Highlights
-
•
The influence of MBBR operational parameters on its microbial population is reviewed.
-
•
The microbial phyla and classes have shown distinguish profiles depending on biomass forms.
-
•
Specific cases of MBBRs used for micropollutants polishing are also overviewed.
-
•
MBBR operational parameters have demonstrated significant impact on Alpha-diversity indices.
Abstract
Moving bed biofilm reactor (MBBR) is currently one of the most substantially studied biofilm-based reactor due to its numerous advantageous in terms of biomass robustness and excellent pollutants removal performances. In order to understand the performance determining factors of MBBR system, it is essential to investigate its fundamental element; the microbial community of the biomass. This review discusses the influence of MBBR operational parameters on the microbiological aspect of the technology at the highest taxonomic rank (i.e., phylum, class). Moreover, a special focus on the microbial community structure of different forms of biomass in MBBR (i.e., initial inoculum, suspended biomass, attached biofilm) is addressed in the review. Proteobacteria and its corresponding class, Alpha-proteobacteria, frequently dominate the microbial community at phylum and class level respectively, regardless of the operating conditions and biomass forms. The diversity of attached biofilm is often higher than in suspended biomass, which is a strong indicator of biofilm development enhancement, thanks to the operating conditions management. Based on these findings, the current challenges and future perspectives of microbiological analysis in MBBR system are presented.
Graphical Abstract
Abbreviations
biological oxygen demand
black polypropylene media
biological phosphorus removal
conventional activated sludge process
chemical oxygen demand
denitrifying phosphorus accumulation organisms
dual anaerobic-anoxic/oxic
extracellular polymeric substances
fluidized bed biofilm reactor
fixed bed hybrid biological reactor
fluorescent in situ hybridization
food to microorganisms
high-density polyethylene
heterotrophic nitrification - aerobic denitrification
hydraulic retention time
integrated fixed-film activated sludge
moving bed biofilm reactor
moving bed membrane bioreactor
moving bed sequencing batch reactor
oil sands process-affected water
operational taxonomic unit
phosphorus accumulation organisms
denaturing gradient gel electrophoresis of polymerase chain reaction
polyethylene terephthalate
porous polymer carrier
rotating biological contactor
sequencing batch reactor
simultaneous nitrification and denitrification
Trickling filter reactor
upflow anaerobic sludge blanket
wastewater treatment plant
Keywords
MBBR
Operational parameters
Microbial community
Biofilm
Suspended biomass
1. Introduction
The moving bed biofilm reactor (MBBR) has increased in popularity over the past 40 years and it is currently utilized in various wastewater treatment plants (WWTP) worldwide [1]. This technology continues to attract vast attention due to its numerous advantageous compared to other biofilm-based technologies (e.g., rotating biological contactor (RBC), trickling filter reactor (TFR), fluidized bed biofilm reactor (FBBR)) and conventional activated sludge (CAS)[2]. Among the frequently highlighted advantages of MBBR systems are the low reactor volume requirements, the high resistance of biofilm to variation in influent compositions (e.g. organic loads, temperature, pH, toxic substances) and the simplicity of operation (e.g. no recirculation of sludge, less clogging, no backwash when compared to fixed-film reactors) [2], [3], [4]. Essentially, the performance of MBBR system in terms of organic matter (i.e., chemical (COD) and biological oxygen demand (BOD)) and nutrients (e.g., nitrogen, phosphorus) removals are comparable or better than other existing technologies used in conventional WWTPs [5], [6]. Moreover, various recent studies in the literature reported that MBBR as tertiary treatment is highly efficient to reduce or eliminate micropollutants (MPs) in treated wastewater [7], [8], [9], [10].
The elimination of such compounds by MBBR is enhanced due to the development of slow growing and consequently more resistant bacteria under the form of biofilm on the suspended carriers in the reactor [8], [11]. Indeed, in MBBR systems, the greatest fraction of biomass is present in the biofilm [12], thus making it the utmost crucial component that assures the efficiency of this system [13]. For this reason, a significant increase of studies on the characterization of microbial community structure and diversity of attached biomass in MBBR system was observed recently [14], [15], [16]. Research focusing on the microbiological aspect of MBBR biofilm is deemed important as it could elucidate the relationship between the microbial community structure in the bioreactor and the wastewater treatment efficiency [17], [18]. Besides, it could also provide a better comprehension of the role of microbial community for MPs biotransformation [11].
Available reviews on the advancement of MBBR technology throughout the past two decades have proved that the operational parameters (e.g., hydraulic retention time (HRT), carbon to nitrogen (C/N) ratio, aeration conditions, nature of carriers) as well as the hybridization (i.e., coupling MBBR system with different physical- or chemical-based processes) of MBBRs have a noteworthy impact on the systems’ performances [2], [3], [19], [20]. In addition, a special focus on the importance of bacterial biofilm and extracellular polymeric substances (EPS) in MBBR technology was encapsulated in a recent review by [20]. Despite the steady augmentation in the number of articles on MBBR systems, to our knowledge so far, no review regarding the microbial population in MBBR at phylum and class level has been published.
Therefore, this review aims to tackle the microbiological aspect of MBBR systems with a chemical engineering approach by investigating the influence of MBBR operational parameters on its bacterial community structure. A particular emphasis is given to the bacterial composition at phylum and class level due to the extensive amount of information available on both taxonomic ranks. Besides, since they are also the least complex taxonomic ranks to be characterized, determining their structure and proliferation pattern could be used as a preliminary approach for metagenomics characterization. This review also pursues to identify operational conditions in which specific functions of the phyla and classes are stimulated. It would then allow researchers to enhance the activation of these functions through efficient management of the process operating parameters. Fundamentally, this review will be a reference to researchers working on MBBRs to understand via a comparative approach, the microbial community structure and diversity in their own systems.
This review firstly recapitulates the impact of MBBR operational parameters on its global performances (part 2) and succinctly describes some general information on the microbiological aspect of MBBR systems (part 3). Then, a comparative study of bacterial population at phylum and class level of different forms of biomass (i.e., initial inoculum, suspended biomass, attached biofilm) is covered (part 4). Furthermore, the influence of MBBR operating parameters on the microbial community structure of the fixed biofilm are thoroughly analysed (part 4). The latter is important, as it will enable us to interlink all the three fundamental elements in a biological process by evaluating the relationship between the microbiological analyses in MBBR systems and their global performances. Fig. 1 illustrates the interdependence between the three major elements that are addressed in this review. This review also includes microbial analysis of biofilms in hybrid MBBR systems such as Integrated Fixed-Film Activated Sludge (IFAS) and Moving Bed Membrane Bioreactor (MBMBR) systems. Finally, the current obstacles and future potential research approaches regarding the microbiological aspects of MBBR process are discussed (part 5).
 Fig. 1
Fig. 12. Overview of operational parameters influence on MBBR general performance
The design of a MBBR system is essential as this phase will predetermine the operational conditions in which the system could be operated. In general, MBBR systems are conceived based on wastewater characteristics and effluents discharge limit requirement [2]. Among the commonly studied operating parameters are HRT, C/N ratio, aeration conditions and nature of carriers (i.e., carrier filling ratio, form of carriers) [19]. Some operational parameters (e.g., C/N ratio, aeration conditions) have been proven to have significant influence on the performance of MBBR systems while others (e.g. nature of carriers) have less consequential impacts on the efficiency of the system. For example, the aeration conditions (i.e., aerobic, anoxic, anaerobic) are important parameters especially in MBBR systems designed to eliminate MPs as each MP undergoes different transformation pathways depending on the aeration conditions [21]. This paragraph assembles findings from diverse works on MBBR systems in the literature as a means to establish an optimal range of values for each operational parameter; and to provide fundamental elements that govern the proper functioning of MBBRs. The synthetic Table 2 gathers the different operational parameters influencing the performance of various MBBR systems treating wastewater. The parameters in bold correspond to the variables that were considered responsible for the change in performance of MBBR system in each respective study.
2.1. Effects of HRT on the global performance of MBBR
In this part of the review, focus is particularly placed on the hydraulic component of MBBR systems. As regards of HRT in MBBR processes, J. Wang et al. [22]studied the effect of HRT on the performance of simultaneous nitrification and denitrification (SND) of a lab-scale MBBR operating under sequencing batch reactor (SBR) mode. The reactor fed with synthetic wastewater was operated at four different HRTs (6, 8, 10, 12 h). The authors noted that the bioreactor operated at HRT of 10 h displayed the highest removals of ammonia-N and Total Nitrogen (TN) at 97.5 % and 83 % respectively. They also stated that the removals of ammonia-N and TN increased gradually with the HRTs (from 6 to 10 h) but at the highest fixed HRT of 12 h, an accumulation of nitrate ions was observed, thus resulting in the decline of TN removal. This is due to the inhibition of denitrification process caused by the excess assimilation of COD by nitrifying bacteria in the bioreactor at the highest HRT. Besides, Jiang et al. [23] reported that their hybrid MBBR-MBR which were operated at various HRTs ranging from 6 to 24 h, showed the highest performance efficiency for the removal of COD (97.4 ± 0.8 %), TN (72.3 ± 6.9 %) and most of the selected MPs including recalcitrant ones (e.g., diclofenac, 83.3 %) at a HRT of 18 h. The authors concluded that at the HRT of 18 h, the organic loading rate (OLR) (i.e., food to microorganisms (F/M) ratio) supplied to the system was adequate for the proliferation of microorganisms responsible for the depollution of wastewater. In a different work by Lusinier et al. [6] on the influence of HRT in two different biological reactors used in oilfield produced water treatment, they reported that the performance of the fixed bed hybrid biological reactor (FBHBR) remained impressive even at the lowest fixed HRT of 12 h when compared to the CAS bioreactor. Indeed, the FBHBR maintained a high removal efficiency of COD (between 77 % and 99 %) and other pollutants (i.e. phenol, volatile organic compounds and polycyclic aromatic hydrocarbons) despite the reduction of experimental HRT from 24 to 12 h. This indicates that the biomass in the FBHBR demonstrates high robustness because it was able to withstand the organic loading shock resulting from the change in HRT. Globally, the interlinkage between HRT and OLR signifies that each MBBR system functions optimally under different operating conditions depending on the process primary objectives and the characteristics of the influent. Nevertheless, in spite of the aforementioned interdependent variables, findings from the cited works emphasize that HRT in the range of 10–18 h has been highlighted to be optimal for MBBR processes.
2.2. Correlation between C/N ratio and pollutants degradation capacity in MBBR
Another essential parameter that determines the performance of a MBBR system is the C/N ratio. In a biological reactor, the C/N ratio is eminently important because it is the determining factor in terms of the reactor’s capacity to eliminate nitrogen compounds. Indeed, in a WWTP, the latter could be efficiently removed when sufficient amount of carbon compounds is available [24]. In the literature, it was reported that the optimal C/N ratio for conventional SND systems ranges from 7 to 20 [25]. Liang et al. [26] conducted a study to enhance the degradation of sulfamethoxazole in a synthetic aquaculture wastewater using a bio-augmented aerobic MBBR system. The C/N ratio ranged from 3 – 6 while the HRT of the reactor varied from 6 – 10 h. It was found that the highest nitrate-N (93.97 %), ammonia-N (96.07 %) and aerobic degradation of sulfamethoxazole (80.49 %) occurred in the reactor at C/N ratio and HRT of 6 and 10 h respectively. Furthermore, in a recent work by Pan et al. [27], a lab-scale MBBR was operated at a HRT of 40 h and fed with synthetic wastewater with various C/N ratios (2−20). It was reported that the MBBR performance was the best and the worst at C/N ratio of 5 and 2 respectively. Indeed, at the C/N ratio of 2, the TN removal was the lowest (46.11 %) and accumulation of nitrate-N in the effluent was observed due to the incomplete denitrification process from the lack of carbon source. This is corroborated by similar observations in a MBBR system where the TN removal decreased with the C/N ratio (from 7.5 to 3.7) [28]. Conversely, J. Wang et al. [22] reported that C/N ratio exceeding 12 could inhibit nitrification process, thus declining the ammonia-N removal in the process. It can be inferred that besides HRT, C/N ratio is also a system-dependant parameter. Nevertheless, a moderate C/N ratio in the range of 5–12 could be opted for the optimal performance of MBBR systems.
2.3. Choice of aeration strategy for the optimisation of MBBR performance
MBBR processes are mostly applied under aerobic condition since aeration system has a dual purpose; to provide dissolved oxygen (DO) to the system and to maintain effective mixing of bio-carriers. On the other hand, anaerobic/anoxic MBBRs utilize mechanical mixing and/or sludge recirculation instead of aeration system to assure homogenization of suspended carriers [2]. Several works combining anoxic/anaerobic and aerobic conditions in various reactors’ configurations (i.e., multi-stage MBBR, anoxic-oxic (A/O) MBBR, SBR mode) have showed outstanding performance in terms of macro-pollutants removals [29], [30], [31]. Indeed, Bassin et al. [29] reported an overall COD and TN removals of around 90 % and 80 % respectively, in a two-stage MBBR system operated under different aeration conditions. Moreover, similar noteworthy COD (91.57 %) and TN (80.20 %) removal efficiencies were also obtained in a hybrid dual-anaerobic-anoxic/oxic MBBR system despite the consequential organic loading shock applied to the system throughout the study [31]. Concerning the influence of aeration conditions on MPs removal, most studied organic MPs has shown to have higher degradation rates under aerobic conditions [10], [32]. However, some MPs such as sulfamethoxazole, trimethoprim, naproxen and estradiol were efficiently bio-transformed under anaerobic condition [10], [33]. In summary, despite the presence of anaerobic and anoxic zones naturally developed in the biofilm, alternating aerobic and anoxic/anaerobic phase in MBBR has proven to be necessary in order to enhance macro- and micro-pollutants removal.
2.4. Impacts of carrier filling ratio and geometrical properties
Another performance determining parameter that also has to be considered when designing a MBBR system is the carrier filling ratio, which is the ratio of the volume of carrier media over the total reactor volume [34]. In general, the filling fraction in a MBBR varies from 10 – 60 % but it could go up to a maximum of 70 % [2]. Moreover, this parameter depends highly on the physical properties (i.e. shape, size) of the carriers in the system. Indeed, Dias et al. [35], reported that faster biofilm formation rates associated with COD (>80 %) and ammonia-N (>50 %) removals were obtained with spherical-shaped carriers compared to cylindrical medias. Hence, they concluded that the design of MBBR systems should not focus solely on the protected surface area of the carriers but equal attention has to be paid on the carrier geometrical properties. This finding is supported by a different work on the usage of carriers with several configurations enabling to control the thickness of biofilm, particularly the series of AnoxK™ saddle-shaped polyethylene (PE) Z-carriers (e.g., Z-50, Z-200, Z-300, Z-400, Z-500), to have noteworthy influence on the overall performance of the MBBR systems [36]. The authors stated that the thickest biofilm (500 µm) demonstrated the highest specific biotransformation rate of most targeted MPs while the thinnest biofilm (50 µm) presented the highest nitrification rate in their lab-scale MBBR systems treating tertiary wastewater. In a more recent work by Arabgol et al. [37] involving AnoxK™ K5, Z-200 and Z-400 carriers, it was observed that the MBBR with cylindrical K5 carriers exhibited significantly higher soluble BOD (59.9 ± 3.0 %) and soluble COD (31.5 ± 4.0 %) removal efficiencies compared to the MBBRs filled with saddle-shaped Z-carriers. Therefore, it can be concluded that the geometrical properties of carriers (e.g., saddle-shaped, cylindrical, spherical) are as important as determining the optimal carrier filling ratio in MBBR systems. The former is necessary to control the biofilm properties (i.e., thickness, density) [37] while the latter is essential to ensure effective mixing properties of bio-carriers [2]. Basically, a carrier filling fraction between 30 % and 50 % in a MBBR process was found to be ideal not only in ensuring an efficient mixing intensity but also in achieving the best MBBR performance [38], [39], [40].
2.5. Conclusion: operating parameters influence on MBBR overall performance
To conclude, the optimal range of values/findings for each operational parameters of a MBBR are compared with those of a CAS system. Considering HRT, MBBR systems used as tertiary treatment, especially for the MPs’ removal, were observed to have excellent performances at higher HRT than those usually fixed in MBBR and CAS systems for secondary treatment. Moreover, the optimal C/N ratio range for MBBR systems (secondary treatment) was reported to be lower than the one generally applied in CAS system. In terms of aeration conditions, both MBBR and CAS processes function optimally and independently under different aeration conditions. Therefore, the latter have to be manage according to the fixed objectives. Finally, regarding the carrier, it was observed that the geometrical properties as well as the carrier-filling ratio play a significant role on the performance of MBBR systems. The essential findings in this second part are summarized in Table 1.
| Operational parameters | Optimal conditions |
|---|---|
| HRT | 10–18 h |
| C/N ratio | 5–12 |
| Aeration conditions | Both aerobic and anaerobic |
| Carriers (form, ratio) | Geometry and filling ratio between 30 % and 50 % for hydrodynamics and carriers homogenisation |
3. General information on the microbiological aspect of MBBR
The microbiological aspects of a biological process used in wastewater treatment system are extremely complex as they vary significantly from one system to another [41]. Indeed, microbial growth and development are influenced by both intrinsic and extrinsic factors [42], [43]. Despite their complexity, it is imperative to deepen our understanding on these intricate features in order to efficiently optimize the performance of bioreactors, particularly the emerging biofilm-based technologies for wastewater treatment. In this third part, some general information regarding the research trends on the microbiological features of MBBR process (3.1) as well as the metagenomics characterization methods (3.2) are provided. Besides, one of the primary elements of MBBR systems, which is the inoculation phase of the bioreactor (3.3), is succinctly described.
3.1. Current research trends on MBBR microbial community
The collection of data for this review was executed on the Web of Science platform. Research articles related to the microbial community in MBBR systems were obtained using the keywords, “Microbial Community” AND “Moving Bed Biofilm Reactor”. As of 26th of September 2023, a total of 303 articles containing both terms either in their title, keywords or abstract, appeared on the database of Web of Science. Then, the pre-selected articles were screened manually and individually, and only articles containing the following terms, “Phylum” AND/OR “Class”, were retained for the review. This resulted in the remainder of 105 articles that are purely related to microbial community at phylum and/or class level in MBBR systems. The number of articles before and after the screening process according to their year of publication are represented in Fig. 2. Over the last decade, the number of articles reporting on microbial community, specifically at phylum and/or class level in MBBR systems appeared to increase progressively, hence demonstrating the constant growth in interest on the topic. This augmentation could also be linked to the increase in accessibility and easiness in terms of analysis execution. It is also worth noting that the number of published papers has seemed to reach a plateau since 2020 but the number of articles discussing microbial community at phylum and/or class level in MBBR systems has doubled in 2022.
 Fig. 2
Fig. 23.2. DNA extraction and characterization methods
Recently, the metagenomics characterization of microbial community structure and diversity of biofilm in MBBR systems are almost systematically carried out to better understand their role in the elimination of pollutants. Previously, methods such as denaturing gradient gel electrophoresis of polymerase chain reaction (PCR-DGGE) and fluorescent in situ hybridization (FISH) were used for such analysis [44], [45], but they gradually became less popular due to other emerging technologies. For example, high-throughput DNA sequencing technologies, especially the amplicon sequencing of bacterial 16 S rRNA gene are currently being largely utilized [46] as it provides a great coverage with adequate sequencing depth and outcomes of high accuracy [47]. On top of that, DNA extraction from attached biomass are more feasible with the existence of diverse commercial DNA extraction kits. Each commercial kit provides its appropriate extraction protocol thus outcomes in terms of DNA yield and purity, and to a lesser extent the 16 S rRNA bacterial diversity, could differ depending on the method applied [46]. Nonetheless, this review only focuses on the resulting microbial community structure and not on the DNA extraction methodology. It is still worth highlighting that a vast majority of articles published over the past decade have performed in-depth investigation of microbial community analysis in MBBR systems using high-throughput DNA sequencing technologies.
3.3. Inoculum of MBBR: Origin and taxonomic structure
The start-up phase of a MBBR generally consists in inoculating the bioreactor with activated sludge (AS), typically collected from a WWTP. In general, the inoculum is obtained either from a secondary sedimentation tank [48], [49], [50]or an aeration tank [5], [17], [51].
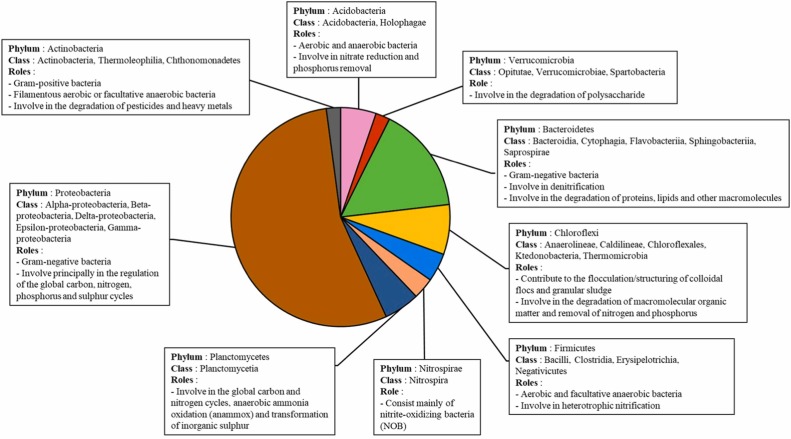
The conventional AS is commonly predominated by Proteobacteria [54], [55], [56], which is a Gram-negative bacterium that has a wide metabolic capacity and plays an important role in the assimilation of nitrogen- and carbon-based pollutants [14], [17]. Besides, the second most abundant phylum in AS is Bacteroidetes [55], [56], [57], another Gram-negative bacterium which has been proven to effectively degrade organic matter [58] and often reported to be closely related to denitrification [27], [50]. Other most frequently detected phyla (average abundance over 1 %) include Actinobacteria, Acidobacteria, Firmicutes, Chloroflexi, Planctomycetes and Verrucomicrobia [59]. In the recent years, isolated microbial strains containing specific bacteria capable of heterotrophic nitrification - aerobic denitrification (HN-AD) are also being used as inoculum in certain MBBR systems to enhance their performance in terms of nitrogen removal [52], [53].
The principal roles of each commonly detected phylum and their corresponding classes are presented in Fig. 3. The figure is constructed using information collected from nine different articles [17], [58], [60], [61], [62], [63], [64], [65], [66]. The relative abundance of each phylum in the pie chart represents actual values (i.e., calculated average) of abundance that are recorded on conventional AS in municipal WWTPs from several recent works [55], [56], [63], [67]. Moreover, the specific functionalities of phyla in AS (i.e., inoculum) presented in the pie chart can be individually enhanced via distinct management of MBBR systems (paragraph 4).
 Fig. 3
Fig. 34. Operational parameters influencing microbial community in MBBR
Operational parameters [2], [19] and diverse microorganisms [20] have been proven to have substantial influence on the performance of MBBR systems. In the two previous paragraphs, the influence of operational parameters on the global efficiency of MBBR systems as well as the general information on the microbiological aspects of MBBR processes were discussed independently. Therefore, in this part, essential operational parameters of MBBR systems namely chemical and physical nature of carriers, process-related parameters (i.e., HRT, C/N ratio, aeration conditions) and the type of influent including a special focus on the presence of MPs, will be discussed to elucidate their impact on the microbial community structure of attached biofilm. Indeed, it is deemed important to understand the interdependence between the operational parameters and the microbial community in MBBR systems in order to optimise their performance. Moreover, a comprehensive comparison between the microbial community of different forms of biomass (i.e., initial inoculum, suspended biomass, attached biofilm) is carried out independently to better assimilate the composition and importance of attached biomass in MBBR processes.
It is important to highlight that we are aware that the MBBR systems in all the reviewed articles could be extensively different from one another on various aspects. For instance, several studies reported that the maturation process of attached biofilm have indeed favoured relatively different microbial communities due to the differences observed between young and mature biofilms [1], [20], [70], [71]. Nevertheless, in this critical review, we have standardized the comparative methodology by taking into account only the microbial profiles of mature biofilms in steady-state bioreactors. Furthermore, in each of the following section dedicated to one specific operational parameter, we will solely focus on that highlighted parameter without deliberately taking into consideration other variables.
Fig. 4 depicts the general statistics on the different types of fixed-film systems as well as their scale of study, based on the total number of selected articles in this review. It can be seen that a majority (>80 %) of the studies were conducted in lab-scale MBBR systems used as either secondary or tertiary treatment. This is logical since the review is based on research articles that were mainly published by academia. In terms of types of fixed-film systems, hybrid MBMBR and IFAS systems represent 9 % and 3 % respectively. 11 % of the studies were based on pilot-scale system while the remaining 6 % were on full-scale system. Moreover, most of the studies (62.5 %) were conducted with synthetic wastewater and this could induce bias on the microbial population due to the simplicity of its composition. This ‘preliminary’ approach is indeed useful for mechanistic interpretations; however, more research on pilot- and full-scale MBBR systems operating with real wastewater would be expected in the near future.
 Fig. 4
Fig. 4| Type of biofilm reactor | Type of wastewater | Operational conditions | Specification of reactor | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| HRT (h) | Filling Ratio (%) | Type of carriers | C/N ratio | Scale | Aeration conditions | |||
| Hybrid MBBR-MBR | Synthetic wastewater | 6 – 24 | 20 | Porous polyurethane sponge cubes | N.A. | Lab-scale | Aerobic | [23] |
| FBHBR | Synthetic oilfield-produced water | 12 – 24 | 33 | AnoxKaldnes™ cylindrical rings | N.A. | Lab-scale | Aerobic | [6] |
| MBSBR | Synthetic wastewater | 6–12 | 30 | Polyurethane foam | 6–14 | Lab-scale |
Anoxic Aerobic |
[22] |
| MBBR | Synthetic wastewater | 7 | 30 | Biocarrier (Zhengzhou, China) | 3.7–7.5 | Lab-scale | Aerobic | [28] |
| MBBR | Synthetic aquaculture wastewater | 6–10 | 40 | YuLong biological filler | 3 – 6 | Lab-scale | Aerobic | [26] |
| MBSBR | Synthetic wastewater | 40 | 40 | Polypropylene YuLong biological filler | 2–20 | Lab-scale |
Anoxic Aerobic |
[27] |
| MBBR | Real chemical industry wastewater | 36 | 50 | AnoxKaldnes™ K1 carriers | N.A. | Lab-scale |
Anoxic Aerobic |
[29] |
| MBSBR | Real textile effluent wastewater | 36–60 | 50 |
AnoxKaldnes™ K3 carriers |
N.A. | Lab-scale |
Aerobic Anaerobic |
[30] |
| Hybrid dual anaerobic-anoxic/oxic MBBR | Real decentralized wastewater | 8–10 | 35 | Porous polymer sponge (PPS) carriers | N.A. | Pilot-scale |
Anaerobic Anoxic Aerobic |
[31] |
| MBBR | Synthetic wastewater | 12 | 20 | Cubic-shaped polyurethane sponge carriers | N.A. | Lab-scale | Aerobic | [32] |
| MBBR | Real secondary treated wastewater | 2 | 25 |
AnoxKaldnes™ Z-400 carriers |
N.A. | Pilot-scale |
Aerobic Anaerobic |
[10] |
| UASB reactor - IFAS system | Synthetic medium-low strength wastewater | 9 | 20–23 |
Synthetic porous foams Porous semi-flexible carriers |
N.A. | Lab-scale |
Anoxic Aerobic |
[33] |
| MBBR | Real settled wastewater | N.A. | 60 | Recycled polypropylene spherical and cylindrical carriers | N.A. | Pilot-scale | Aerobic | [35] |
| MBBR | Synthetic medium strength domestic wastewater | 12 | 10–30 | Cubic-shaped polyurethane sponge carriers | 6.5 ± 0.5 | Lab-scale | Aerobic | [38] |
| MBBR | Real WWTP effluent | 12 | 20–50 | Cylindrical PE carriers | N.A. | Lab-scale | Anaerobic | [39] |
| MBBR | Real palm oil mill effluent (POME) | 24–72 | 25–100 |
Polypropylene black plastic media PE hexafilter |
N.A. | Lab-scale | Aerobic | [40] |
4.1. Comparison between MBBR biomass - Suspended and Attached, and Initial Inoculum
Biomass in inoculated and acclimatized MBBR systems exist in the form of suspended sludge and/or biofilms developing on suspended carriers that are constantly in motion in the mixed bioreactor [11], [19]. The dissimilarity in terms of environmental conditions exerted on both types of biomass could have a significant impact on their microbial community structure and diversity [72]. For example, in biofilm-based systems utilized for wastewater treatment, biofilm communities are described to be more biodiverse than those of suspended biomass [69]. Besides, in order to evaluate the influence of MBBR process on the natural selection of bacterial species at phylum and class level, it is essential to compare the bacterial population of the initial inoculum used in the start-up phase with the acclimatized biomass. It is important to note that even though we identified 14 different works, out of the 105 reviewed articles, addressing the microbiological properties of the initial inoculum in MBBR systems, only five out of these 14 articles are cited in this review because they provide extensive and insightful findings on the initial inoculum’s microbial structure and diversity. A synthetic table on microbiological analysis at phylum and class level as well as the alpha-diversity indexes of different forms of biomass in MBBR processes is presented in Table 3.
| Type of biofilm reactor | Form of biomass | Microbial Analysis | Reference | |||
|---|---|---|---|---|---|---|
|
Inoculum (IN) Attached biofilm (AB) Suspended biomass (SB) |
Dominant Phylum | Subdominant Phylum | Dominant Class | Diversity and Richness indexes | ||
| MBBR |
AB SB |
Proteobacteria |
Bacteroidetes Firmicutes |
γ-proteobacteria | Highest richness and diversity in SB | [73] |
| MBBR |
AB SB |
Proteobacteria |
Nitrospirae Bacteroidetes |
α-proteobacteria γ-proteobacteria |
Highest richness and diversity in AB | [11] |
| MBBR-NF-MBR |
AB SB |
Proteobacteria | Chloroflexi |
γ-proteobacteria Chloroflexia |
Highest richness and diversity in AB | [74] |
| SB-HMBR |
AB SB IN |
Bacteroidetes | Proteobacteria | [5] | ||
| MBBR |
AB SB IN |
Proteobacteria |
Bacteroidetes - Chloroflexi |
α-proteobacteria γ-proteobacteria β-proteobacteria |
Highest richness and diversity in AB | [75] |
| MBBR |
AB IN |
Proteobacteria | Bacteroidetes | [50] | ||
| MBMBR |
AB SB |
Proteobacteria | Bacteroidetes |
β-proteobacteria Sphingobacteria |
[78] | |
| Plug-flow A/O MBBR |
AB IN |
Proteobacteria |
Bacteroidetes; Nitrospirae Bacteroidetes |
[76] | ||
| IFAS |
AB SB |
Proteobacteria |
Acidobacteria Acidobacteria; Firmicutes |
α-proteobacteria γ-proteobacteria |
Highest diversity in AB | [80] |
| MBBR |
AB SB IN |
Bacteroidetes Proteobacteria Proteobacteria |
Proteobacteria Bacteroidetes Planctomycetes |
Highest diversity in IN Highest richness in AB Lowest diversity in SB |
[81] | |
| IFAS |
AB SB |
Ignavibacteriae Bacteroidetes |
Planctomycetes Ignavibacteriae |
Highest richness in SB | [77] | |
| Anaerobic MBBR |
AB SB |
Proteobacteria |
Firmicutes Bacteroidetes |
[79] | ||
A majority of studies on MBBR systems comparing the microbial structure of suspended biomass with those of attached biofilm at phylum level has reported the dominance of Proteobacteria on both forms of biomass [11], [73], [74]. Moreover, the similar phylum was recorded with the highest relative abundance in the initial inoculum of MBBR systems [50], [75], [76]. These observations are consistent because Proteobacteria is often identified as the most dominant phylum in AS of WWTPs [56], [68]. Nevertheless, S. Yang et al. [77] reported the dominance of an unusual phylum, Ignavibacteriae in the attached biomass while Bacteroidetes was dominant in the suspended biomass of the IFAS system used in their study. Besides, other works also emphasize differences in terms of sub-dominant phyla, dominant classes as well as alpha-diversity [74], [78], [79]. For example, Wolff et al. [11] stated that six phyla and 12 classes were significantly enriched in the attached biofilm compared to the suspended sludge. The authors noticed that the relative abundance of Bacteroidetes was significantly higher in the suspended biomass whereas Nitrospirae was more abundant in the fixed biofilm. The latter finding is corroborated by other works indicating that the suspended carriers provided favourable conditions for the growth of Nitrospirae [78], [80], [81]. On the other hand, some studies recorded the dominance of Bacteroidetes on attached biomass [81] as well as on all forms of biomass including the initial inoculum [5], while others reported it as the second most abundant phylum on the carriers [50], [75], [78]. Divers sub-dominant phyla such as Chloroflexi [74], Acidobacteria [80], Planctomycetes [77], Firmicutes [79] and Nitrospirae [11], [76] were also observed in the attached biofilm. Furthermore, L. Li et al. [73] observed that Actinobacteria was exclusively present in the biofilm while Planctomycetes was particularly detected in the suspended sludge. Other noteworthy differences recorded at the phylum level include the enrichment of Verrucomicrobia [50] and the significant decrease in abundance of Saccharibacteria [76] in the biofilms, compared to the bacterial population of inoculum.
As regards the dominant classes, Ribera-Pi et al. [75] reported that Beta-proteobacteria was the most dominant in the inoculum but Alpha-proteobacteria and Gamma-proteobacteria dominated the biofilm and suspended biomass respectively. Correspondingly, similar observations were highlighted concerning the dominant classes on both forms of biomass in an IFAS system [80]. Moreover, in a different study by L. Li et al. [73], the authors reported the dominance of Gamma-proteobacteria on both suspended and attached biomass while Cao et al. [74] observed the dominance of Gamma-proteobacteria and Chloroflexia in the biofilm and the suspended sludge respectively. It was also noticed that some classes (i.e., Alpha-proteobacteria, Delta-proteobacteria) were only detected or enriched in the biofilm whereas other classes (i.e., Planctomycetacia, Beta-proteobacteria) were solely observed in the suspended biomass [11], [73].
Besides, alpha-diversity analysis conducted in diverse works demonstrated that the richness and diversity of bacteria were significantly higher for the fixed biofilm over the suspended biomass [11], [74], [75]. Moreover, the observed OTUs were higher in the biofilm than in the suspended sludge samples thus indicating that the attached biofilm has enhanced the complex of microbial community [15], [74]. It was also reported that several notable differences in terms of microbial community compositions were observed between the initial inoculum and the fixed biomass [76], [81]. Indeed, T. Liu et al. [81] affirmed that the major phyla in the MBBR system were quite distinct from the seed sludge with the highest biodiversity (i.e., highest Shannon, lowest Simpson indexes) and the highest richness (i.e., highest Chao1 index) being recorded in the initial inoculum and the attached biofilm respectively.
Overall, we conclude that in most of the reviewed articles, Proteobacteria is cited as the most dominant phylum on both forms of biomass in MBBR systems. This is expected as Proteobacteria is repeatedly reported as the most abundant phylum in AS of WWTPs and the latter is generally used to inoculate MBBR during its start-up phase (mentioned in paragraph 3.3). Indeed, all the concerned studies in this review have mentioned that their MBBR systems were inoculated with AS taken from a WWTP. Nevertheless, it is worth highlighting that the MBBR process itself has the capacity to favour the proliferation of different phyla that are uncommon in AS, to the extent of changing not only the sub-dominant phyla but in certain cases, the dominant phylum. For example, in several studies, Nitrospirae was abundantly detected in attached biofilm while it was not present on suspended biomass or the inoculum. This is due to the fact that Nitrospirae is a slow growing bacteria that needs a longer time to proliferate [82] and this essential condition is fulfilled by the biofilm attachment process which lengthens the contact time between the biomass and its support. Furthermore, at class level, all the cited works have reported different dominant and sub-dominant classes according to the form of biomass. Indeed, we can deduce that the attachment of biomass has the capacity to promote the growth of different classes of bacteria. It would have been more interesting to compare the dominant classes of the inoculum to those on MBBR biomass but up to this day, related works have only investigated the bacterial communities at lower taxonomic level (e.g., order, genus). As regards the alpha-diversity aspects, the majority of the studies have mentioned that the microbial composition in fixed biofilm has indeed higher diversity and richness compared to the inoculum and the suspended biomass, which is coherent due to the longer residence time of the attached biomass in the bioreactor. Hence, this proves that the attachment of biomass on carriers has a prominent effect on the natural selection and enrichment of certain groups of bacteria. This also indicates that the implementation of MBBR process for wastewater depollution is of high interest, as it allows diverse functional bacteria carrying out different activities to coexist.
4.2. The influence of MBBR key operating parameters (C/N ratio, HRT, Aeration conditions) on its microbial community structure
As seen in part 2, process-related parameters such as C/N ratio, HRT and aeration conditions are important variables due to their notable influence on the performances of MBBR systems. Indeed, studies have reported that C/N ratio, HRT and aeration conditions have a great influence on the microbial community structure and diversity. Therefore, it is imperative to elucidate the significance of such parameters on the bacterial community of biofilms primarily at phylum and class level. A synthetic table on the process-related parameters influencing the microbiological analysis in various MBBR systems is given in Table 5. The parameters in bold correspond to the variables that were considered responsible for the change in microbial diversity and structure of attached biofilm in each respective study.
4.2.1. Impact of C/N ratio on the microbial community structure in MBBR
Regarding the C/N ratio, several studies on lab-scale MBBR systems treating synthetic wastewater with various C/N ratios reported the dominance of Proteobacteria at phylum level [17], [26], [27], [76]. According to Jia et al. [28], Proteobacteria dominates most biological N-removal systems regardless of the C/N ratio. The authors also observed a gradual increase in abundance of Proteobacteria and Acidobacteria when C/N decreased while Bacteroidetes abundance remained unchanged at different C/N ratios. On the other hand, Pan et al. [27] reported that Proteobacteria slightly decreased while Bacteroidetes and Planctomycetes abundances were unmodified when the C/N ratio dropped from 15 to 5. However, they stated that Nitrospirae and Chloroflexi were more abundant at the C/N ratio of 5, which is the lowest C/N ratio fixed in the study. Correspondingly, Ding et al. [76] stated a similar trend where Nitrospirae was the most abundant (29.33 %) at a C/N ratio of 5.1. Hence, the C/N ratio of 5 is the optimal value that could promote the growth of nitrifying bacteria in aerobic MBBR systems. These findings are coherent because as reported in part 2, the best performance of MBBR systems in terms of TN, ammonia-N and nitrate-N removals were reported at C/N ratios of 5 and 6 [26], [27]. At the class level, Yuan et al. [17] and Pan et al. [27] identified Beta-proteobacteria as the dominant class at diverse C/N ratios. However, Jia et al. [28] recorded the dominance of Alpha-proteobacteria at highest C/N of 7.5 while Gamma-proteobacteria became the most abundant class at the lowest C/N ratio of 3.7.
In a recent work by Xiang et al. [53], they observed the decrease in Shannon, Chao1 and ACE values as the C/N ratio decreased (from 16 to 4) thus indicating that the diminution of C/N ratio affects negatively the diversity and richness of the bacterial community in the biofilm. This could be due to growth inhibition of certain bacteria, mainly heterotrophs, caused by the reduction of carbon concentration in the influent. Indeed, at C/N ratio lower than 5, accumulation of nitrate ions and suppression of ammonium removal are frequently observed due to the limited growth of heterotrophic organisms (e.g., denitrifiers and nitrifiers) [27], [28]. Nevertheless, the abundance of Proteobacteria and Bacteroidetes (>50 %) remained unchanged regardless of the C/N ratio. It was also showed that the increase in ammonia concentrations in synthetic domestic wastewater has only a slight influence on the relative abundance of phyla and almost no significant impact on the predominant phyla [17]. The authors stated that in the case of Proteobacteria, its relative abundance is less affected by the nutrient content but more easily affected by the DO concentration in the influent. On the contrary, J. Wang et al. [22] described Chloroflexi as the most dominant phylum in a lab-scale MBBR fed with synthetic wastewater and operated under different C/N ratios, pH and DO concentrations. The authors stated that Proteobacteria was the second most dominant phylum in all set of experiments except those under different HRTs in which it became the most dominant phylum replacing Chloroflexi.
4.2.2. Interdependence between HRT and the microbial community structure in MBBR
In terms of HRT, Xiong et al. [83] operated a MBBR system treating real mature landfill leachate with extremely long HRT of 10 and 20 days. They noticed that Proteobacteria (52 %) followed by Bacteroidetes (32 %) were the two most dominant phyla whereas Alpha-proteobacteria and Sphingobacteriia were the predominant classes belonging to each phylum respectively. Other works with high HRT (i.e., 32–36 h) corroborate this finding as both aforementioned phyla were identified with the highest relative abundances [29], [84]. Nevertheless, the dominance of a less common phylum, Ignavibacteriae, was observed in the biofilm of an IFAS system under SBR mode treating real pre-treated ammonia rich lagoon supernatant at a HRT of 60 h [77]. The phylum Ignavibacteriae is an organotrophic and facultative anaerobic bacterium that utilizes diverse sources of carbon substrates and electron acceptors for growth [85]. Indeed, a noteworthy proportion of Ignavibacteriae was particularly recorded in anoxic/anaerobic MBBR systems [86], [87]. In a different study by X. Liu and Li [88], a lab-scale anoxic/oxic MBBR system was operated at two HRTs (8, 20 h) and fed with synthetic wastewater to study its impact on the microbial community. The authors reported that the dominant phyla remained unchanged but their relative abundances were modified under different HRTs. It was observed that when the HRT was reduced from 20 to 8 h, the relative abundances of Proteobacteria increased significantly (36.9–74.8 %) in the anoxic reactor but it decreased (from 66.3 % to 44.8 %) in the aerobic tank. Similarly, studies conducted under short HRTs (i.e., 1–2 h) also indicated the dominance of Proteobacteria (>50 %) regardless of the type of influent or the MBBR system configurations [1], [89]. Concerning the alpha-diversity, X. Liu and Li [88] and Mazioti and Vyrides [15] deduced that a higher diversity of microbial population observed during lower HRTs could be due to higher availability of nutrients in the system. Therefore, the alpha-diversity aspects of a bacterial community in MBBR systems are more likely influenced by the C/N ratio (see previous part 4.2.1.).
4.2.3. Influence of aeration conditions on the microbial community structure of MBBR
Another deterministic factor that could affect the microbial community of biomass in MBBR systems is the aeration conditions. For example, Gilbert et al. [90] indicated the highest abundance of Planctomycetes during the entire operation of a lab-scale anoxic MBBR fed with synthetic wastewater containing only ammonium (i.e., without organic carbon). In a different work by Ma et al. [91], the influence of DO concentrations on the biofilm’s microbial composition of two different systems, MBBR and IFAS, were investigated. They observed that the changes of DO levels modified to a certain extent the dominance of phyla in both reactors. In the MBBR, Proteobacteria was the most abundant phylum at intermediate (2.13–3.02 mg/L) and highest (4.31–5.16 mg/L) DO concentrations but Actinobacteria became the most dominant phylum at the lowest DO concentrations (0.71–1.32 mg/L). On the contrary, in the IFAS biofilm, Bacteroidetes was dominant when DO level was the lowest (0.71–1.32 mg/L) and Actinobacteria was dominant at intermediate and highest DO concentrations.
In contrast, in other rare research works involving strict anoxic/anaerobic MBBR systems, Proteobacteria usually dominated the bacterial community followed by Bacteroidetes and Firmicutes as the subdominant phyla [77], [92], [93], [94]. Moreover, in a hybrid dual anaerobic-anoxic/oxic MBBR (D-A² MBBR) system treating real decentralized wastewater from the sewer network of a university, Proteobacteria (43.27–48.61 %) was the dominant phylum followed by Bacteroidetes in all the compartments during the stable operation [31]. The authors recorded the increase in abundance of Bacteroidetes and Chloroflexi in the anaerobic and anoxic reactor. Indeed, the enrichment of both phyla were typically observed in anaerobic processes as they were reported to contain phosphorus accumulation organisms (PAOs) and denitrifying PAOs (DPAOs) [31], [95]. It was also stated that Bacteroidetes was closely related to TN removal specifically for the denitrification in the anoxic reactor [93]. Alpha-proteobacteria, Beta-proteobacteria and Flavobacteriia were the commonly identified dominant classes under anoxic/anaerobic conditions [29], [93], [96]. In addition, according to X. Wang et al. [96], significant relative differences (>60 %) of ACE and Chao1 values for samples from each MBBR chambers were recorded thus distinguishing the microbial community richness of aerobic biofilms from those developed under anoxic condition. Similar findings by J. Li et al. [31] described that the alpha-diversity indexes varied significantly according to the aeration conditions applied in the reactors; the highest and lowest species richness (i.e., ACE and Chao1 indexes) were observed in the aerobic and anoxic MBBR chambers respectively.
In summary, most of the cited works on MBBR systems working under different C/N ratios, HRTs as well as aeration conditions have highlighted the dominance of Proteobacteria on the attached biomass, except in certain studies that were executed under specific operational conditions. This is mainly observed in works involving MBBRs operated under anoxic/anaerobic conditions. For example, phyla such as Ignavibacteriae and Planctomycetes became dominant on the attached biofilm of certain MBBR systems because several essential conditions such as the DO concentration and the type of available nutrients were respected for their optimal growth. Indeed, Iganvibacteriae is known to be a facultative anaerobic bacterium [85] while Planctomycetes is commonly classified as either chemoheterotrophic or chemoautotrophic (annamox) [60]. From the latter finding, we can infer that the dominance of Planctomycetes could be achieved in a system where only ammonium is available as the primary nutrients (i.e., absence of organic carbon). Besides, we would like to emphasize that in several studies, the C/N ratio of 5 was reported to have a positive effect on the proliferation of nitrifying bacteria such as Nitrospirae. Therefore, it is highly recommended to maintain a C/N ratio of 5 in order to optimise the performance of nitrifying MBBRs. Moreover, the dominance of Alpha-proteobacteria and Beta-proteobacteria, two classes from the phylum of Proteobacteria, in the majority of the studies is not surprising because Proteobacteria are well known to be widely involved in the degradation of carbon- and nitrogen-based compounds. Due to the latter, the impacts of C/N ratios and HRTs are marginally visible on the microbiological aspects, at least at phylum and class level. Concerning the microbial diversity and richness aspects, the highlighted findings are summarized in Table 4.
| Operational parameters | Alpha-diversity aspects | Justification |
|---|---|---|
| C/N ratio | higher richness and diversity when higher C/N ratio | Better proliferation of certain bacteria, mainly heterotrophs due to increase in concentration of carbon (nutrients) in the influent |
| HRT | higher richness and diversity when lower HRT | |
| Aeration conditions | Inconclusive | Different types of bacteria (i.e., aerobic, anoxic or anaerobic) need different aeration conditions to proliferate |
| Type of biofilm reactor | Highlighted parameter | Microbial Analysis | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| C/N ratio | HRT (h) | Aeration conditions | Dominant Phylum | Subdominant Phylum | Dominant Class | Diversity and Richness indexes | ||
| MBBR | 3.7–7.5 | 7 | Aerobic | Proteobacteria |
Bacteroidetes Firmicutes |
α-proteobacteria γ-proteobacteria |
Highest diversity at C/N of 3.7 Highest richness at C/N of 5.6 |
[28] |
| MBSBR | 2–20 | 40 |
Anoxic Aerobic |
Proteobacteria | Bacteroidetes | β-proteobacteria | [27] | |
| A/O MBBR | 2.7–5.1 | 8 |
Anoxic Aerobic |
Proteobacteria |
Bacteroidetes Nitrospirae |
[76] | ||
| MBBR | 1.5–15 | 12 | Aerobic | Proteobacteria |
Bacteroidetes Verrucomicrobia |
β-proteobacteria | [17] | |
| MBBR | 4–16 | 8 | Aerobic | Proteobacteria | Bacteroidetes | Highest diversity and richness at highest C/N of 16 | [53] | |
| MBSBR | 6–14 | 6–12 |
Anoxic Aerobic |
Chloroflexi | Proteobacteria |
Chloroflexia β-proteobacteria |
[22] | |
| MBBR | N.A. | 10–20 days |
Anoxic Aerobic |
Proteobacteria | Bacteroidetes |
α-proteobacteria Sphingobacteria |
[83] | |
| IFAS | N.A. | 60 | Anoxic | Ignavibacteriae | Planctomycetes | [77] | ||
| A/O MBBR | 6 | 8–20 |
Anoxic Aerobic |
Proteobacteria | Bacteroidetes | Highest richness at lowest HRT of 8 h | [88] | |
| MBBR | N.A. | 24–48 | Aerobic | Proteobacteria |
Bacteroidetes Firmicutes |
α-proteobacteria | Highest diversity at lowest HRT of 24 h | [15] |
| Two-stage MBBR | N.A. | 36 |
Anoxic Aerobic |
Proteobacteria | Bacteroidetes | α-proteobacteria | Highest diversity and richness in aerobic MBBR | [29] |
| MBBR | N.A. | 24–120 | Anoxic | Planctomycetes | Proteobacteria | [90] | ||
|
MBBR (M) IFAS (I) |
3.3 | 11.5 |
Aerobic 0.71–1.32 mgO2/L 2.13–3.02 mgO2/L 4.31–5.16 mgO2/L |
Actinobacteria -M Bacteroidetes -I Proteobacteria -M Actinobacteria -I Proteobacteria -M Actinobacteria -I |
Proteobacteria -M Proteobacteria -I Actinobacteria -M Proteobacteria -I Bacteroidetes -M Proteobacteria -I |
Highest diversity in MBBR and IFAS at intermediate DO | [91] | |
| AnoxMBBR - AeMBR | N.A. | 24–48 |
Anoxic Aerobic |
Proteobacteria |
Bacteroidetes Firmicutes |
α-proteobacteria | [93] | |
| D-A2MBBR | 4–12.5 | 8–10 |
Anaerobic Anoxic Aerobic |
Protoebacteria |
Bacteroidetes Bacteroidetes Actinobacteria |
Highest diversity in anaerobic MBBR Highest richness in aerobic MBBR |
[31] | |
| Multi-stage MBBR | 14 | 6 |
Anoxic Aerobic |
Proteobacteria | Bacteroidetes |
Flavobacteriia β-proteobacteria |
Highest richness in anoxic MBBR | [96] |
4.3. MBBR carriers-related parameters (Filling ratio, Material, Physical properties) play non-negligible roles on the microbial community structure
Carriers are the core elements in MBBR technology as they act as a support that allows the development of biofilm. In general, the latter occurs via three main stages, namely biofilm attachment, growth and maturation, and biofilm detachment [97] and it is greatly influenced by biotic and abiotic factors [98]. For the design of a MBBR system, the carrier filling ratio as well as the chemical and physical nature of carriers (i.e., materiel, form) are important parameters that have to be taken into consideration because they provide good hydrodynamic conditions for better control of biofilm development and microbial activity. Hence, it is beneficial to analyse the significance of these parameters on the biofilms bacterial community principally at phylum and class level. A synthetic table on the carriers-related parameters influencing the microbiological analysis in various biofilm systems is given in Table 6. The parameters in boldcorrespond to the variables that were considered responsible for the change in microbial diversity and structure of attached biofilm in each respective study.
| Type of biofilm reactor | Highlighted parameter | Microbiological Analysis | Reference | ||||
|---|---|---|---|---|---|---|---|
| Carrier filling ratio (%) | Type of carriers | Dominant Phylum | Subdominant Phylum | Dominant Class | Diversity and Richness indexes | ||
| MBBR | 20; 30; 40 | PU foam cubic carriers | Proteobacteria | Bacteroidetes | [99] | ||
| MBMBR | 20; 35 | AnoxKaldnes™ K1 HDPE carriers |
Proteobacteria Actinobacteria |
Bacteroidetes |
α-proteobacteria β-proteobacteria Acidimicrobiia Actinobacteria |
Highest diversity and richness at CFR of 35 % | [100] |
| RBC | 50–90 | K3 PE carriers | Proteobacteria |
Bacteroidetes Firmicutes |
Highest diversity at CFR of 50 % | [101] | |
| MBBR | 20; 40 | Mutag BioChip PE carriers | Proteobacteria |
Bacteroidetes Firmicutes |
α-proteobacteria | [15] | |
| MBBR | 40 |
AnoxKaldnesTMK1 HDPE carriers Polyvinyl alcohol gel |
Proteobacteria |
Bacteroidetes Actinobacteria |
α-proteobacteria | Highest richness in MBBR with K1 carriers | [58] |
| MBBR | 40 |
PU soft sponge carrier PP hollow ball carrier PPC |
Proteobacteria Bacteroidetes Bacteroidetes |
Bacteroidetes Proteobacteria Proteobacteria |
γ-proteobacteria α-proteobacteria γ-proteobacteria |
Highest richness in MBBR with PU soft sponge carrier | [14] |
| MBSBR | 30 |
K3 polystyrene carrier LEVAPOR porous cubic foam carriers |
Proteobacteria |
Nitrospirae Bacteroidetes |
Highest diversity in MBBR with porous cubic foam carriers | [47] | |
| MBBR | N.A. |
Activated carbon Lignite activated coke |
Proteobacteria |
Planctomycetes Acidobacteria |
Highest diversity in MBBR with lignite activated coke | [102] | |
| MBBR | 30 |
Suspended biochar HDPE carrier |
Proteobacteria | Bacteroidetes | Highest richness in MBBR using suspended biochar as carriers | [49] | |
| Hybrid MBBR-constructed wetland (CW) | 20 |
PPC Cylindrical PE carrier |
Proteobacteria |
Bacteroidetes Planctomycetes |
[51] | ||
| MBBR | 20 |
AnoxKaldnesTMK5 carrier Sponge biocarrier |
Proteobacteria Nitrospirae |
Nitrospirae Proteobacteria |
Nitrospira | Highest richness and diversity on sponge carriers | [4] |
| MBBR | 50 |
PP black plastic media PE hexafilter |
Chloroflexi Proteobacteria |
Firmicutes Bacteroidetes |
Higher diversity on the PE hexafilter carriers | [40] | |
| MBBR | 25 |
HDPE carrier Chemically-modified HDPE carrier |
Proteobacteria Bacteroidetes |
Bacteroidetes Proteobacteria |
Highest richness on the chemically-modified HDPE carriers | [81] | |
| MBBR | N.A. | Plastic suspended carrier treated with different enzymes and surfactants | Actinobacteria | Proteobacteria | [71] | ||
4.3.1. Effects of carrier filling ratio on the microbial community structure in MBBR
In terms of carrier filling ratio, Feng et al. [99] evaluated the microbial community structure on polyurethane foam cubic carrier at three filling ratios (20 %, 30 %, 40 %). They reported that at phylum level, Proteobacteria was the most dominant phylum followed by Bacteroidetes and Verrucomicrobia regardless of the filling ratio. Nevertheless, the relative abundance of each phylum varies in a different manner with the filling ratio. For example, Planctomycetes decreased progressively in abundance when the carrier filling ratio increased while the abundance of Proteobacteria firstly increased when the filling ratio increased to 30 % but later decreased when it was fixed at 40 %. Hence, the authors concluded that the packing ratio only influences the abundance of phyla but not their dominance. This observation is corroborated by other studies in which Proteobacteria remained dominant despite the different filling ratios applied, but the latter had noteworthy effects on the bacterial community diversity and richness instead [100], [101]. Indeed, J.-H. Wang et al. [101] indicated that the bacterial community had the highest richness at the lowest filling ratio of 50 % while the highest diversity was attained at the highest filling ratio of 90 %. This finding is logical because at a low filling ratio, bacteria could be better enriched on the carriers because of the limited availability of surface (i.e., lesser carriers for colonization). On the other hand, at a high filling ratio, an improved diversification of bacterial community could be achieved because the attached biofilms are less exposed to shear force produced by the aeration system (i.e., longer contact time between carriers and microorganisms). The latter is possible due to the decrease in efficiency of mixing intensity resulting categorically from the increase in carriers’ quantity.
Besides, Mazioti and Vyrides [15] reported the predominance of Proteobacteria at phylum level and Alpha-proteobacteria at class level in two pilot double-staged MBBR systems, MBBR-A and MBBR-B, filled with porous PE bio-carrier at a ratio of (A) 20 % and (B) 40 % respectively. The authors noticed that instead of the carrier filling ratio, other factors could have influenced the bacterial population since several different sub-dominant phyla (e.g., Firmicutes, Spirochaete) and classes (e.g., Clostridia, Spirochaetes) were detected only in MBBRs placed on the first-stage of the systems. In a different work on a pilot-scale MBMBR operated at two carrier filling ratios (20 %, 35 %), Reboleiro-Rivas et al. [100] described that the community richness and diversity significantly increased at higher filling ratio of 35 %. The authors stated that the two predominant phyla were Proteobacteria (20.9–53.8 %) and Actinobacteria (20.6–57.6 %) in all the experiments. Moreover, the major classes detected were Alpha-proteobacteria (8.1–25.6 %) and Beta-proteobacteria (2.7–26.8 %) from the Proteobacteria phylum, and Acidimicrobiia (1.5–35.35 %) and Actinobacteria (4.5–33.04 %) belonging to the Actinobacteria phylum.
In conclusion, we deduce that the carrier filling ratio has insignificant impact on the dominance of the microbial population at phylum level since Proteobacteria was repeatedly reported to be the most abundant phylum in various reviewed studies. However, several cited articles have stated that the carrier filling ratio has an impact on the relative abundance percentage of the dominant and sub-dominant phyla, but not to the extent of modifying their dominance. Besides, in terms of diversity and richness indexes, we corroborated our deduction made in paragraph 2.4 stating that a carrier filling ratio between 30 % and 50 % has shown to be the most optimal filling ratio in achieving better hydrodynamic conditions and the best MBBR performance. Indeed, the highest microbial richness and diversity of attached biomass were reported in studies with MBBR systems operated at this range of filling ratio. Furthermore, we strongly think that the shear force exerted on the attached biomass due to the hydrodynamic turbulence has an impact on its microbial diversity and richness. It is indeed observed that a lower biofilm thickness induces a higher community diversity and richness and vice versa. This is explained by the higher diversification of the bacterial community when the biofilm is exposed to a higher shear force, thus promoting thinner biofilm (< 400 µm).
4.3.2. Interlinkage between the physicochemical properties of carriers and the microbial community structure in MBBR
As regards the material and form of bio-carriers, Chen et al. [58] observed a higher bacterial richness on plastic K1 carriers than on polyvinyl alcohol gel carriers while L. Zhu et al. [14] noticed the highest microbial richness on polyurethane (PU) sponge carriers compared to classic polypropylene (PP) hollow ball carriers and porous polymer carrier (PPC). Moreover, Zheng et al. [102] reported a more diverse and complex bacterial community in MBBR using lignite activated coke as carrier. Biofilms attached to suspended biochar also demonstrated higher microbial richness and diversity compared to carriers made out of high-density polyethylene (HDPE) [49]. Nevertheless, in terms of bacterial composition at phylum level, the typically observed dominant phylum was Proteobacteria regardless of the carriers materials or geometrical properties, and only their relatives abundances were different from one carrier model to another [47], [49], [51], [58], [102]. Indeed, in a recent work by Jang et al. [103]they also reported the dominance of Proteobacteria and its two major classes, Gamma- and Beta-proteobacteria on both types of carriers (i.e., PE and polyethylene terephthalate (PET)) used in their MBBRs despite the different carriers’ materials. However, the authors reported that a higher abundance of Proteobacteria was recorded on the PE carriers compared to the PET carriers. Hence, they concluded that the high ammonia removal efficiency (>99 %) observed in the MBBR with PE carriers was due to the aforementioned observation.
However, it is worth highlighting that the physical and chemical properties of the carriers exhibited a significant influence on the sub-dominant phyla of the microbial communities. Indeed, several studies comparing MBBR systems filled with different types of carriers recorded the sub-dominance of Actinobacteria [58], Nitrospirae [47], Planctomycetes [51], [102] and Acidobacteria [102]instead of the commonly detected Bacteroidetes. On the other hand, Chen et al. [58] identified Alpha-proteobacteria as the most abundant class on both types of carriers in their MBBR system whereas Zhu et al. [14] observed the abundance of Gamma-proteobacteria on two out of the three types of carriers utilized in their study.
On the contrary, in a work by Abu Bakar et al. [40], Chloroflexi was reported to be the dominant phylum on black polypropylene media (BPM) while Proteobacteria was the dominant phylum on PE hexafilter (HEX) carriers. The authors concluded that different types of polymer used to make the carriers and to a lesser extent their form, could have an influence on the microbial compositions because the carriers were the only highlighted variable in their study. This result is supported by a different study in which Nitrospirae was identified as the dominant phylum on sponge bio-carriers whereas Proteobacteria had the highest relative abundance on AnoxKaldnes™ K5 carriers [4]. Moreover, the authors stated that Nitrospira was the dominant class in both MBBR followed by Beta-proteobacteria and Alpha-proteobacteria. Correspondingly, T. Liu et al. [81] described that Bacteroidetes was the most abundant phylum on the chemically modified HDPE carriers of a lab-scale MBBR system. Chemical substances such as polyquaternium-10 and Fe2O3, which enhance the hydrophilicity and electrophilicity of the carriers have seemed to favour the proliferation of Bacteroidetes over Proteobacteria. Although there are no findings to corroborate this observation, we hypothesize that those substances could amplify the affinity between the carriers and Bacteroidetes. Furthermore, in a study conducted by Huang et al. [71] to elucidate the detachment and activity recovery of aging biofilm, the authors observed the dominance of Actinobacteria on plastic bio-carriers that were treated with enzyme and surfactants. The filamentous structure of Actinobacteria and its strong EPS secretion provided them a firm adsorption onto carriers’ surface thus resulting in a higher resistance to detachment during treatments or biofilm sloughing process [70], [71], [104]. Concerning exclusively the form of carriers, biofilms on four different configurations of cylindrical polyethylene carriers were found to be mainly dominated by Proteobacteria (52.2–64.97 %) and Bacteroidetes (7.12–10.82 %) in anoxic lab-scale MBBR systems [87]. Hence, it can be deduced that the form of carriers alone has little to no impact on the dominance of bacterial phyla, but their materials as well as the combination of both elements (i.e., material, form) were described to favour the dominance of certain phyla and classes in MBBR processes.
As regards the physicochemical properties of the carriers, we can distinguish two principal types of carriers’ materials, conventional polymers (i.e., PE, PU, PP, HDPE) and non-conventional materials such as polyvinyl alcohol gel, lignite activated coke, activated carbon and suspended biochar. Upon critical analysis of the different results presented in the cited articles, we can conclude that the majority of the carriers’ materials has inconsequential effects on the dominance of microbial populations at phylum and class level. Indeed, most conventional and non-conventional materials displayed the dominance of Proteobacteria and its corresponding classes, which is logical due to its widely known function in the depollution of wastewater. However, it is worth highlighting that abundance of this dominant Proteobacteria was observed to vary according to the types of the carriers. Based on in-depth analysis, we notice that these variations have a notable impact on the performance of the MBBR systems in terms of pollutants removal. Indeed, we can deduce that MBBR processes yield higher efficiency when a higher abundance of Proteobacteria is detected on its biomass. Furthermore, from certain cited works in this review, we also observe that some carriers can promote the growth of different phyla other than Proteobacteria. For example, it was seen that the sponge-type carriers has a high capacity to retain microorganisms due to its porous structure hence promoting the growth of slow nitrifying bacteria such as Nitrospirae. Therefore, these kind of carriers are highly recommended for MBBR systems designed for ammonium removals. Moreover, these sponge type carriers are often reported to have the highest microbial richness and diversity in the reviewed articles. Globally, the choice of carriers with varying physicochemical properties in MBBR processes has indeed a notable influence on the abundance and in some cases even on the dominance of microbial communities despite the lack of clarity on the proliferation pattern.
4.4. The interdependence between MBBR influent characteristics (Type of influent, Presence of MPs) and its microbial community structure
The type of influents fed in the MBBR systems could be categorized as either synthetic or real wastewater. Collected data from the literature showed that more studies were conducted with synthetic wastewater (62.5 %) compared to real wastewater (37.5 %). This is due to the fact that the composition of artificial influents is easier to control as interferences originating from meteorological and/or seasonal factors are entirely excluded. Moreover, the process of collecting real wastewater in significant volume for experimental purposes is a complex task especially concerning the sanitary aspects. These two main categories could then be differentiated according to the source of wastewater such as domestic, industrial, hospital and agricultural wastewater. In addition, certain studies related to MBBR systems were carried out with less conventional influents including landfill leachate, stormwater, seawater and reverse osmosis (RO) concentrate. Recently, wastewater spiked with organic MPs, particularly pharmaceutical products has also been the primary attention of diverse works.
4.4.1. Correlation between the type of influent and the microbial community structure in MBBR
A study conducted by An et al. [18] on lab-scale MBBR systems treating real stormwater mixed with different proportions of river water according to the defined strategy showed that Proteobacteria (>70 %) was the dominant phylum and its relative abundance greatly increased at the end of experimental cycles. They stated that the abundance of subdominant phyla such as Bacteroidetes and Acidobacteria decreased after long-term operation due to the frequent change in environments. Furthermore, Proteobacteria (35.1–46.4 %) was also identified as dominant phylum in MBBR processes fed with two different real effluents, raw and ozone-treated oil sands process-affected water (OSPW) collected from a water pumping station [105]. The authors’ major observations were the similar relative abundance of Nitrospirae in both MBBRs (24.6 %, 23.7 %) and the high abundance of Acidobacteria (21.9 %) in the MBBR treating raw OSPW. However, Zhuang et al. [106] reported the dominance of Firmicutes (15.2 %) followed by Bacteroidetes (7.92 %) and Proteobacteria (2.5 %) on attached biomass community of a MBBR co-treating real pre-treated produced water (PW) from a typical shale gas well and synthetic domestic wastewater. On the other hand, a recent work by Chen et al. [107] demonstrated similar dominant microbes among all the samples despite certain variations in terms of relative abundances. They noticed that Proteobacteria was the dominant phylum in the MBBR treating synthetic swine wastewater but Actinobacteria had the highest abundance during the treatment of real swine wastewater. Correspondingly, the dominant classes in synthetic and real swine wastewater system were Gamma-proteobacteria and Actinobacteria respectively.
Moreover, in MBBR processes treating seawater or wastewater with high salinities, commonly identified predominant phyla were Proteobacteria followed by Bacteroidetes and Nitrospirae [108], [109], [110]. On the contrary, the study by Ganesan et al. [111] reported that Firmicutes became the most abundant phylum in a MBBR when the salinity of the synthetic wastewater attained 21.35 g/L NaCl but Proteobacteria regained its dominance at the salinity of 31.53 g/L NaCl. This shows that Proteobacteria is the prevalent species in high salinity water. Besides, several works were carried out in MBBR processes treating synthetic wastewater containing diverse sources of carbon to investigate their influence on the performance of the systems especially on the biological phosphorus removal (BPR) and their microbial community structure. Indeed, the latter could be affected because the change in carbon sources might alter the metabolism pathways of certain microorganisms, thus influencing the taxonomic structure of the bacterial community. It was observed that MBBRs fed with acetate, ethanol or starch were typically dominated by Proteobacteria at varying relative abundance (39–88.92 %) [95], [104]. A different study by M. Zhang et al. [112] corroborated this observation as they recorded the dominance of Proteobacteria (77.6–81.79 %) in MBBR fed with propionate as the carbon source. As regard the phyla playing important role in BPR, Bacteroidetes and Chloroflexi were reported to dominate enhanced BPR systems and include DPAOs [95], [104]. In addition, at the class level, the microbial communities of MBBRs were dominated by Gamma-proteobacteria (20–42 %) and Alpha-proteobacteria (15–32 %) regardless of the carbon sources [95]. According to Peng et al. [113], Proteobacteria was the dominant phylum while Bacteroidetes was the second most abundant phylum in lab-scale MBBR systems treating two different synthetic wastewater (i.e., low C/N ratio, antibiotic).
4.4.2. Influence of MPs on the microbial community structure in MBBR
In a recent work by Atasoy et al. [16], a real effluent of a secondary clarifier was spiked with a mixture of 16 MPs at a concentration of 2 µg/L, and then used as feed for three different bioreactors including a MBBR. The authors reported the abundance of Proteobacteria (24 ± 11 %) followed by Cyanobacteria (23 ± 17 %) and Actinobacteria (12 ± 10 %) on the attached biomass of the MBBR. This finding is supported by various studies in which the dominance of Proteobacteria was notably recorded in MBBR systems fed with wastewater containing MPs [11], [32], [52], [114]. Besides, Song et al. [32] noticed that the presence of pharmaceuticals stimulated the proliferation of Actinobacteria as the second dominant phylum thus indicating that they could withstand the biological toxicity of such products. Correspondingly, Liang et al. [52] reported the enrichment of Actinobacteria and Bacteroidetes with the addition of diverse antibiotics in the MBBR system. In contrast, X. Zhang et al. [114] described the decrease in abundance of Actinobacteria, the third most abundant phylum in their system, when the concentration of MPs increased but opposite trend was observed with Bacteroidetes, particularly in the MBBR spiked with carbamazepine. This indicates that the phylum Bacteroidetes could be particularly resistant to this pharmaceutical molecule while the growth of other phyla were notably inhibited in the presence of carbamazepine. Other reported tendencies at phylum level include the proportional increase in relative abundance of Firmicutes with MPs concentration [32], [115] and the decline of Acidobacteria abundance at higher concentration of MPs [52], [114]. Concerning the microbial community at class level, Gamma-proteobacteria was identified as the most dominant class in MBBR systems treating MPs [52], [74], [115]. Nevertheless, the dominance of certain classes such as Alpha-proteobacteria [11], [116] and Actinobacteria [52] were also observed in other MBBR systems developed to eliminate MPs. In terms of the alpha-diversity of microbial populations, X. Zhang et al. [114] stated that the OTUs increased when MPs concentration increased from 1 mg/L to 2 mg/L but they reduced when the concentration of MPs reached 5 mg/L due to the microorganisms tolerance limit towards MPs. Furthermore, it is noticed that the Shannon index was the lowest (i.e., lowest biodiversity) in MBBRs containing pharmaceuticals at the highest concentration while the highest Chao1 index (i.e., highest microbial richness) was recorded in MBBRs with the intermediate concentration of MPs [32].
To summarize, Proteobacteria is the most commonly detected dominant phylum on the attached biofilm of MBBR processes regardless of the nature of influent and the type of external carbon source added into the influent. This is highly likely due to its excellent adaptability to constant changes in environment and its high resistance to influent with high salinity or oligotrophic characteristic. Nevertheless, it is worth highlighting that the composition of a real swine wastewater has favoured the proliferation of Actinobacteria over Proteobacteria while a real pre-treated PW from a shale gas well has promoted the abundant growth of Firmicutes instead of Proteobacteria. As regards the real swine wastewater, we believe that the presence of pharmaceutical products such as antibiotics, which are generally used in livestock industry, could have enhanced the dominance of Actinobacteria. Furthermore, this hypothesis is corroborated in other reviewed articles because Actinobacteria was abundantly detected on fixed biofilms and its abundance has been positively altered in MBBR systems treating wastewater with MPs. We also observe a similar trend for Bacteroidetes in such systems thus indicating that some MPs could improve its growth while inhibiting other phyla. However, more studies need to be executed to confirm the effect of MPs on these aforementioned phyla. It would also be interesting to evaluate the impact of one specific MP, instead of a cocktail of MPs, on the microbial communities in order to distinguish and validate the effect (i.e., inhibition or enhancement) of that particular MP on the bacterial growth. At class level, we can deduce that Gamma-proteobacteria and Actinobacteria dominated the majority of the cited works. Once again, we could correlate this findings to the fact that both classes are either resistant to changes in composition of influents or favour the presence of atypical pollutants such as pharmaceutical compounds for its proliferation. Nonetheless, at this state of the research, we are still unable to firmly conclude on the participation of these bacteria in the degradation of such MPs. As regards the alpha-diversity aspects, it can be inferred that a very high concentration of MPs (> 5 mg/L) negatively affects the microbial diversity and richness. Therefore, it is important to monitor the concentration of MPs in a bioreactor because it could disrupt with the development of functional microorganisms.
4.5. Conclusion: operational parameters influence on microbial community in MBBR
Overall, based on the majority of the results reported in the literature over the past few years, Proteobacteria is the most dominant phylum on both attached and suspended biomass of pure and hybrid MBBR processes regardless of the applied operational parameters. Moreover, Alpha-proteobacteria and Gamma-proteobacteria are the two most abundant classes observed in MBBR systems biomass. These findings are coherent since Proteobacteria are well identified to be largely involved in the degradation of carbon- and nitrogen-based compounds in different biological treatment processes including MBBR. However, we have provided useful insights that could promote the growth of different phyla such as Nitrospirae, Actinobacteria, Bacteroidetes, Ignavibacteriae as well as Planctomycetes on attached biofilms in MBBR systems. Indeed, repetitive outcomes and in-depth critical analysis done on most of the reviewed articles have helped to enlighten these observations thus turning them into new discoveries. Besides, we would also like to emphasize that MBBR systems have proven to greatly favour the proliferation of other phyla and classes especially on the attached biofilm. Indeed, in comparison with CAS systems, the taxonomic structure of attached biofilm from one MBBR system to another is completely different in terms of abundances (i.e., proportion of dominant and sub-dominant phyla and classes) and diversity. This variety of microbial population would probably explain the improved degradation capacities exhibited by MBBR, especially for recalcitrant pollutants.
5. The present and future of microbiological analysis in MBBR system
The perpetual research efforts to deepen the current knowledge on the microbiological aspects of MBBR systems are crucial to better comprehend the influence of the process itself on the bacterial communities, and to elucidate the correlation between the microbial taxonomy and the efficiency of the technology. In recent studies, the characterisation of microbial communities in MBBR systems have demonstrated that the implementation of MBBR technology plays an important role in enhancing the selection and enrichment of certain functional groups of bacteria particularly under extreme or abnormal conditions [117], [118], [119]. Based on the microbial profiles highlighted in various cited works, the alteration of bacterial communities resulting from natural selections and other process-related parameters is significantly distinguishable at phylum level despite the irregular and yet obscure trend. Most of the studies (80 %) identified Protoebacteria as the dominant phylum while only a small proportion recorded the dominance of uncommon phyla such as Firmicutes [106], [111], [120], [121], Actinobacteria [91], [71], [104], [107], Chloroflexi [22], [40] Nitrospirae [4], [13]and Bacteroidetes [5], [81], [91], [120]. Besides, some works on MBBRs have reported not only the presence but also the dominance of an uncommon phylum, Ignavibacteriae [77], [86], [87], which shows that the MBBR process itself, principally the fixation of biomass on the carriers has seemed to favour metabolic pathways that enable the microorganisms to develop specific functions. Despite insufficient clear trends on the influence of deterministic factors on the bacterial communities in MBBR system, metagenomics characterizations of microbial compositions at phylum level have shown remarkable distinctions between the different forms of biomass in MBBR and the initial inoculum (i.e., conventional AS).
Moreover, as the analysis goes further into the class level, a more prominent variation in terms of bacterial classes’ dominance was observed. Indeed, we have seen in some reviewed articles that the change in C/N ratio, aeration conditions as well as the characteristics of the influent could either increase or decrease the abundance of certain classes to the extent of modifying their dominance. For instance, in one third of the reviewed articles, Alpha-proteobacteria is identified as the most dominant class on both forms of biomass, while in some MBBRs working under specific conditions, the dominance of other classes such as Gamma-proteobacteria, Nitrospira and Actinobacteria are also distinguished, with specific phylum’s functions and activities as presented in Fig. 3. Insightful findings emphasizing the operational parameters that are positively linked to the dominance of respective classes are summarized in Fig. 5. Based on these outcomes, the characterizations of microbial compositions at class level have indeed proven to be more sensible to changes in operational parameters compared to those at phylum level.
 Fig. 5
Fig. 5Following this trend, it is logical to further analyse lower taxonomic ranks particularly at genus and species level to obtain better insight on the impact of operating parameters on the bacterial community structure in MBBR processes. This approach could substantially help to establish correlations between the role of specific bacterial genus or species and the performance of MBBR process in terms of macro- and micro-pollutants eliminations [11], [32], [122]. Indeed, recent studies have started to include this characterization approach to better identify the responsible genera or species in specific pollutants degradation mechanisms [50], [74], [103], [123]. Nevertheless, at the current research state, specific bacterial taxonomic structure at lower level (i.e., genus, species) could not be deduced in MBBR systems due to the complexity of the microbial biocenosis. Indeed, this intricacy is well reflected in some recent works on MBBRs that addressed microbial community at genus level in attached biofilm as the authors reported high abundances of either unknown or unclassified genus in their system [18], [124], [125]. On top of the information scarcity, numerous variables susceptible of influencing the microbial community have to be taken into consideration when implementing this approach. Nevertheless, based on the general research trend on this topic, which seems to be positive due to the progressive increase in number of studies (Fig. 2), it will definitely be possible in the near future to establish a reliable correlation between the impact of MBBR operating parameters on its microbial structure at genus and species level. The latter presents major benefits as researchers working on MBBR processes would finally be capable of regulating the growth and development of functional microbial population through the control of MBBR operating conditions.
6. Conclusion
Microbiological analysis in MBBR systems is essential as it provides valuable insights on the bacterial community structure and biomass diversity. Both elements are indeed categorically correlated to the performance of MBBR processes. The metagenomics characterization at phylum level has shown distinguish profiles according to the biomass state despite the absence of a clear trend regarding the deterministic factors. Nonetheless, the operational parameters of MBBR systems, in their optimal ranges, have showed to have a compelling influence on the bacterial class composition and the Alpha-diversity indices. Indeed, Alpha-proteobacteria is identified as the most dominant class in almost one third of the reviewed articles but the emergence of other dominant classes in MBBRs operating under specific conditions indicates that the microbial characterization at class level is more operational parameters-dependent. Hence, it would be pertinent to analyse the microbial community of MBBR biomass at a lower taxonomic rank in order to obtain a better overview of the influence of operational parameters on the functional dominant genus and species. This will be a breakthrough in terms of MBBR process management because the control of its operating conditions will make the regulation of microbial development possible.
Funding
This study is part of the ongoing industrial PhD work (CIFRE 2020/1501, Nereus S.A.S) by Mukhlis Eshamuddin in the framework of the SAVE project (https://filiere-save.com/), funded by Agence de l’eau Adour-Garonne, Agence de l’eau Rhône-Méditerranée-Corse, the Occitanie Région and ADEME.
CRediT authorship contribution statement
Gaetano Zuccaro: Formal analysis, Writing – review & editing. Mukhlis Eshamuddin: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Conceptualization. Claire Albasi: Validation, Supervision, Writing – original draft, Writing – review & editing, Conceptualization, Investigation. Guillaume Nourrit: Project administration, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
© 2024 The Author(s). Published by Elsevier Ltd.