1. Introduction
The global aging of the population is increasing at an accelerating rate. These demographic changes are leading to an increased prevalence of age-related degeneration and chronic diseases. Traditional medicine is trying to solve all these problems. In fact, successful organ transplantation (allografts) is considered among one of the major milestones of medicine of the last decades. However, success of transplantation is limited by shortage in the supply of transplantable organs and by the potential organ rejection.
As social and health needs are changing, the pressure to develop new alternatives, designed to address unmet medical needs, increases. Over the last years, the gradual understanding of the biological processes involved in wound healing has paved the way for developing new regenerative therapies with the ultimate goal of promoting and accelerating tissue regeneration [1], [2]. In this context, personalized medicine is emerging as a promising and appealing therapeutic option. It is based in the complex uniqueness of each patient offering a tailor-made treatment for improving the management of patients [1], [3]. Already in ancient Greece, the father of medicine, Hippocrates, mentioned the importance of personalizing the medicine: “it is more important to know what sort of person has a disease than to know what sort of disease a person has”.
Autologous plasma-based therapies may be a form of personalized medicine. These therapies employ human-based cells or tissues, may or not manipulate them outside the body, and reintroduce them into the same donor [4]. This emerging field, by being autologous, provides new advantages in the clinical setting: (i) the donor is immediately available, (ii) no immunosuppression is required, (iii) no rejection occurs, (iv) elimination of graft versus host disease, (v) risks associated to disease transmission are eliminated and (vi) in general, does not present ethical conflicts [5].
Platelet rich plasma is included within these types of autologous therapies. It is based on properly selecting, from human blood, a pool of cells and growth factors, together with fibrin forming proteins to create a three-dimensional (3D) scaffold, necessary to support tissue regeneration. As derived from patient's own blood, platelet rich plasma is an affordable and minimally invasive technique [2].
In this review we describe the potential of plasma-based medicine and in particular platelet rich plasma as an autologous therapy. We provide insight about their biological effects as well as some pivotal preclinical and clinical applications.
2. Mimicking the healing process
Following any tissue injury, one of the main priorities of the organism is the restoration of tissue integrity and function. For this purpose, the process of wound healing goes through a sequence of continuous, overlapping and precisely programmed phases involving rapid hemostasis, controlled inflammation, cell migration to the injury site and subsequent cell proliferationand differentiation, and finally, formation and remodeling of extracellular matrix (ECM) [6], [7], [8]. During the healing process some remarkable events must be specially mentioned.
2.1. Fibrin scaffold
Polymerized fibrin that is structured from soluble fibrinogen, is the principal component of blood clots, and provides a provisional scaffold which enables formation of a temporary matrix in the wound bed. Fibrinogen is a key protein for both hemostasis and homeostasis. In fact, fibrinogen assembles into the ECM at sites of tissue damage, where it may be involved in cell type-specific mechanisms of wound repair [9]. Its contribution largely depends on the interactions between specific-binding sites on fibrin(ogen), pro-enzymes, clotting factors, enzyme inhibitors, and cell receptors as well as on the structural composition of fibrin [10]. Fibrinogen circulates in normal human plasma in a high and low molecular weight form (HMW and LMW, respectively). This fibrinogen heterogeneity strongly influences coagulation rate, fibrin structure and endothelial cell behavior. HMW fibrin matrices display a porous and malleable network with thicker fibers whereas the thinner fibers bundles of LMW fibrin matrix form a dense structure. Moreover, HMW fibrins stimulate endothelial cell proliferation and tube formation more than LMW matrices do. These differences could be exploited for therapeutic applications [11], [12].
2.2. Growth factors
The aggregated platelets, which are trapped in the provisional fibrin matrix, release multiple growth factors that exert outstanding roles in modulating the inflammatory stage and cell recruitment to the damaged area (Table 1). In addition, these bioactive molecules are key regulators of the main tissue regenerative processes such as cell migration, proliferation, differentiation, angiogenesis and ECM biosynthesis. Growth factors modulate their effects through binding to specific receptors on the target cell surface. Following this binding, signal-transduction pathway involves a complex array of events such as second messengers, protein phosphorylation, gene expression and protein synthesis [13]. Multiple growth factors may share mechanisms while the same growth factor may deliver different messages depending on the cell and receptor type they bind to.
Table 1. Main growth factors involved in human tissue regeneration [24], [25], [26].
| Growth factor | Molecular weight (kDa) | Types | Receptors | Mechanisms of activation and signaling | Main function |
|---|---|---|---|---|---|
| EGF [14], [15] | 6.4 | – | EGFR (ErbB1) | Receptor tyrosine kinases | Cell growth, proliferation, differentiation and survival. |
| FGF [16], [17] | 7–38 | FGF1-14, FGF16-23 | FGFR1, FGFR2, FGFR3, FGFR4 | Receptor tyrosine kinases | Cell proliferation, migration, differentiation, angiogenesis and survival. |
| PDGF [18], [19] | 32–35 | -AA, -BB, -AB, -CC, -DD | PDGF-α and PDGF-β | Receptor tyrosine kinases | Chemotactic, cell proliferation and extracellular matrix production. |
| TGF-β [20], [21] | 25 | -β1, -β2, -β3 | TGFβRI and TGFβRII | Serine/threonine kinase receptors. | Wound healing, angiogenesis and immune suppression promotion. Differentiation. Extracellular matrix production, and wound contraction. |
| VEGF [22], [23] | 34–42 | -A, -B, -C, -D and placental growth factor | VEGFR-1, VEGFR-2 and VEGFR-3. Neuropilins (Nrp-1 and Nrp-2). | Receptor tyrosine kinases. Receptors for semaphorins. | Endothelial cell proliferation and migration. |
2.3. The active role of cells
After hemostasis and inflammatory stages, damaged tissue restoration process is intensified. This process is carried out by several phenotypes through different procedures. Tissue-specific adult stem cells gain special importance in the response to regulatory signals from the injured tissue [27]. In addition, bone marrow-derived stem cells significantly contribute to tissue regeneration by providing trophic factors that modulate the local environment and by promoting angiogenesis, reepithelialization and granulation tissue formation [28]. Stem cell behavior is profoundly modulated by different signals such as cell-cell interactions and the ECM, components all of them of the stem cell niche, a dynamic structure that includes immune cells during inflammation and wound healing process [29]. It is also necessary to mention the role of macrophages during the inflammatory response and tissue repair. Considered more than immune cells, macrophages have the ability of acquiring distinct functional phenotypes in response to the microenvironmental cues. Traditionally, two main phenotypes have been identified: the classically activated macrophages (M1) that produce many inflammatory cytokines, reactive oxygen species and nitrogen intermediates and the alternatively activated macrophages (M2) that are characterized by low levels of pro-inflammatory cytokines and high IL-10 expression. M1 supports pathogen killing and drives the inflammatory response whereas M2 sustains tissue remodeling. Both phenotypes also differ in the metabolic pathway, since glycolytic pathway is involved in M1 polarization, whereas fatty acid oxidationoccurs in M2. Macrophages show different activation states, therefore, a continuum of phenotypes is expected to occur at the wound site. This macrophage plasticity suggests a complex molecular system that enables them to play multiple roles in inflammation and regeneration [30], [31], [32].
2.4. ECM biosynthesis
ECM presents a unique composition and topology that is generated through a dynamic and reciprocal, biochemical and biophysical dialogue between various inner cellular components and the evolving microenvironment [33]. Moreover, the ECM is a highly dynamic structure which molecular components are subjected to post-translational modifications generating the particular biochemical and mechanical properties of each organ [34]. Far from being just an inert supportive structure, the ECM, by anchoring cell and growth factors, directs cell fate in a well-orchestrated manner [29]. Cell-ECM interactions are primarily mediated by integrins, the main cell adhesion receptors, which in turn, control growth factor signaling pathways in a synergistically manner [35]. The emerging field of tissue engineering seeks to develop biomaterials that closely resemble the characteristics and functions of the native ECM.
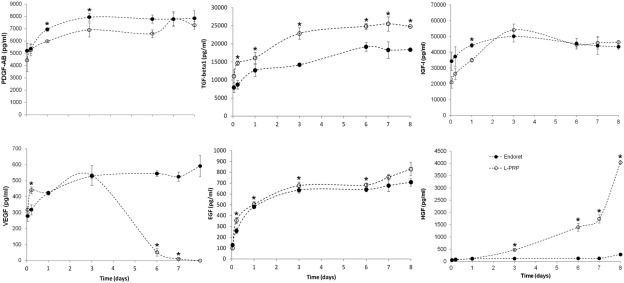
Since fibrin glue was originally described in 1970, artificial acellular scaffolds have been considered as a promising field in tissue regeneration therapies due to their ability to create and maintain an space for tissue growth, to provide mechanical stability and to also support cell adhesion and migration [36], [37]. However, synthetic polymers, as well as their degradation products, can activate the complement cascade of inflammatory response [38]. On the contrary, the fibrin scaffolds derived from plasma rich in growth factor (PRGF), a particular platelet rich plasma, are biocompatible matrices and contain a plethora of biologically active substances provided by platelets that actively participate in the regenerative process [39]. Platelet rich plasma reabsorbable matrices combine the sealant properties with the ability to gradually release the main promoters of tissue regeneration [40]. In this sense, the kinetic of GFs delivery from platelet rich plasma scaffolds have been already described [41]. Thus, it has been probed that after a quick delivery of GFs (PDGF-AB, VEGF, HGF and IGF-I), the release is maintained more than a week, being the GF retention by platelet rich plasma fibrin of almost 30% on the 8th day (Fig. 1).

Fig. 1. PDGF-AB, TGF-β1, IGF-I, VEGF, EGF and HGF release from PRGF and L-PRP fibrin scaffolds during 8 days.
Reproduced, with permission from [41].3. Autologous platelet rich plasma therapies
Biological therapies provide new opportunities for developing personalized medicine. Platelet-rich plasma is included within the autologous therapies based on the reparative ability of platelets. Not long ago it was thought that the role of platelets was limited to maintaining tissue hemostasis. However, recent evidences suggest that platelets participate in a wide range of physiological processes, including the immune response, inflammation and wound healing through the large number of substances present in their secretome and the high amount of receptors that are present in their surface [42], [43]. In fact, high-throughput proteomic approaches have identified over 300 proteins released by human platelets upon activation [44]. Following activation, proteins, cytokines, exosomes and microparticles are released from platelets after rapid translation of pre-existing mRNA. This process delivers bioactive mediators and also modifies the platelet surface, which provides them with the ability to modulate the function of many cells which is essential for tissue repair. This ability of platelets beyond hemostasis confers them a great ability to be applied in multiple medical fields [43], [45].
Basically, platelet rich plasma is defined as a portion of plasma fraction obtained from the patient's own blood having a platelet concentration above baseline. The ultimate goal of these therapies is to facilitate, optimize, and accelerate the body's innate ability to repair tissues by the progressive delivery of autologous growth factors and proteins by the platelet rich plasma scaffold (Fig. 2). Despite the great number of possible protocols for platelet rich plasma preparation with multiple commercially available products, most share a common sequence of basic steps: (i) blood collection by simple venipuncture, (ii) blood centrifugation to obtain the platelet concentration plasma fraction and (iii) activation of platelets to release growth factors from the temporary scaffold [46]. Nevertheless, there is no consensus regarding the conditions of centrifugation, the method for platelet activation, the optimal concentration of platelets, or the inclusion or not of leukocytes. All of these variables lead to different products with different biological potential [2], [46]. Therefore, PRGF is a platelet rich plasma obtained by a single centrifugation and in which activation process calcium chloride is used. This personalized technology is characterized by a specific platelet concentration that leads to an optimal biological effect and by the absence of leukocytes thus avoiding the promotion of inflammation. Due to its great therapeutic potential, this pioneering technology is widely used in several medical fields [47], and was firstly used worldwide in 2003 in the area of orthopaedics [48].
 Fig. 2. Illustration representing the regenerative potential of PRGF, a particular platelet rich plasma. A) PRGF obtaining to be applied in several injured tissues. B) Wound healing main cell processes positive modulated by the temporary scaffold and growth factors provided by PRGF preparations.
Fig. 2. Illustration representing the regenerative potential of PRGF, a particular platelet rich plasma. A) PRGF obtaining to be applied in several injured tissues. B) Wound healing main cell processes positive modulated by the temporary scaffold and growth factors provided by PRGF preparations.Cells, growth factors and scaffolds are the three components considered as essential for tissue regeneration. These autologous therapies provide natural, biodegradable and transient scaffolds with a wide range of mechanical properties that combine with a sustained release of proteins and growth factors to induce host cells to replace the lost tissue structurally and functionally. Additionally, platelet rich plasma fibrin matrix provides anti-inflammatory factors and anti-bacterial peptides avoiding the inflammatory response as well as bioactive factors that play important roles in regulating cellular processes such as mitogenesis, chemotaxis, differentiation and metabolism [49], [50]. On the other hand, both the highly organized structure that confers to platelet rich plasma scaffold rigidity, porosity and tolerance properties and its content in several proteins such as fibrin, fibronectin and vitronectin, favor the migration and adhesion of cells that are actively involved in the regeneration process [51].
The multiple formulations that can be obtained from platelet rich plasma autologous therapies confer this approach a great versatility to be applied in different medical fields, including orthopedic, sports medicine, bone reconstruction, tissue engineering, maxillofacial surgery, periodontal regenerative medicine and ophthalmology [52] (Table 2). Additionally, platelet rich plasma technology is beginning to be used in autologous cell therapies for ex vivo expansion and subsequent transplantation of stem cells as an alternative to the use of xenogeneic products [53].
Table 2. Main clinical applications for autologous platelet rich plasma.
| Pathologies | Outcome | Administration | |
|---|---|---|---|
| Orthopedic [48], [54], [55], [56] | Achilles tendinopathy, anterior cruciate ligament (ACL) reconstruction, osteoarthritis, lateral epicondylitis, patellar tendon healing, osteochondral lesions, plantar fasciitis, hip fracture, rotator cuff repair, knee arthroplasty. | Pain reduction, improvement of functional status and joint stiffness, significant improvement in several scores (VAS, AOFAS, FAAM, WOMAC…). | Injection. |
| Dentistry [57], [58], [59], [60], [61], [62], [63] | Temporomandibular disorders, extraction socket, sinus elevation, horizontal bone defect, bone ridge expansion, bisphosphonate related osteonecrosis of the jaw (BRONJ). | Pain reduction, improve temporomandibular joint mobility, ridge thickening, inflammation decrease, enhance healing process, superior bone density, enhance vascularization, and regeneration of osseous and epithelial tissues. | Injection, fibrin. |
| Ophthalmology [64], [65], [66], [67] | Dry eye, graft versus host disease, Sjögren's syndrome, persistent epithelial defects, corneal ulcers, macular holes, ocular surface pathologies associated with LASIK surgery. | Improvement or disappearance of symptoms, reduction of photophobia and inflammation, improvement in the resolution of keratitis punctata, increase in the lacrimal volume production, improvement in the tear break-up, re-epithelization, ocular pain decrease, visual acuity improvement. | Eyedrops, fibrin scaffold, injection. |
| Dermatology [68], [69], [70], [71], [72] | Androgenetic alopecia, diabetic and other chronic ulcers, aesthetic dermatology, vitiligo. | Increase in the follicular density, reduction in hair loss, hair growth stimulation, prevention of dermal papilla apoptosis, hair density increase, enhancement of the re-epithelialization process, increase healing rate, repigmentation improvement. | Injection, spray, fibrin topical application. |
| Others | The versatility of this autologous technology is opening up new fields of application where the use of this biological therapy may be an alternative to current treatments (peripheral neuropathies [73], gynecological issues [74]…). | ||
4. Concluding remarks and future perspectives
Personalized medicine and specifically autologous therapies represent promising new medical technologies that offer the potential to reduce risks and increase safety using proteins and cells from the own patient. The concept is to transform the way in which medicine is practice to optimize and enhance the body's innate ability to repair tissues in order to improve medical outcomes.
The last decades have witnessed an explosion in the use of platelet rich plasma products for tissue regeneration purposes. Efficacy and safety have been demonstrated in a wide range of medical fields. Nevertheless, there is still a long way to go in regulatory and standardization terms. The goal to be achieved in the coming years should be a more predictable and efficient treatment by reducing the variability of preparation and administration protocols thus leading to completely different biological effects and to misinterpretation of results.
Most successful treatments in regenerative medicine take advantage of the synergistic effects of combining therapies. In the last years, stem cells have adopted a relevant role in this field. Therefore, an integrated strategy that combines the autologous technology of platelet rich plasma and the emerging use of stem cells may represent a new challenge to develop new applications.