passive as well as active targeting moiety. Other targeting ligands can also attach in these carriers to provide site specificity [11]. Different albumin based carrier systems are depicted in Fig. 2 and their roles in the treatment of cancer will be discussed in subsequent sections of this review.
 Fig. 2. Albumin based nanocarriers.
Fig. 2. Albumin based nanocarriers.3.1. Albumin nanoparticles
Albumin nanoparticles can be prepared by several methods like desolvation, emulsification, thermal gelation, nano spray drying, nab technology and self-assembly etc. All preparation methods are summarized in Table 2. The selection of the method is based on several factors such as type of system, area of application, required size, type of drug (hydrophilic or hydrophobic), etc.
Table 2. Methods of preparation of albumin based nanoparticles [36], [37], [41], [42], [43], [44], [45], [46], [47].
| Sr. no. | Method | Key factors/variables | Advantages | Disadvantages |
|---|---|---|---|---|
| 1. |
Desolvation |
|
|
|
| 2. |
Emulsification |
|
|
|
| 3. |
Thermal gelation |
|
|
|
| 4. |
Nano spray drying |
|
|
|
| 5. |
Nab-technology |
|
|
3.2. Albumin micelles
Protein derived copolymers are used in preparation of micelles (core/shell structure) in which core acts as a reservoir for drug or gene and hydrophilic side chains form shell that reduces nonspecific interactions, immunogenicity and antigenicity of proteins and peptides. These micelles are used for gene delivery, targeted anticancer drug delivery, surface engineered multimodal therapy etc. [48]. Wu et al. developed albumin copolymer micelles for delivery of doxorubicin in which polycationic albumin precursor protein cBSA-147 was used to formulate nanosized micelles and doxorubicin (DOX) was loaded by hydrophobic interaction with polypeptide scaffold. The obtained results showed higher drug cytotoxicity and cellular uptake as compared to free DOX and also higher pH dependent stability in various physiological buffers [48]. In another study, amphiphilic adriamycin-human serum albumin (HSA-ADR) conjugates were developed by Chen et al. This amphiphilic HSA-ADR conjugate self assembled into redox sensitive micelles like nanoparticles. These redox sensitive micelles showed higher in vitro cytotoxicity in gastric cancer cell line and also higher intra tumor accumulation as compared to HAS/ADR NPs. An in-vivo tumor suppression was also observed after i.v. administration which showed the promising potential of glutathione sensitive (redox) micelles in gastric cancer therapy [49]. Apart from drugs, proteins can be also delivered by the albumin based micelles to their target site. Jiang et al. prepared albumin (BSA) based polyionic complex micelles for protein delivery. Although BSA itself directly formed complex with the positively charged proteins and self assembled into nanoparticles, but these nanoparticles were not stable and aggregated into large particles after some days. To overcome this drawback, maleimide functionalized poly(oligo (ethylene glycol) methyl ether methacrylate) was conjugated with BSA and this was used to deliver Spry 1 by forming polyionic complexed micelles. These micelles showed improved cytotoxicity of Spry1 on the breast cancer cell lines (MCF7 and MDA-MB-23) and exhibited high anticancer efficacy by inhibiting the growth of three dimensional MCF-7 multicellular tumor spheroids [50]. The formed pegylated albumin based polyionic complex micelles also revealed their potential in gene delivery [51]. Jiang et al. also developed albumin micelles for drug delivery. They formulated albumin - poly(methyl methacrylate) (PMMA) conjugate which self assembled into micelles for delivery of curcumin [52].
3.3. Albumin based nanoconjugates
Apart from nanoparticles, various albumin based nanoconjugates have also been investigated for drug and gene delivery in past few years. These nanoconjugates are formed by the interaction of albumin with different conjugation moieties like polymers, drugs, metals, DNA or RNA and known as albumin-polymer conjugate, albumin-drug conjugate, albumin-metal conjugate and albumin based nanoplexes respectively. These interactions may be of two types: either non covalent interaction or covalent interactions and are possible because of the unique structure of albumin as it has various binding sites (Fig. 3). The non-covalent interaction between albumin and conjugation moiety is because of hydrophobic and electrostatic interaction which act as driving force in the formation of albumin conjugates. Albumin contains different functional groups due to presence of different amino acid residues and these functional groups are utilized to prepare albumin based nanoconjugates by different chemical coupling reactions like thiol-maleimide coupling, Michael addition reaction and carbodiimide coupling reactions which form covalent bondsbetween the albumin and conjugation moiety. Such nanoconjugates are more stable as compared to nanoconjugates formed by non-covalent interactions. Among the various amino acid residues present in the albumin structure, cysteine (Cys) and lysine (Lys) residues are most widely explored targeting residues for the preparation of covalently conjugated albumin nanoconjugates [53].
 Fig. 3. Interactions involved in the synthesis of albumin based nanoconjugates.
Fig. 3. Interactions involved in the synthesis of albumin based nanoconjugates.3.3.1. Albumin based prodrugs and albumin-drug conjugates
Albumin based prodrugs and drug conjugates are widely used in cancer therapy. The unique properties of albumin like biodegradability, solid tumor accumulation ability, non-toxicity etc. makes it suitable drug carrier. Albumin also has different binding sites which can bind drugs by covalent or non-covalent bonding. Binding of drugs with albumin alters the properties of the drug molecule like toxicity profile, circulation half life and other pharmacokinetic properties [54]. Albumin-drug conjugate enhances the circulation half of drug due to long plasma half life (~ 19 days) of albumin in human body. Apart from this, it also overcomes the multi drug resistance of anticancer drugs [55]. On the basis of above mentioned properties of albumin-drug conjugates, lots of research work is carried out in last few years for the treatment of cancer and is also in clinical trials.
Many anticancer drugs like methotrexate (MTX), doxorubicin (DOX), cisplatin, oxaliplatin, carboplatin, docetaxel etc. were used to prepare albumin-drug conjugates. Stehle et al. prepared MTX-HSA conjugate for the treatment of cancer and this was the first albumin-drug conjugate that was under gone clinical trials. MTX-HSA conjugate was prepared by the direct coupling of MTX with the lysine residue of HSA and found to be more effective in targeting tumor [56], [57]. 7-Ethyl-10-hydroxycamptothecin (SN38) - HSA conjugate was prepared by Sepeheri et al. to improve the solubility as well as tumor tissue targeting as compared to active form of SN38 by chemical coupling using carbodiimide chemistry (EDC/NHS) [55]. The results revealed better solubility and stability of SN38-HSA conjugate along with prolonged circulation time in blood as compared to free SN38. Esmaeili et al. developed docetaxel-albumin conjugate for enhancing the solubility and tumor targeting [58]. Apart from drugs, different dyes and photosensitizing agents have also been conjugated to albumin for cancer diagnosis and therapy. Jeong et al. prepared chlorin e6 (Ce6) conjugated HSA nanoparticles for photodynamic therapy. Ce6-HSA conjugate was prepared by the carbodiimide chemistry using EDC/NHS. This conjugate was able to form self-assembled nanoparticles which upon illumination with specific wavelength light produced singlet oxygen which damaged the target tumor cells in cell culture. The in vivo results also revealed superior biodistribution of Ce6-HSA conjugate at the tumor site as compared to free Ce6 [59].
Different prodrugs have been designed to target the cancer cells that utilized endogenous albumin as drug carrier. These prodrugs are designed in such a way that binds rapidly and selectively to the cysteine-34 position (Cys-34) of circulating serum albumin after administration because approximately 70% of blood circulating albumin is mercaptalbumin containing an accessible Cys-34 [34]. Many platinum based prodrugs have been designed to selectively bind with the serum albumin for cancer treatment. Zheng et al. designed Pt (IV) prodrugs to bind non-covalently to HSA for drug delivery. In this study, axial ligand of cisplatin was asymmetrically functionalized by two different groups i.e. succinate and an unbranched aliphatic carbamate. Optimized compound of this study (compound 4e) bound with serum albumin non-covalently and after conversion of active form demonstrated 9 to 70 time better anticancer activity than parent compound cisplatin in lung and ovarian cancer cell lines along with prolonged half life (6.8 h) in blood as compared to cisplatin (t1/2 ∼ 20 min) or satraplatin (t1/2 ∼ 6 min). This prodrug compound (compound 4e) was interacted with the biological reductants, present in the cancer cells, which convert it into Pt(II) form. This Pt(II) form further interacted with DNA and causes DNA damage, cell cycle arrest and apoptosis [60]. In another study, Mayr et al. prepared oxaliplatin prodrugs to bind with HSA and improve the anticancer activity. Prodrugs were designed by the functionalization of the maleimide group in the parent compound which converted the Pt (II) form of oxaliplatin to Pt (IV) prodrug. Apart from oxaliplatin, cisplatin prodrug was also prepared with the same functional moiety and results were compared. The results indicated the faster reduction of cisplatin analogue as compared to oxaliplatin analogues. Apart from the reducibility, tumor-targeting potential of the albumin-bound drugs 24 h after i.v. administration also varied which may be due to different reducibility of the drugs. All these results revealed the potential of prodrug of oxaliplatin in enhancing plasma half life of drug by avoiding fast renal clearance and better tumor accumulation of the drug due to EPR effect [61]. Apart from the platinum based prodrugs, ferric prodrugs have also been designed to interact with serum albumin and targeting cancer. Qi et al. designed the ferric prodrug based on N-donor residues (Lys199 or/and His242) of human serum albumin (HSA) carrier IIA subdomain for cancer treatment. They synthesized six Fe (III) compounds derived from 2-hydroxy-1-naphthaldehyde thiosemicarbazone and compared their potential alone as well as after binding with the HSA. Among these compounds, compound 12 and compound 12-HSA complex demonstrated better in vivo performance. As compared to compound 12, compound 12-HAS complex exhibited better targeting ability and activity in liver cancer [62]. All these results suggest that HSA carrier prodrug strategy for intravenous administration of novel anticancer compounds may be a promising approach for targeted cancer therapy.
In the development of albumin based prodrugs and albumin-drug conjugates, several factors may affect the activity of the compound. The major factors are: molar ratio of drug and albumin, structure and stereochemistry of the drug, nature of the drug and substitution moiety etc. For example, presence of hydrophobic moiety in the drug may enhance the affinity of drug to bind with the serum albumin which leads to stabilization of the drug-albumin conjugate [47], [59].
3.3.2. Albumin-polymer conjugates
Apart from tremendous advantageous properties, some draw backs are also associated with albumin that restrict its use in drug delivery such as lack of intrinsic targeting group, limited proteolytic stability and lesser suitability for hydrophilic and charged drugs [63], [64]. These drawbacks can be overcome by conjugating albumin with another polymer. Similar to albumin-drug conjugates and other protein polymer hybrids, albumin-polymer conjugates can be prepared either by chemical conjugation using covalent or non-covalent conjugation strategies or simply by the physical attachment of different polymers with albumin. Interactions between albumin and polymers are possible because of the presence of various active groups present on the surface of albumin like amino (Lys residues), carboxyl (Asp and Glu residues) and thiol (Cys residue). As compared to non-covalent conjugation strategy, covalent conjugates of albumin-polymer are more stable under physiological conditions. But on the basis of preparation method, non-covalent conjugation methods are relatively simple and flexible [47], [64]. There are two basic techniques for preparing the albumin-polymer conjugates: 1) conjugation of polymer to albumin by various coupling reactions like maleimide coupling, carbodiimide chemistry etc. [65] and 2) in-situ polymerization method (atom transfer radical polymerization (ATRP), reversible addition fragmentation chain transfer polymerization (RAFT), ring opening polymerization (ROP) etc.) [65], [66], [67], [68]. Albumin-polymer conjugates have been prepared by conjugating several polymers like polyethylene glycol (PEG) and its derivatives [69], poly(methyl methacrylate) (PMMA) [66], [70], poly (lactide co glycolide) (PLGA) [71], poly(N-isopropylacrylamide) (PNIPAAm) [68], poly(ε-caprolactone) (PCL) [65], hydroxyethylacrylate (HEA) [67] etc. Albumin-polymer conjugates based nanoparticles can be prepared by different methods like self assembly, coating/conjugation of polymer over the albumin nanoparticles and vice-versa [65], [70], [71] and have been extensively explored in the treatment of cancer. Dag et al. developed polymer-albumin conjugate for the delivery of macromolecular platinum drugs and its effectiveness was tested against the ovarian cancer. In their study, two monomers N-(2-hydroxypropyl)methacrylamide (HPMA) and Boc protected 1,3-diaminopropan-2-yl acrylate (Ac-DAP-Boc) were copolymerized using RAFT polymerization to form macromolecular ligand and this was further conjugated with the platinum drug. This polymer‑platinum conjugate was further conjugated with albumin to form self assembled core (polymer‑platinum)-shell (albumin) nanoparticles having the size of 80 nm. Albumin coated polymer‑platinum conjugate was readily taken up by the ovarian cancer cells and demonstrated superior toxicity as compared to albumin coating free polymer‑platinum conjugate [72]. In another study, Liu et al. prepared DOX-encapsulated cetuximab-functionalized BSA–PCL nano vesicle as a tumor-targeted nanocarrier. BSA-PCL conjugates were synthesized by maleimide–sulfhydryl coupling reaction. DOX-encapsulated cetuximab-functionalized BSA–PCL nano vesicle showed enhanced antitumor activity as compared to free DOX [65]. Camptothecin (CPT) encapsulated BSA-PMMA nanoparticles were prepared by the simple nanoprecipitation method and showed enhanced anti-tumor activity both in vitro and in animals [70].
3.3.3. Albumin-metal conjugates (albumin coated inorganic nanoparticles)
Inorganic nanoparticles are widely used in the drug delivery system not only for delivering the active moiety but also for diagnostic and imaging application because of their unique optical, magnetic and other properties. For target specificity, effective bioavailability and colloidal stability of these inorganic nanoparticles, surface modification with suitable targeting moiety or biocompatible moiety is necessary [47], [73]. Albumin coated inorganic nanoparticles have been explored extensively because of the unique properties of albumin. It provides the stability, enhanced circulation and better accumulation of inorganic nanoparticles to the desired target site and can also provide target specificity due to the presence of reactive surface functional groups for the attachment of targeting ligands [74]. Chen et al. developed pH-/H2O2-Responsive albumin-MnO2 Nanoparticles for combinational cancer therapy to modulate tumor hypoxia. To prevent the decomposition of MnO2nanoparticles in acidic environment, premodified HSA either with Ce6 or with c,t,c-[Pt(NH3)2-(O2CCH2CH2COOH)(OH)Cl2] (cis‑Pt(IV)SA), as pro-drug of cis‑platinum, were used which acted as a template and coating molecules to induce the formation of MnO2 nanoclusters through biomineralization in alkaline conditions, obtaining multicomponent HSA-MnO2-Ce6&Pt (HMCP) nanoparticles. These HMCP nanoparticles acted as a multimodal therapeutic system in which pH-/H2O2-responsive behaviors of MnO2 simultaneously generated O2 in situ by reaction with endogenous H2O2 inside the tumor and overcame the tumor hypoxia-associated resistance of photodynamic therapy. On the other side, HMCP nanoparticles within the acidic tumor microenvironment were gradually degraded into individual therapeutic albumin-drug complexes with small sizes (< 10 nm) and exhibited greatly enhanced intratumoral permeability for improved effectiveness in combined photodynamic and chemotherapy [75]. Attachment of albumin with the inorganic nanoparticles can alter the biological behavior like cellular uptake of nanoparticles, blood circulation time, higher penetration and retention of nanoparticles in the tumor cells etc. and these were also verified by different studies [47], [74], [76], [77], [78], [79].
4. Surface modification of albumin nanocarriers
Surface modification of protein based nanocarriers is necessary to alter the surface properties and enhance the targeting potential of the delivery system. Presence of different binding sites and functional groups like carboxyl and amino groups on albumin offers several possibilities for surface modification of albumin based nanocarriers. Surface modification of albumin nanocarriers with the specific ligand can be done by conjugating functional group of albumin with the ligand by covalent bond. For surface modification of albumin nanocarriers, electrostatic adsorption or surface coating techniques may be utilized as non-covalent attachment of ligands. In surface modified albumin nanocarrier, albumin plays a role of carrier for delivering therapeutic moiety whereas the ligand is used to modify the pharmacokinetic parameters, improve stability, prolonging circulation half life, modifying the release pattern of therapeutic moiety or as a targeting agent [11], [36]. For the surface modification of protein based systems, different ligation strategies are utilized viz. thiol–maleimide Michael addition ligation, biotin/avidin ligation, carbodiimide coupling strategy and disulfide bridging [80].
4.1. Ligands as a targeting agent
Various ligands like small molecules, carbohydrates, peptides, proteins or antibodies have been utilized for targeting of different nanocarriers. These act as receptor mediated targeting ligand for cancer treatment because several receptors like folate (FR), transferrin (TfR), epidermal growth factor receptor (EGFR), and lipoprotein receptors etc. are over expressed in the cancer cells and these ligands bind with the receptor and shows their action [80]. Some examples of ligand conjugated albumin nanoparticles for targeted delivery in cancer are summarized in Table 3.
Table 3. Ligand conjugated albumin nanoparticles for targeted delivery in cancer.
| Albumin based architecture | Ligand | Ligand category | Formulation method | Morphology | Size (nm) | Target cancer | Ref. |
|---|---|---|---|---|---|---|---|
| HSA-biotin | Biotin | Small molecule | Cross linked HSA with EDC | Nanoparticles | 125–145 | Breast and cervical | [81] |
| BSA-lysine-galactose | Galactose | Carbohydrate | Desolvation/crosslinking | Nanoparticles | 180–200 | Liver | [82] |
| HSA-PEG-cyclic RGD | Cyclic RDG | Peptide | Self assembly | Micelles | 30 | Skin | [83] |
| BSA-cyclic RGD | Cyclic RDG | Peptide | Desolvation/crosslinking | Nanoparticles | 130 | Pancreas | [84] |
| HSA-PEG-anti-HER-2 Mab | Anti-HER-2 MAb | MAb | Desolvation/crosslinking | Nanoparticles | 390–410 | Breast | [85] |
| HSA-PEG-anti-HER-2 Mab | Anti-HER-2 MAb | MAb | Desolvation/crosslinking | Nanoparticles | 220 | Breast | [86] |
| HSA-PEGDI17E6/IgG | DI17E6 MAb | Mab | Desolvation/crosslinking | Nanoparticles | 165–180 | Skin | [87] |
| FA-BSA-SPIO NPs | Folate | Small molecule | Chemical coprecipitation then coating of BSA | Albumin-magnetic nanoparticles | – | Brain tumor (MRI imaging) | [88] |
| Cationic and Mannose modified HSA | Mannose | Carbohydrate | High-pressure homogenization | Nanoparticles | 90.3 | Brain tumor | [89] |
| cRGD conjugated HSA | Cyclic RGD | Peptide | Nab technology | Nanoparticles | 160 | Pancreatic | [90] |
| Glycyrrhetinic acid-BSA | Glycyrrhetinic acid | Carbohydrate | Desolvation | Nanoparticles | 258.8 | Hepato-cellular carcinoma | [91] |
4.1.1. Small molecules
Small molecules like folate and biotin are widely used as targeting ligand for cancer cell targeting because of their easy availability, inexpensiveness, ease in handling and flexibility in chemical modification and characterization [80]. Folic acid, FR binding ligand, is an inexpensive and non-immunogenic small molecule having molecular weight of 44 Da. Folate conjugated drugs or biomarkers retain their ability to bind specifically with FR because of the high stability of folate over a wide range of temperatures and pH values [92]. It significantly binds with the glycosylphosphatidylinositol-linked FR which is over expressed (100–300 times higher than those observed in normal tissues) in different cancerous cells [80]. Folate conjugated proteins like albumin are selectively internalized into cytoplasm by receptor mediated endocytosis and promotes cellular uptake of therapeutic moiety like drug into cancerous cells [93]. Biotin is another vitamin used to target the cancer cells. It is basically cell growth promoter and present in significantly higher concentration in the cancer cells as compared to normal cells. Biotin receptors are also over expressed on the cancer cell surface because rapid proliferation occurs in cancer cells which need a higher quantity of biotin so it can also be utilized as a cancer targeting ligand [94].
4.1.2. Carbohydrates
Carbohydrate molecules (galactose, lactose and mannose) specifically bind to asialoglycoprotein receptors (membrane lectin receptors) commonly found in liver cells and are used for targeting hepatic and cervical cancer cells [95]. Hyaluronic acid which is a polysaccharide is also used as ligand for CD44 receptor mediated cancer targeting [96], [97].
4.1.3. Peptides
Integrin receptors (αvβ3) (membrane receptor for extracellular matrix ligands) are over expressed on the cancer cells surface and in tumor proliferating neovascular endothelial cells while lesser expressed in normal quiescent endothelial cells. These integrin receptors are very well targeted by the use of peptides. Among the various peptides, cyclic arginine-glycine-aspartic acid (RGD) is mostly used to target cancer cells because of high affinity to αvβ3integrin which can enhance cellular uptake and prolong retention of drug in the cancer cells [80], [83]. Some examples are mentioned in Table 3. Apart from RGD, other peptides like cell penetrating peptide (i.e. TAT), tumor homing peptide like CREKA and LyP-1 are also used in the targeted delivery of albumin based carrier for cancer [98].
4.1.4. Proteins
Proteins like transferrin, lactoferrin and Apolipoprotein E (Apo E) bind with the transferrin receptor, lactoferrin receptor and low density lipoprotein receptor respectively. These ligands are used for brain targeting because of over expression in blood brain barrier also. Apo E and transferrin conjugated HSA nanoparticles loaded with loperamide were also prepared for brain targeting. The results indicated higher concentration of drug after the conjugation of these ligands with HSA nanoparticles in the brain [99], [100], [101], [102]. Zhigui et al. prepared lactoferrin-modified polyethylene glycol grafted BSA nanoparticles as a dual targeting system for gliomas. Results showed higher permeability and cellular uptake in both primary brain capillary endothelial cells (BCECs) and glioma cells (C6) [103].
4.1.5. Monoclonal antibody (MAb)
MAbs (Y-shaped proteins) have been also utilized as a ligand for tumor targeting because of their unique properties like higher affinity (Kd ~ 0.1 nM) even with a low density and ability to recognize a specific part of their target [104]. Different MAbs used as ligands for tumor targeting are: anti-CD3 MAb, Herceptin, EGFRMAb, DI17E6 MAb, anti FAS MAb, anti-antigen rich breast cancer cells MAb, AMB8LK MAb, anti-CD20 MAb, anti-CEA MAb etc. [80]. MAb functionalized albumin nanocarriers are also summarized in Table 3.
4.2. Ligands as pharmacokinetic parameter modifier
Surfactants like polysorbate 80 (Tween 80), poloxamer etc. are used to alter the pharmacokinetics of the formulation. These surfactants are coated over the surface of the nanoparticles which significantly reduces various toxicities of the drug [11]. This can be explained by tween 80 coated DOX loaded HSA nanoparticles. Coating of tween 80 significantly reduced the toxicities (hematological, cardiac and testicular) of DOX which may be due to alteration of pharmacokinetic properties of drug [36], [105], [106], [107].
4.3. Ligands as circulation half life enhancer
Polyethylene glycol (PEG) coating or chemical coupling, known as pegylation, to protein or particles (the delivery system) is used to improve the circulation half life and reduce the immunogenicity. It also enhances the accumulation of carrier system in the tumor because of EPR effect [108]. Methoxy PEG2000 was used for the pegylation of BSA nanoparticles. In vivo pharmacokinetic studies demonstrated long circulation time and higher uptake and cytotoxicity of doxorubicin in both primary brain capillary endothelial cells (BCECs) and glioma cells (C6) [103]. Moreover, pegylation also modified the release of drug from the nanoparticles. The release of 5-Flurouracil from methoxy PEG-succinimididyl propionate modified BSA nanoparticles was much slower than non-pegylated nanoparticles due to presence of mPEG which acted as a barrier to drug diffusion [108]. Pegylation of HSA nanoparticles were done by conjugation of two different PEG: poly (thioetheramido acid) poly (ethylene glycol) and methoxy poly (ethylene glycol) [109]. The loading of Rose Bengal in HSA-mPEG nanoparticles was much lesser as compared to unmodified HSA due to lesser drug-protein binding sites in modified HSA. The drug release from the modified HSA was also slow due to steric stabilization of nanoparticles provided by PEG which prevented the enzymatic degradation of nanoparticles [109].
4.4. Ligands used to modify the drug release and stability of nanocarrier
Different polymers are used to modify the drug release from the albumin based carrier and to stabilize them. The polymers can be chemically or physically attached with the albumin which potentially alters the properties of albumin like solubility, biocompatibility etc. and enhances its stability. These polymers can be either coated on the surface of the nanocarrier or conjugated with the albumin prior to the preparation of nanocarrier [64]. A large number of polymers viz. PEG, PLGA, PCL, PLA cationic polymers like polyethylenimine (PEI), poly-l-lysine (PLL), thermoreversible and thermosensitive polymers like poly(N-isopropylacrylamide) (PNIPAAm), poly(methylmethacrylate) (PMMA) etc. have been exploited to alter the drug release and stability of nanocarriers [64], [110], [111], [112], [113], [114], [115], [116], [117]. Cationic polymers are mainly used to alter the release behavior of drug from the nanocarriers. These are used to retard and control the drug release which depends on the concentration of coated polymer [11]. Zhang et al. prepared bone morphogenetic protein − 2 (BMP-2) encapsulated PEI coated BSA nanoparticles and study results showed release alteration due to PEI [118], [119]. As PEI is a cationic polymer, surface charge of BSA nanoparticles shifted towards the neutral or slightly negative side and facilitated in vivo application of nanoparticles due to reduced opsonization and improved stability of system [119]. In another study, PEI coating over the HSA nanoparticles improved the therapeutic index of DOX against MCF-7 breast cancer cells because PEI coating enhanced cellular uptake of the particles [120]. PLL is another cationic polymer used to coat the albumin based nanocarriers. PLL coating enhances the nanoparticles stability by providing proteolytic resistance. This was reported for SiRNA encapsulated BSA nanoparticles in which stability of nanoparticles in aqueous solution was improved with increasing PLL molecular weight and concentration [11], [121]. Apart from cationic polymers, thermoresponsive polymers are also used for controlling the release of encapsulated therapeutic active from the albumin nanocarrier. Poly (N-isopropylacrylamide)-block-polyallylamine (PNIPAM-AAm-b-PAA) conjugated albumin nanospheres were developed by Shen et al. to control the release of adriamycin. The results indicated that as compared to unconjugated nanoparticles, release of adriamycin from conjugated nanoparticles in trypsin solution was slower and decreased with increasing the conjugation amount or molecular weight of PNIPAM-AAm-b-PAA. This may be due to steric stabilization of nanoparticles which makes the degradation of unconjugated nanoparticles more difficult [122], [123].
5. Role of albumin and albumin nanocarriers in cancer therapy
Albumin has been widely explored for cancer diagnosis and therapy due to its unique properties like biocompatibility, biodegradability, non-toxicity and low immunogenicity [36]. Albumin accumulates in the malignant tumor tissue/cells because of leaky vasculature and impaired (absent or defective) lymphatic drainage system of tumor cells. These properties of tumor cells enhance the accumulation of albumin in tumor by EPR effect [124], [125], [126]. Albumin or albumin nanocarrier binds with gp60 (60 kDa glycoprotein also known as albondin) receptors over-expressed on epithelium wall of tumor blood vessels. This ligand-receptor binding initiates transcytosis and leads to formation of invagination at the surface of membrane and ultimately form caveolae (transcytotic vesicles). Then fusion of these caveolae with the plasma membrane takes place by the extra vascular deposition and passing through other side of cell followed by release of albumin into the extracellular space (tumor interstitium). In the tumor interstitium, SPARC (secreted protein, acidic and rich in cysteine) are over expressed which enhance the accumulation of albumin inside the tumor by providing a mediated albumin transport pathway to the subendothelial space [127]. The process of accumulation of albumin-drug complex in the solid tumor is demonstrated in the Fig. 4.
 Fig. 4. Accumulation and uptake of albumin-drug conjugate in tumor cell [reproduced from B. Elsadek and F. Kratz [220]].
Fig. 4. Accumulation and uptake of albumin-drug conjugate in tumor cell [reproduced from B. Elsadek and F. Kratz [220]].5.1. Albumin nanocarriers for cancer therapy
Albumin based nanoparticles are widely explored protein based nanocarriers for cancer therapy. Several categories of drugs can be encapsulated in albumin via covalent conjugation, electrostatic interaction or hydrophobic interaction. This makes albumin a versatile drug delivery carrier. Apart from this, its accumulation in the tumor also makes it suitable candidate for cancer therapy. Different albumin based nanocarriers and their applications in cancer therapy are summarized in Table 4.