1. Introduction
Bone loss has, in general, significant effects on patient's quality of life. As a consequence, bone-related medical treatments and costs are increasing. Moreover, because of a prolonged life expectancy and an aging world population, there is a rapid increase in musculoskeletal pathologies such as fractures, low back pain, scoliosis, osteoporosis, bone infection or tumors, congenital defects and oral and maxillofacial pathologies, as well as rheumatic diseases like osteoarthritis [1].
Recently, more attention has been given to osteochondral defects, mostly concerning a young, athletic population. Such lesions, if untreated within subchondral bone, do not heal properly and osteoarthritis may develop over time [2].
Bone dynamic structure displays remarkable regenerative properties [3], [4]. However, complicated larger defects can delay or impair healing, thus needing additional treatment before they can regenerate. In particular, a main issue yet to be solved is represented by the decrease in vascular supply [5].
The development of bone lesion treatment techniques starts from the understanding of bone biology [6], [7]. Bone is a mineralized connective organ exerting important functions in the body: locomotion, soft tissue support and protection, calcium and phosphate storage and bone marrow harboring. Mechanical properties vary according to location and function [4]. Due to its complex and hierarchical structure, such a tissue can be defined as a nanocomposite consisting of inorganic nanocrystalline hydroxyapatite (HA), organic components (mainly collagens) and water. Proteins assemble together to form a nanostructured extracellular matrix (ECM) which influences adhesion, proliferation, and differentiation of several cell types: osteoblasts, bone lining cells, osteocytes, and osteoclasts [8], [9], [10], [11].
This review gives a state of the art description of the scaffold-based strategies used in Bone Tissue Engineering (BTE). Critical issues and obstacles are highlighted. Applications and advances are described, figuring out possible scenarios and future directions. Our finality is to consider not only the engineering point of view, but all aspects of the BTE complex scenario: medical, economic-industrial, legal-ethical and patient perspective.
2. Strategies for bone regeneration
Different techniques have been proposed over the years to cure bone lesions, but they still represent a challenge in the orthopedic field [1].
Bone is the most commonly transplanted tissue after blood [12]; however, despite having been used for over a decade in the clinical setting, bone grafts display some disadvantages that limit their applications in therapy. The drawbacks in autograft technique (either vascularized or not), which represents the current gold standard, are: supply limitation, variable resorption, risk of donor site morbidity, high rate of failure in specific sites and the need of a second surgery [1]. The problems associated with allografts (from human cadavers or living donors), such as Demineralized Bone Matrix and xenografts (animal source) can be pathogen transmission and rejection by the recipient's body [1], [13]. Metal implant strategy (titanium alloys and stainless steel) such as joint prostheses, plates and screws, currently utilized to provide mechanical and structural support for joint arthroplasties and long bone and vertebral fractures [1], presents limitations due to non-degradability, high rigidity, fatigue, fracture, lack of integration into the host tissue, extrusion and infection.
In the last decades, tissue engineering and regenerative medicine have emerged as promising strategies for bone reconstitution, with the ambition to circumvent the complications associated with traditional techniques [14], [15], [16], [17]. Recently, their role has become more strategic, due to an ever-increasing demand for organ transplantation and, at the same time, to a severe shortage of donor availability [1], [12]. Indeed, a potential solution to fix these problems is the development of engineered structures through the combination of scaffolds, cells and/or soluble/mechanical factors.
In BTE, a biomaterial can be defined as a temporary matrix that provides a specific environment and architecture for bone growth and development. A scaffold can be described as an artificial structure used to support three Dimensional (3D) tissue formation [16], [18]. Scaffolds can be used as acellular systems or as vehicles for cells and/or drugs. Once implanted into the injured site, acellular materials should allow proper host cell colonization for regeneration purposes. Alternatively, scaffolds can be combined with different types of cells able to promote bone formation in vivo either by differentiating towards the osteogenic lineage or releasing specific soluble molecules. These cells may be expanded ex vivo before the implant. The most commonly utilized expanded cells are adult stem cells (bone marrow-, adipose tissue-, tooth-, peripheral blood-derived Stem Cells), Embryonic Stem Cells, induced Pluripotent Stem Cells and genetically modified cells. Conversely, non-expanded cells are, for instance, Bone Marrow aspirate concentrate supplemented with Platelet-Rich Plasma [14], [17], [19]. Another possibility is the use of scaffolds primed with soluble molecules such as antibiotics, chemotherapeutic agents or growth factors (i.e. Bone Morphogenetic Proteins - BMPs - and Vascular Endothelial Growth Factor) that can be delivered in the environment exerting their therapeutical/regenerative effects.
2.1. Scaffold features for bone regeneration
Despite the increasing number of researches, discoveries and innovations, there is often a gap between research and clinical application/commercialization which is commonly termed the “Valley of Death” due to the large number of ventures that “die” between the scientific technology development and its actual commercialization [17].
One of the key factors to bridge this gap is the possibility to modulate scaffold characteristics so that specific biological, clinical, manufacturing, economic and regulatory prerequisites can be met. An ideal scaffold suitable for BTE applications should allow or improve cell viability, attachment, proliferation and homing, osteogenic differentiation, vascularization, host integration and, where necessary, load bearing [19]. Moreover, it should enable easy handling without extensive preparatory procedures in the operation theatre and allow minimally invasive implantation. It should be sterilizable by industrial techniques and reproducible on a large scale with cost effective processes. Finally, all its properties should meet the applicable Agency or competent authority requirements.
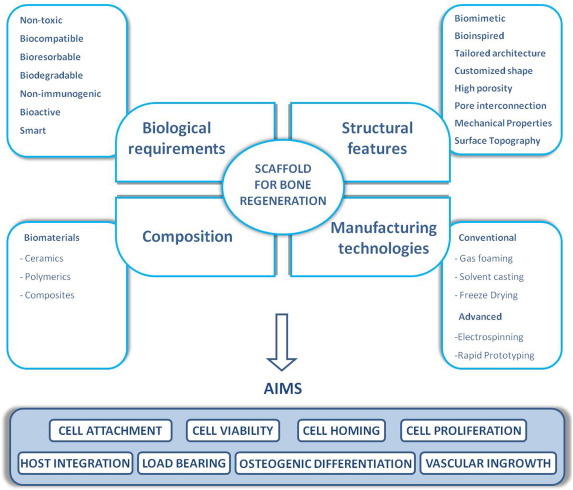
Scaffold characteristics that can be modulated, improved or changed to make a scaffold suitable for BTE applications can be grouped into four main types: biological requirements, structural features, biomaterial composition, and types of fabrication process (Fig. 1).

Fig. 1. Properties that an ideal scaffold should display for Bone Tissue Engineering applications.
In the upper part of the scheme the main characteristics concerning scaffold design for bone regeneration are highlighted. Clockwise from the left they are: biological requirements, structural features, manufacturing technologies and biomaterial composition. Biological requirements: an ideal scaffold should be non toxic, biodegradable and able to integrate and interact with the surrounding environment, at the same time minimizing the risk of any adverse reaction. Structural features: scaffolds should reflect the anatomical and physiological hierarchical 3D network structure of native bone ECM. Manufacturing technologies: the fabrication technologies now available to generate 3D scaffolds are summarized (conventional and advanced ones). Composition: the majority of scaffolds currently in use are naturally derived or synthetic polymers or a combination of them (composite materials).
In the lower part of the scheme the main purposes of bone regeneration applications by means of proper scaffolds are indicated: to allow cell engraftment and restore physiological structure and functions.
2.1.1. Biological requirements
First of all, a scaffold must be biocompatible and non toxic. In particular, cells must adhere, function normally, proliferate, differentiate and produce new matrix [16], [17], [20]. Bioresorbable and biodegradable features are also important in order to allow tissue formation to occur in tandem with degradation. In addition, even degradation products should be non-toxic and able to be excreted by the body without or with negligible interference with other organs. Variation of mechanical strength during degradation is critical in load bearing implants, since the load should be gradually transferred to the regenerating tissue [21], [22].
Likewise, it is important for a scaffold to avoid the host immune response. Recently, the concept of immune-inert biomaterials has been implemented with that of immuno-modulatory ones able to regulate the immune system (i.e.decreased NK cell activity and T and B cells mediated immunity) [19].
Another key feature is scaffold bioactivity i.e. its ability to interact with the surrounding living tissues or organs. Conventional passive biomaterials display low or ineffective capacity to cross-talk with the environment. Bioactive scaffolds are designed to promote proper cell migration or differentiation, tissue neoformation and integration in the host, avoiding undesirable processes such as scarring [23], [24].
More recently, attention has been directed towards “osteoinductive or smart biomaterials” that seem to hold great potential for bone tissue regeneration. In fact, they display the ability to instruct and respond to the surrounding in vivoenvironment to ectopically form bone. The biological mechanisms of this phenomenon have not been fully elucidated yet and two main hypotheses have been proposed. The first is based on the ability of the biomaterial surface to absorb and present osteoinductive factors to the surrounding cells. The second concerns calcium and phosphate ions release from calcium phosphate–based materials, which can induce stem cell differentiation towards bone phenotype [25], [26], [27], [28].
2.1.2. Structural features
Recent advances in BTE have lead to the development of novel biomaterials that allow to better mimic the 3D bone structure, in terms of mechanical properties as well as osteoinductive, osteoconductive and osteogenic features [29]. In this perspective, the development of vascularized engineered scaffolds is one of the greatest challenges, since the lack of, or an inadequate vasculature, easily results in an ineffective osseointegration [30], [31]. Recently, nanomaterialshave emerged as promising biomimetic candidates, since bone itself is a typical example of a nanocomposite. The commonly accepted definition of nanomaterials refers to materials with features between 1 and 100 nm, such as nanopatterns, nanofibers, nanotubes, nanopores, nanospheres and nanocomposites. They display excellent torsion and tensile modulus. In particular, nanoceramics appear to be promising to solve the brittleness of bioactive ceramics structure since bubbles and defects decrease with the size of crystalline grain into nanometer [32], [33], [34], [35].
Recently, also the design of biomaterials has become significant. This is inspired by natural processes of developmental biology and is aimed at promoting tissue remodeling, rather than simply supporting final tissue form and function (biologically-inspired biomaterials) [19]. For instance, the use of self-assembling materials is proposed to assist functional bone regeneration in cases of larger defects such as exposed fractures due to trauma, spinal disorders dealing with high loadings and replacement of big bone structures due to tumors [36]. In particular, self-assembling peptides have been shown to support osteogenesis both in vitro and in vivo [37], [38], [39], [40].
Another important aspect to consider is the non-homogeneous structure of bone. In this perspective, multilayered, stratified or gradient scaffolds can better mimic its original structure [41]. Such biomaterials can be useful especially when it is necessary to regenerate complex orthopedic interfaces, like the osteochondral compartment [42], [43], [44], [45], [46], [47].
Scaffold architecture is of critical importance for bone regeneration. By combining computer assisted design (CAD) with computer assisted manufacturing (CAM), custom-made, personalized and anatomically-shaped scaffolds can be manufactured especially for the repair of complex defects that are often encountered in craniomaxillofacial surgery [19]. Micro-architecture plays a role too. Scaffolds should have distributed, interconnected pores and display a high porosity in order to ensure cell penetration, vascular ingrowth and nutrient diffusion, as well as waste products elimination [19], [48]. Another key component to allow proper cell colonization (cell bound to ligands within the scaffold) is mean pore size. For any scaffold, a critical range exists, depending on the cell type and the tissue being engineered. Optimal pore size, ranging from 200 to 500 μm, is considered ideal for bone regeneration and vascularization [49].
Scaffold mechanical properties, such as elastic modulus, tensile strength, fracture toughness, fatigue, and elongation percentage, are considered crucial in BTE and should be modulated or tailored in order to match with those found at the site of implantation, minimizing, at the same time, the risk of stress shielding, implant-related osteopenia, and subsequent re-fracture [14], [15], [16], [17], [20]. A further challenge is posed by healing rates that vary with age: in young individuals, fractures normally heal to the point of weight-bearing in about six weeks, while in the elderly the repair process is slower [29].
Another relevant feature is surface topography which can be influenced by modulation/treatment/incorporation of artificial ECM and/or bioactive molecules (growth factors, anti-inflammatory drugs etc.) to be delivered in the environment after implantation. Such an approach seems not only to enhance bone tissue regeneration, but also to modulate the immune system. The biomaterial surface is the first, most critical factor for host acute immune response upon implantation and should be designed to limit macrophage adhesion and activation, as well as their fusion into foreign body giant cells. Biomaterial surface treatments may also be employed to shield it from protein absorption (i.e., coating with microparticle hydrogels) [19].
2.1.3. Composition
The majority of scaffolds that are currently used for BTE applications are polymers, bioactive ceramics (glasses) and hybrids (composites). Depending on composition and intended use, they may be injectable or rigid [50], [51].
Polymers can be natural or synthetic. Naturally derived polymers, such as fibrin, hyaluronic acid, chitosan and collagen exhibit good biocompatibility, osteoconductivity and low immunogenicity [52], [53]. However, disadvantages are represented by a degradation rate difficult to control and low mechanical stability. Synthetic polymers, like polyanhydride, polypropylene fumarate (PPF), polycaprolactone (PCL), polyphosphazene, polylactic acid (PLA), polyether ether ketone (PEEK) and poly(glycolic acid) (PGA) display a controlled degradation rate, the possibility to design or tune bone mechanical properties and to fabricate complex shapes, cell attachment improvement (negatively-charged chemical groups) and the potential to deliver soluble molecules. Moreover, this type of polymers can be produced at reduced costs, in large uniform quantities and have a long shelf life. A critical drawback is its lower ability to interact with cells in comparison with natural polymers that, because of their intrinsic nature, show better bioactive properties [52]. An important class of polymers utilized in BTE is represented by hydrogels, hydrophilic polymer networks that may absorb water from 10 to 20% up to thousands folds their dry weight. This property allows cells to adhere, proliferate, and differentiate. Hydrogels, both naturals (agarose, alginate and gelatines) and synthetics (poly(vinyl alcohol)-based), are able to mimic ECM topography and to deliver bioactive molecules [50]. Gelatin, produced through the partial hydrolysis of collagen, is mainly utilized for the production of microparticles, one of the most extensively used drug carriers due to their non-toxicity, storage stability, cost-effectiveness as well as the simplicity in preparation.
Bioactive ceramics can be of natural or synthetic origin (coralline, HA, tricalcium phosphate - TCP - sulphate, bioactive glass - BG - and calcium silicate). Chemically similar to bone, they show high compressive strength and low ductility, thus providing high resistance to deformation but, at the same time, brittleness [54]. We recently studied a synthetic putty, nanocrystalline, ceramic, biomimetic biomaterial made of HA enriched with magnesium. The scaffold had been previously utilized as a bone defect filler in a patient affected by jaw ameloblastoma, [55]. Twenty-five months later, we investigated the quality of the newly-formed tissue. Histological analysis gave evidence of a bone remodeling process, showing a mature and compact tissue. Immunohistochemical evaluations confirmed the presence of the main phenotypical bone tissue markers. Despite the inherent limitations of a case report, these data would support the healing potential of this bone substitutefor the management of mandibular ameloblastoma.
Composites consist of a combination of two or more materials with different properties, each displaying only some advantages and specific drawbacks [50], [51]. The combination can be in the form of co-polymers, polymer–polymer blends, or polymer–ceramic composites. Co-polymers derive from two or more monomeric species, such as PLGA that is a combination of poly lactide and polyglycolide and is regarded as an excellent candidate for BTE applications due to its biodegradability and ease of fabrication. Polymer–polymer blends are mixtures of two polymers, like a PLGA-polyphosphazenes blend that allows to overcome the problems due to PLGA's acidic degradation products that may induce tissue necrosis and implant failure, since polyphosphazenes release only neutral or basic products. As a result, the blend produces near-neutral degradation products. Polymer-ceramic composites are really biomimetic, since bone is, in fact, a composite material made of a mix of inorganic HA crystals and organic collagen fibers. They have been successful in bone regeneration, exceeding the results obtained when these materials are used separately. The incorporation of inorganic inclusions such as bioceramic and bioglass particles, carbon nanotubes, or magnesium metallic or alloy particles seems to positively affect scaffold mechanical properties [50], [51].
2.1.4. Manufacturing technologies
3D fabrication available technologies can be divided into two main categories, conventional and Rapid Prototyping (RP), producing different scaffold's characteristics [49].
Conventional techniques use subtractive methods in which parts of the material are removed from an initial block to reach the desired conformation. A key issue is a limited ability to control shapes and geometries or to incorporate internal architecture or curved channels [56]. In addition, the use of organic solventsmay compromise cell viability or functions, even when only residues remain [49].
RP techniques, recently introduced with the aim of overcoming the limitations of standard ones, are additive fabrication processes (also defined as “additive manufacturing” or “solid free form fabrication”) that manufacture the final 3D object via deposition of overlying layers. Materials to be used can be liquid-, solid-, and powder-based.
In general, these techniques do not utilize toxic organic solvents, allowing a significant enhancement in scaffold biocompatibility [57]. A more punctual control of porosity, pore size and mechanical and chemical properties is possible, permitting to better mimic natural bone tissue structure. These methods allow also for variation of composition of two or more materials across the surface, interface, or bulk of the scaffold. RP technology is based on the possibility to obtain objects (CAM) starting from a 3D mathematical model definition (CAD) (Fig. 2). CAD/CAM systems encloses three components: a digitalization tool/scanner that transforms geometry into digital data that can be processed by a computer; a software that produces a data set readable by a fabrication machine; and a manufacturing technology that transforms the data set into the desired product [58]. Recently, such a system has been applied in the clinical practice, starting from patient's medical images acquired with non-invasive techniques like Magnetic Resonance Imaging (MRI) and Computerized Tomography (CT).

Fig. 2. A flow chart illustrating the Rapid Prototyping process steps.
A computer based 3D model is generated using imaging scan data (A); the model is then imported into a manufacturing system software to be sliced into thin horizontal layers (B); finally, the 3D scaffold is built layer by layer based on the shape of the computer model for the manufacturing of tailored 3D structures (C).
3. Current manufacturing technologies for scaffold fabrication
3.1. Conventional technologies
Conventional techniques, include solvent casting and particle leaching, freeze-drying, Thermally Induced Phase Separation, gas foaming, powder-forming, sol-gel, and electrospinning [49].
3.1.1. Solvent-casting and particle leaching
Solvent-casting and particle leaching are techniques where a polymer solutionis dissolved in a solvent with uniformly distributed salt particles of a specific size. The solvent evaporates leaving a salt particles-embedded matrix; this is immersed in water where the salt leaches out to produce a highly porous structure. This technique is relatively easy to perform and equipment costs are sustainable. Key advantages are high scaffold porosity and the feasibility to tune pore size that may allow to better reach bone-like tissue structure [49], [56]. Mehrabanian and collaborators developed nanoHA-nylon composite scaffolds displaying porosity values that were comparable to those of natural bone. The use of a composite allowed to overcome singular material disadvantages and to add up advantages. The nanoHA structure is very close to that of natural bone however, its brittleness and poor mechanical stability limit its use for the treatment of load-bearing defects. Nylon displays good biocompatibility (probably due to its chemical structure similar to collagen) and excellent mechanical properties, but its degradation is difficult to control. The combination of both results in a scaffold material that could be a good candidate as a bone substitute due to its histocompatibility, osteoconductivity and osteoinductivity [59]. In the study of Cao and colleagues, 3D PGA/β-TCP composite scaffolds were fabricated using this method. The scaffolds, displaying high porosity and interconnected pores, were investigated for their ability to repair critical bone defects in rat femoral medial-epicondyles. Quantitative image analysis and qualitative histological evaluations showed that bone formation began within 14 days of surgery and was completed after 30 days. By day 90 bone replacement was almost completed [60].
Disadvantages of this technique are represented by the fact that the process can only form simple shape scaffolds (flat sheets and tubes) and that the residual solvent could be harmful to cells [60], [61], [62], [63].
3.1.2. Freeze drying
In the freeze drying process, also known as lyophilization, a synthetic polymer is firstly dissolved into a suitable solvent. Then, the polymer solution is cooled down below its freezing point, leading to the solidification of the solvent that is evaporated via sublimation, leaving a dry scaffold with numerous and interconnected pores. A benefit of the technique is the possibility to avoid high temperatures that could reduce the activity of incorporated biological factors. Moreover, pore size can be easily controlled by tuning the freezing regime. This technique has been utilized to fabricate a BG-Collagen-Phosphatidylserine scaffold with interconnected pores with sizes of about 300 μm. Phosphatidylserine, like several phospholipids, has been demonstrated to be able to form complexes with both calcium and phosphate and nucleate HA formation. The scaffold was seeded with rat MSCs and either cultured for few days or implanted in rat femurs defects. In vitro results at day 21 showed that cells had grown within the scaffold, showing osteogenic features. In vivo results showed good biocompatibility and extensive osteoconductivity with host bone after 6 weeks [64]. Chen and colleagues utilized freeze-dried-produced calcium-Alginate scaffolds to seed human osteoblasts. The engineered tissues were then cultured in a perfusion-based bioreactor system, with the aim to mimic in vivo bone environment. Results highlighted the formation of bone-like tissues. Moreover, the Authors stated that the bioreactor strategy could provide a safe and economic tool for possible clinical BTE applications [65]. Disadvantages of this technique are the lengthy timescales, high energy consumption, the use of cytotoxic solvents [50], [51], [56] and the production of irregular, small size pores (typically ranging from 15 to 35 μm). To overcome this last issue, techniques modifications have been tested such as varying the freezing temperature (− 10 °C ÷ − 70 °C) and introducing an annealing additional step in order to increase the rate of ice crystal growth. The collagen-glycosaminoglycan scaffolds produced with these modifications showed a substantial improvement in pore size (85–325 μm) [66].
3.1.3. Thermally induced phase separation (TIPS)
TIPS is a low temperatures process where a polymer solution is quenched and undergoes a liquid-liquid phase separation to form two phases: one polymer-rich and the other polymer-poor. The polymer-rich phase solidifies and the polymer poor one is removed, leaving a highly porous, nanoscale fibrous network. Low temperatures favor the incorporation of bioactive molecules. Smith's group developed a novel TIPS process where a solution of biodegradable polymer has been cast into a porous scaffold, resulting in a nanofibrous pore-wall structure with 50–500 nm fibers. This process was then combined with a porogen leaching technique, to engineer poly(l-lactic acid)-based scaffolds able to incorporate nanoparticles as carriers of recombinant human BMP-7. In vitroexperiments showed that protein delivery in cell culture enhanced osteogenic differentiation [67]. In the study of Qui et al., aminated mesoporous silica nanoparticles were prepared and used as microcarriers for dexamethasone loading. A PLLA/PCL nanofibrous scaffold was fabricated via TIPS and served as template onto which the drug-loaded nanoparticles were deposited by electrophoretic deposition. In vitro results indicated that bone marrow MSCs cultured on the scaffolds exhibited a high degree of osteogenic differentiation, in terms of alkaline phosphatase activity, mineralized matrix formation and osteocalcin expression. Furthermore, in vivo results in a calvarial defect model of Sprague-Dawley rats demonstrated a significant induction of defect healing [68].
3.1.4. Gas foaming
Gas foaming technique was developed to avoid organic, cytotoxic solvents. The process uses relatively inert gas-foaming agents (carbon dioxide, nitrogen) to pressurize molded biodegradable polymers with water or fluoroform, until they are saturated and full of gas bubbles. This technique typically produces sponge-like structures with an average pore size in the range of 30–700 μm and a porosity up to 85% [49]. The group of Giannitelli recently developed a polyurethane-based, functional-graded material to be used for the treatment of oro-maxillary bone defects. The scaffold was composed by a dense shell representing a barrier to gingival tissue ingrowth and a porous core allowing bone regeneration. Results demonstrated the ability to support human Bone Marrow MSC viability and adhesion. Mechanical tests suggested that the material, although not matching the mechanical properties of natural cancellous bone, could be able to cope with the stresses present at the implant site [69].
Drawbacks of gas foaming technology include the use of excessive heat during compression molding, close, non-interconnected pore structures and a nonporous skin layer at the scaffold surface. Harris and colleagues have combined gas foaming with particulate leaching with the aim of increasing porosity. In particular, they were able to produce PGLA scaffolds displaying an overall porosity of up to 97% [70].
3.1.5. Powder-forming process
The powder-forming process allows the fabrication of porous ceramic scaffolds. In details, a ceramic particle suspension in a suitable liquid (such as water or ethanol) called slurry, is used to prepare green bodies. The methods for forming green bodies can be classified as dry or wet processes. Among these processes, the replication technique, also named the ‘polymer-sponge’ method, including a crystallization phase, offers the potential of forming uniform dispersion of ceramic powder within a template, resulting in controllable pore size, high porosity and interconnectivity. Using this technique, the group of Chen [71] has produced for the first time a silicate BG scaffold with a porosity of about 90% and a pore size ranging from 510 to 720 μm. Fine silicate crystals conferred competent mechanical strength to the scaffolds. The scaffold were also biodegradable and bioactive, but with a reduced protein-binding ability. Therefore, in a subsequent work, the same group toughened the structure by applying a 3-aminopropyl-triethoxysilane coating [72].
3.1.6. Sol–gel technique
Sol–gel technique is based on the inorganic polymerization of metal alkoxides. A sol is formed by the addition of a surfactant, followed by condensation and gelation reactions. This process allows the fabrication of ceramic or glass materials in the form of ultra-fine or spherical-shaped powders, thin-film coatings, ceramic fibers, microporous inorganic membranes, monolithic ceramics and glasses and highly porous aerogel materials [49]. To overcome the disadvantage posed by the fact that these scaffolds do not possess high mechanical strength, Chen et al. [73] developed a modified sol–gel process to fabricate sodium oxide-containing BG ceramics. The structures showed improved mechanical strength, without losing biodegradability.
3.1.7. Electrospinning
Electrospinning utilizes electrical charges to draw, by means of a syringe pump, fine fibers up to the nanometer scale and create, with a collector, a nanofibrous architecture with surface areas able to adsorb proteins and binding sites to cell membrane receptors. A standard system requires four major components: a spinner with a metallic needle, a syringe pump, a high-voltage power supply, and a grounded collector. The electric field strength overcomes the surface tension of the droplet and generates a charged liquid jet that is then elongated and whipped continuously by electrostatic repulsion until it is deposited on the grounded collector. The solvent evaporates in the process and the jet solidifies to form a nonwoven fibrous membrane [51], [56].
The electrospinning technique has the versatility to process a wide range of materials in order to produce scaffolds with required morphology and porosity, including fibers with diameters from few microns down to the nanometer range. Yang et al. combined PCL with various amounts of chitosan to create bioactive nanofibers. Pure electrospun chitosan has a low mechanical resistance, while pure PCL displays reduced cell adhesion. A combination of them resulted in a significant increase of both characteristics and therefore in a better ability to promote bone tissue formation. In fact, the biomaterial demonstrated to be able to improve both adhesion and proliferation of MC 3 T3-E1 cells and to allow elevated calcium deposition, alkaline phosphatase activity and expression of osteopontin [74]. In a recent paper, Dhand and collaborators reported a bio-inspired preparation of collagen containing catecholamines (i.e.dopamine) and calcium composite structures. The presence of divalent cations induces simultaneous partial oxidative polymerization of catecholamines and crosslinking of collagen nanofibers, thus producing mats that are mechanically robust and confer photoluminescence properties. In fact, such scaffolds displayed mechanical properties similar to that of cancellous bone. Seeded human fetal osteoblasts enhanced osteogenic marker expression (Fig. 3). The Authors concluded that these smart multifunctional scaffolds could potentially be utilized to repair and regenerate bone defects and injuries [75].

Fig. 3. Overall summary of a collagen-dopamine scaffold by electrospinning technique.
Electrospinning of a dope solution containing collagen (8% w/v), dopamine (10% w/w of collagen) and 20 mM CaCl2 in 90% HFIP triggered the formation of polydopamine supported by the appearance of brown coloration in the mats. Subsequent exposure of the mats to (NH4)2CO3 vapors resulted in intensification of brown coloration and precipitation of CaCO3. For comparison, the photograph of mat electrospun without CaCl2 is also shown in the lower bottom. The nanofibrous composite mats displayed excellent mechanical properties, blue and green fluorescence, surface wettability and osteoblast cell proliferation and differentiation [75]. Reproduced with permission from Elsevier. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Another important feature of this technique is that the nanofibers can be further functionalized via incorporation of bioactive species. Li et al. developed a new nanoparticle-embedded electrospun nanofiber scaffold for the controlled dual delivery of BMP-2, a potent osteoinductive factor, and dexamethasone able to differentiate MSCs into osteogenic lineage. In particular, BMP-2 was encapsulated into bovine serum albumin nanoparticles to maintain bioactivity. In vitro study results demonstrated a strong ability to induce osteogenic differentiation, in vivo results revealed a significant degree of repair of rat calvarial defects [76].
A main disadvantage of electrospinning is the use of organic solvents.
3.2. Rapid Prototyping
The main RP technologies developed within the last years are: Stereolithography (SL), Fused Deposition Modeling (FDM), Selective Laser Sintering (SLS), 3D Printing (3DP) and Bioprinting, also defined as 3D plotting or Direct-Writing [77], [78], [79], [80].
3.2.1. Stereolithography
The term SL was coined by Charles Hull who invented the technique described in his U.S. Patent issued in 1986. He defined a method and apparatus for making solid objects by successively printing thin layers of an ultraviolet (UV) curable material one on top of the other [81]. Such an approach allowed to overcome some well known shortages of the subtractive methods, for example it avoided discarding the unused portions of raw material and heavy abrasions provoked by milling tools. Schematically, a SL system consists of a tank of photo-sensitive liquid resin, a moveable built platform, an UV laser to irradiate the resin and a dynamic mirror system. The process starts with the UV laser depositing a layer of a photo-sensitive liquid resin onto the platform. Once the layer is completely solidified, the platform is vertically lowered. Subsequently, another layer is deposited onto the first one. These steps are repeated until a complete 3D scaffold is formed. Finally, uncured resin is washed off and the scaffold is post-cured under UV light, yielding a fully cured part. In the study of Castro and colleagues, an osteoconductive nanocrystalline HA material was produced by means of SL. Results showed that human bone marrow-derived MSC adhesion, proliferation, and osteochondral differentiation were greatly improved in vitro[82]. However, the use of bioceramic scaffolds can be problematic due to viscosity that may hamper the process. For this reason an indirect fabrication method has been developed where an epoxy mold is first created by SL and then a suspension of ceramic acrylate is cast into the mold that is then removed by thermal treatment. As a result, the 3D scaffold with the inverse shape of the mold is obtained [49].
With the reduction in the laser power and the improvement of both lateral and vertical resolutions, new SL techniques (more efficient and with higher speed building generation) have emerged, such as: micro-stereolithography (lSL), two-photon polymerization (TPP), and digital light processing (DLP). lSL employs a single photon beam that can be focused more precisely with a reduced spot size of the laser. PPF scaffolds were fabricated by this technique, showing mechanical properties similar to those of human trabecular bone [83] In TPP, when a near-infrared ultra-short-pulsed laser is closely focused into a volume of photo-curable resins, real 3D microstructures can be fabricated using a layer-by-layer accumulating technique. Using such a system, a novel biodegradable star-shaped PLA scaffold has been recently fabricated showing to be capable to support in vitro osteogenic differentiation and in vivo bone tissue formation [84]. In the DLP process, dynamic masks are used to cure a whole layer at a time. Advantages include an efficient process for filling a large amount of ceramic particles and no need for expensive specialized equipment such as a laser or a heating chamber [49]. Skin irritation and cytotoxicity caused by photo-sensitive resins appears to be a major problem of this technique and, for this reason, alternative resins based on vinyl esters that possesses better in vivobiocompatibility, have been explored [85].
3.2.2. Fused deposition modeling
In FDM process molten thermoplastic materials, extruded from a nozzle, are deposited onto a base platform following a path, which is predefined by CAD and CAM. When each layer in the xy plane is finished, the platform (z axis) is lowered and the procedure is repeated. 3D scaffolds with controllable pore size and porosity can be fabricated by changing the material deposition amount, the spacing between the material paths and the height interval (z axis). The main advantages are: material high porosity and good mechanical strength, no toxic solvent requirement and flexibility in material handling and processing. Chou and colleagues tested tailored PLA-based cages to treat femoral segmental bone defects in rabbits. Results showed an improved bony ingrowth. The Authors concluded that the availability of cages of any geometric shape would represent an advantage, in terms of outcome, in the treatment of large segmental bone defects in humans [86].
FDM can be also utilized to produce composites like PCL-HA or PCL-TCP that are used in BTE for their mechanical and biochemical properties [77]. A case report described a calvarial reconstruction by means of CAD/CAM fabrication of a customized medical grade PCL-TCP implant. After six months the implant was well integrated and an initial bony consolidation process could be detected by CT [87].
The main difficulty of the FDM technique is the requirement for preformed fibers with a consistent size and material properties to feed through the rollers and nozzle. Furthermore, its application to biodegradable polymers, apart from PCL, may be limited [88]. To overcome these issues, various modified FDM processes have been developed such as the novel low-temperature deposition manufacturing technique by Xiong et al. who produced porous poly (l-lactic acid) and TCP scaffolds showing good biocompatibility, bone conductivity, and appropriate biodegradation properties for bone repair [89].
3.2.3. Selective laser sintering
SLS, developed by the University of Texas in Austin in 1986, binds together powder particles in thin layers with a high-power laser, such as carbon dioxide. Subsequent formed layers are then bound to the previous ones, following the cross-sectional information carried by the pre-defined CAD data. Because the powders are maintained with low compaction forces after the sintering process to form new layers, structures have an internally porous structure suitable for a bone scaffold. SLS-manufactured scaffolds, based mainly on PCL and a combination of PEEK and HA, display an anatomically shaped external architecture and porous interior structure that can be utilized for load-bearing applications in which high fracture toughness and mechanical strength are needed. Williams and colleagues designed and fabricated a prototype mandibular condyle scaffold based on an actual pig condyle [90]. In a case report, a personalized 3D-printed bioresorbable scaffold made of medical-grade PCL was used to treat a large periodontal defect. For the fabrication, a CT model of the patient's cone beam was utilized. Results at 12 months showed that the structure remained in situ, without signs of chronic inflammation or dehiscence [91]. The recent paper by Roskies et al. reported, for the first time, the fabrications of customized porous PEEK scaffolds with a trabecular microstructure. PEEK is widely used in orthopedic applications as a result of its high strength, fatigue resistance, radiolucency, and good biocompatibility in osseous environments. In general it displays problems due to its poor integration with adjacent bone upon implantation. The novel scaffold structure was able to improve the bone-implant interface and thus demonstrated to be suitable for craniofacial reconstruction [92]. Disadvantages of SLS include the post processing phase necessary to remove trapped powder and the high operating temperature.
3.2.4. Three dimensional printing
The 3DP technique has been primarily used to create engineering prototypes, but recent advances have enabled the fabrication of printers able to make products comparable with traditionally manufactured items. To date, a large number of files containing information about the object to be printed, such as color, texture, layer thickness, etc., is commercially available. Advantages of this printing technology in terms of costs, time and set up involve not only the possibility to enable mass customization of goods on a large scale, but also competitiveness for smaller production runs. Moreover, a high degree of customization is allowed since the cost of the first item is the same as the last [77], [79], [80], [81], [82].
Invented at the Massachusetts Institute of Technology, 3DP technique is able to ink-jet print a liquid binder solution onto a powder bed at room temperature conditions. The process begins with a binder jetting machine distributing a layer of powder onto a platform. Liquid droplets of a bonding agent are deposited onto the powder layer through inkjet print heads, bonding the particles together. The platform is then lowered and a next layer of powder is laid out on top. By repeating the process of laying out powder and bonding, the parts are built up in the powder bed. Removal of the unbound powder reveals the fabricated part. The 3DP process can be direct or indirect by printing the actual part or a mold, respectively [93], [94]. Direct 3DP allows a more punctual control over both the micro- and macro-architecture. Drawbacks are represented by the limited available pore size and by the dissolving action of organic solvents on most print heads. To overcome this issue, stencils may be used; however, this compromises the ability to fabricate highly complex shapes or small features. In the indirect technique, molds are printed using commercially available plaster powder and biodegradable polymers are cast into the printed molds. Indirect 3DP involves the fabrication of a simple 3D sacrificial primary template that works as a mold for a secondary one. This template is then removed by physical, chemical or thermal basis, thus generating the desired 3D scaffold. Such a process overcomes many of the limitations of the direct technique: the use of aqueous binders eliminates the need for stencils; the porogen size is not limited since it is introduced into the mold cavity after printing, and it does not affect printing resolution or layer interconnectivity. Limitations are high density packing of porogen in complex features (i.e. intricate internal undercuts or intersecting channels) and restrictions on shape or feature design due to demolding difficulty [77], [79], [80], [81], [82].
The possibility to room temperature processing of 3DP technology allows the use of a wide range of powder materials and the incorporation of a variety of pharmaceutical and biological agents such as peptides, proteins (e.g. fibrinogen, collagen), polysaccharides (e.g. hyaluronan, alginate), DNA plasmids and cells that can enhance the process of bone formation. Moreover, the opportunity of scaffold local drug delivery is important since systemic delivery requires the periodic intake of high-dose due to low bioavailability. In 2014, Tarafder and Bose evaluated the effect of alendronate (AD) release through PCL-coated 3D printed interconnected porous TCP scaffolds in the distal femoral defect of Sprague–Dawley rats for 6 and 10 weeks. AD is a member of the bisphosphonate family and it is utilized in the treatment of various skeletal disorders because of its potent inhibition of bone resorption. A higher bone formation was observed after 6 weeks from histomorphology, compared to bare TCP and TCP coated with PCL only. After 10 weeks, the newly formed bone was even more compact [95]. Carrel et al. tested the ability of a 3D printed porous block of layered strands of TCP and HA to promote cortical bone vertical growth in a sheep calvarian model. Two standard granular substitutes, one natural, derived from bovine bone, and the other synthetic, made of pure beta-TCP, were used as comparison controls [96]. Histomorphometric analyses showed that 2 months after implantation, the 3D construct's osteoconductivity was significantly higher than in the two substitutes. Moreover, the porous structure of the block favored the formation of vessels inside its channels. However, after 4 months, there was no statistical difference between the 3 materials. The Authors hypothesized that a time period of 4 months was insufficient for bone to fully mature and for the material to resorb. Therefore, in a subsequent work, they decided to prime the 3D printed blocks with BMP-2. Results at 4 months evidenced, for the primed block, the formation of an haversian-like architecture, while the material resorbed [97]. Wang and colleagues investigated the regeneration properties of an anti-inflammatory structure in mice calvarial bone defects [98]. In particular, they evaluated if 3D-printed Atsttrin-incorporated alginate/HA scaffolds can promote bone healing by affecting Tumor Necrosis Factor (TNF)/TNF-Receptor (TNFR) signaling. Atsttrin, (Antagonist of TNF/TNFR Signaling via Targeting to TNF Receptors) is a progranulin-derived engineered protein. Progranulin, a crucial mediator of tissue repair, is able to bind TNFR, thus exerting an antagonistic effect on the growth factor. It is known that high expression levels of pro-inflammatory TNF-α within bone defects can decelerate and impair bone regeneration. 3D-printed Atsttrin-loaded structures demonstrated to be able to significantly reduce the number of TNF-α positive cells within wound sites, thus increasing tissue regeneration [99], [100]. Other advantages of 3DP are represented by the feasibility to fabricate internal channels and by the multi-“color” printing option that allows to simultaneously arrange multiple types of cells, deposit multiple ECM materials, and exert control over bioactive agents. Drawbacks are the difficulty in removing unbound powder from small or curved channels and the use of print heads that can make finer features but are more prone to clogging.
An important application of 3DP is the fabrication of customized, anatomically shaped constructs based on CT medical information. Kim and collaborators [101] fabricated anatomically shaped human molars and rat incisors from a hybrid of poly-ε-PCL and HA. Stromal-derived factor-1 (SDF-1), that regulates osteogenic differentiation of mesenchymal stem/progenitor cells (MPCs) induced by BMP2, and BMP-7 were delivered in the scaffold microchannels. Constructs were ectopically implanted into the rat dorsum, whereas incisors were orthotopically implanted after mandibular extraction. After 9 weeks, a putative periodontal ligament and new bone tissue were observed at the interface between rat incisor scaffold and native alveolar bone. The Authors hypothesized that the two soluble factors delivery not only recruited significantly more endogenous cells, but also favored a higher angiogenesis. Ju-yeon Lee et al. [102] were able to create custom scaffolds made of PCL and chitosan and coated with bioactive apatite, mimicking human mandibular condyle. CAD models were utilized in order to precisely control the internal morphology of the material and to match patient's external contours. In vitroexperiments, performed by adding bone marrow derived MSCs to these scaffolds, showed good cell viability.
The 3DP also allows a construction of a multilayered scaffold to regenerate hybrid tissue systems. Sherwood and collaborators developed an osteochondral porous biphasic composite construct in which the upper region is composed of D, L-PLGA/L-PLA for cartilage regeneration, and the lower region consisted of L-PLGA/TCP to enhance bone ingrowth [103].
3.2.5. Bioprinting
Bioprinting technology has gained much attention due to its ability to overcome some of the challenges encountered in the traditional BTE approaches. Indeed, despite good results, all these methods still do not allow to accurately control cell distribution, thus lacking the ability to generate an homogeneous and appropriate ECM and, ultimately, to build the desired tissue.
Bioprinting is a form of additive manufacturing where small units of cells and biomaterials are dispensed together with micrometer precision, in order to form tissue-like structures. Such a new technology allows accurate cell distribution control, together with scalability and cost-effectiveness [104], [105], [106], [107]. Moreover, it enables the fabrication of engineered tissues constructs at ambient conditions.
To date, this approach has been mostly used to process hydrogels [108]. Poldevaart et al. [109] bioprinted, in a laminar flow-cabinet, non-cytotoxic, biodegradable gelatin microparticles adsorbed with human recombinant BMP-2. Such a sustained release system was supplemented with goat multi-potent stromal cells and calcium phosphate. Osteogenic differentiation and bone formation were detected both in vitro and after subcutaneous implantation in mice or rats. This study, utilizing a sustained release of the BMP-2 protein at lower dosage, may suggest an alternative and effective strategy in enhancing osteogenic differentiation and bone formation while reducing the risk of side effects and complications. In clinical practice, BMP-2 has already been applied to spinal fusion surgery and tibial fracture healing. However, some studies have shown that the dosage is much higher than the effective need in order to compensate for the fast protein wash-out. Such a situation can be associated with an increased risk of bone overgrowth or malignancies in patients. Wang and colleagues encapsulated SaOS-2 cells into a biodegradable alginate/gelatin hydrogel [110]. Results revealed a significant enhancement of the entrapped cells potency to mineralize. In the study of Gao and collaborators [111] human MSCs were evenly distributed in a polymerized poly(ethylene glycol) (PEG) dimethacrylate-gelatin metacrilate scaffold, during layer-by-layer assembly. The bioprinting procedure demonstrated to be biocompatible, with > 80% viability. The construct revealed to provide a strong support to the embedded cells that showed osteogenic and chondrogenic differentiation ability.
However, hydrogel application in orthopedics may be problematic, due to the texture that can be inadequate to bear relevant loads in vivo or to the low stiffness that could inhibit osteogenic differentiation [112]. Other disadvantages are: limited critical timing of gelation, specific matching of material and liquid medium densities and low resolution. For these reasons, researches are focusing on the development of alternative materials. Sawkins et al. [108] investigated a new 3D construct that they had previously developed at ambient conditions starting from a thermo-responsive micro-particulate material based on PLGA. Results demonstrated that the construct displays yield stresses up to 1.22 MPa and Young's moduli up to 57.3 MPa, which are within the range of human cancellous bone properties. Moreover, the microspheres were incorporated with protein lysozyme, which was chosen on the basis of its similarity with BMP-2, in terms of molecular weight and isoelectric point. Results evidenced that the protein release was sustained for a period of 15 days and a high degree of activity could be measured up to day 9. A further step was the incorporation of human MSCs, showing high degree of viability.
A new frontier could be represented by the use of genetically modified phagi as ink material. Lee et al. genetically engineered the M13 bacteriophage, a filamentous virus, by creating, on its major coat protein, new motifs that were crosslinkable with calcium ions in order to form a gel-phase providing an appropriate microenvironment for pre-osteoblasts growth and maturation [113].
To date, the most used bioprinting technologies are jetting-, extrusion- and laser- based printing [104], [105], [106], [107].
Jetting-based (or ink-jet or droplet-based) bioprinting is a non-contact technique in which 2D or 3D structures are fabricated by using picolitre bio-ink droplets layered onto a substrate. Some examples of material jetting techniques include piezoelectric or thermal inkjetting, acoustic wave jetting and electro-hydrodynamic jetting. The advantages are: low costs due to similar structure with commercial printers, high printing speed as a consequence of printer heads' ability to support parallel work mode and relatively high cell viability (80/90%). Disadvantages are represented by a narrow material selectivity and frequent clogging of the print head. The group of Gao developed acrylated peptides and co-printed with acrylated PEG hydrogel by means of simultaneous photopolymerization. Bone marrow-derived human MSCs were simultaneously printed during the scaffold fabrication process. The resulted construct demonstrated excellent biocompatibility, with a high cell viability. Nozzle clogging was minimized due to the low viscosity of the PEG polymer. Bioprinted bone and cartilage tissue demonstrated excellent mineral and cartilage matrix deposition, as well as significantly increased mechanical properties [114]. However, it has to be underlined that material low viscosity, requested for the manufacturing process, can also represent an important limitation for BTE applications requiring mechanical integrity. One solution in order to overcome such a limitation could be to crosslink the structure after printing [104], [105], [106], [107].
Extrusion-based bioprinting systems dispense continuous filaments of a material consisting of cells mixed with hydrogel through a micro-nozzle, using piston or pneumatic pressure to fabricate two dimensional (2D) or 3D structure. After printing 2D patterns, hydrogels are physically or chemically solidified and 3D structures are fabricated by stacking 3D patterns layer by layer. Most existing commercial bioprinters are based on this technology. The advantages of extrusion-based bioprinting are: a wider selection of biomaterials since the micro-nozzle allows to dispense high viscous bio-inks, the use of synthetic polymers, a scalable production and a higher cell viability (> 90%). The extrusion bioprinting technology has been utilized to fabricate heterogeneous scaffolds for osteochondral regeneration. In particular, Fedorovich and colleagues built alginate hydrogel-based porous constructs by encapsulating human osteogenic progenitors and chondrocytes in different parts of the constructs, which were then implanted in mice. Results evidenced that the cells remained viable and stayed in their compartment within the scaffold. Distinctive tissue formation (cartilage/bone) was observed [115]. Roohani-Esfahani et al. have recently utilized the technique to fabricate glass-ceramic scaffolds with sufficient mechanical strength for the treatment of bone defects under load. The hexagonal pore geometry permitted the achievement of a larger contact area among printed layers and an enhanced load transfer capability, in comparison with other conventional patterns (rectangular, curved and zigzag). Moreover, the novel design demonstrated an in vivo elevated fatigue resistance, failure reliability and flexural strength. Such an application would open avenues for the treatment of load bearing bone defects in orthopedic, as well as dental and maxillofacial applications [116]. The main disadvantage of extrusion-based bioprinting is a relatively low resolution.
Laser-based bioprinting uses the energy of pulsed laser to transfer materials [104], [105], [106], [107] to a receiving substrate. A typical system consists of a pulsed laser beam with a focusing system; a “ribbon” with a donor transport support and typically made of glass covered with a laser-energy-absorbing layer (e.g. gold or titanium) and a layer of biological material containing cells and/or hydrogel; a receiving substrate facing the ribbon. Laser-assisted 3D bioprinter focuses laser pulses on the absorbing gold layer of the ribbon and this generates a high-pressure bubble, which in turn propels cell-containing materials towards the collector substrate. This is a nozzle-free procedure, and therefore does not have the problems of nozzle clogging with cells or materials. Another advantage is its compatibility with a wide range of biomaterial viscosities (1–300 mPa/s). This technique has been applied for the first time to print a nanoHA scaffold in mice calvaria defects, directly in vivo. A workstation dedicated to high-throughput biological laser printing has been set up for printing inside mouse defects. Prior to laser printing experiments, the absence of inflammation due to laser irradiation onto mice dura mater was shown by MRI. Preliminary results demonstrated that in vivo bioprinting is possible and that it may prove to be helpful in the future for medical robotics and computer-assisted medical interventions [117]. Although this technique is able to produce relatively higher resolution patterns, it results in lower cell viability in comparison to the ink-jet mechanisms.
4. Conclusion
Although the rapid evolution in BTE, there are still challenges to face that hamper the progress towards clinical application and new approaches need to be actively pursued. These challenges will drive future developments in the field.