1. Introduction
1.1. Total joint replacement
Severe joint degeneration might occur as a consequence of trauma, pathological or degenerative diseases such as osteoarthritis and rheumatoid arthritis, to such an extent that total knee (TKR) and hip (THR) replacements alone reach every year > 1 million cases in the USA and about 200,000 in the UK [1], [2]. Future projections indicate that THR and TKR are expected to grow by 174% and 673%, respectively, by 2030 [3], corresponding to about 572,000 hip and 3.48 million knee interventions only in the USA [4], [5]. However, revision rates for total joint replacement (TJR) procedures are still unacceptably high, 10 years survival rate being around 90% [6]. The number of revision interventions is expected to grow with the same rate as primary interventions [4], [5]. Further, revision surgery is associated with higher occurrence of postoperative complications such as infections compared to primary interventions, as well as with more complex rehabilitation [7]. Indeed, implant infections occur for about 1–2% of primary interventions and up to 3–4% in case of revisions, leading, in the latter case, to the need for “re-revision procedures”. Notably, patients with revisions are about 5–6 times more likely to undergo re-revision than in the case of primary intervention [8]. Together with the risks that derive from the need for retreatments, infections at both early or late stage have a strong economic impact [8], [9], [10], [11], [12], [13]: considering primary interventions and revisions together, the sum of societal costs related to TJR treatments has been estimated to about 15 billion $ per year [6]. Due to the high social burden, it is of paramount importance to boost bone implant stability and decrease the number of revisions.
Cemented prostheses involve the use of polymethylmethacrylate (PMMA) to fill the gap between the implant and the surrounding bone [14], [15]. However, the ability of self-curing PMMA bone cements to favor implant fixation is controversial, as a mismatch exists between its elastic modulus and that of bone, which prevents implant-bone bonding. Moreover, other drawbacks are generally highlighted, namely scarce bioresorbability, possible induction of necrosis in the surrounding tissue due to exothermic polymerization reactions during on-site curing and high susceptibility to wear, that arises because implant movement at the micro-scale results in the detachment of a significant amount of particles for polymeric materials [14].
Cementless prostheses, instead, rely on the ability of the implant to self-integrate in the surrounding bone [16]. The factors that determine the ability to create a stable bonding with the host tissue, hence the implant success, are the nature and the integrity of the bond that forms, and the quantity of bone around the implant that contributes to the implant fixation: early stabilization by direct bone/implant contact, instead of the formation of a fibrous tissue capsule, is a key parameter in determining long-term durability of the prosthesis.
Since metal-based implant are inert materials, thus lacking osteoconductivity and osteoinductivity, the common approach is to coat the implant with thick (30–300 μm) layers of hydroxyapatite (HA, Ca10(PO4)6(OH)2), which is generally believed to resemble the inorganic part of human and mammals' bones and teeth and thus to provide a suitable environment for bone bonding [15], [17], [18].
Clinical success of cementless prostheses depends upon initial fixation, that in turn depends on osseointegration achieved in the first few months after intervention, and on long term fixation. Osseointegration depends on a series of events (mesenchymal cell attachment, spreading, proliferation and differentiation into osteoblasts) that eventually lead to the formation of mineralized bone around the implant [16]. Lack of osseointegration is particularly harmful for patients with conditions that suppress bone formation, such as osteoporosis, diabetes, immunosuppressive therapy, smoking and also for patients that have undergone revision surgeries [16].
1.2. The biomimetic principle
According to the “biomimetic principle”, a biomaterial engineered for bone replacement should be as similar as possible to the real bone, in terms of composition, crystallinity, lattice dimensions and Ca/P ratio, in order to elicit optimal biological behavior [19]. The inorganic phase of bone is normally referred to as hydroxyapatite; however biological apatite differs from pure HA in terms of composition, stoichiometry, crystallinity degree, crystal size/morphology and, as a direct consequence, ions availability in the biological medium [19], [20]. Indeed, bone apatite is a carbonated-HA, containing significant amounts of foreign ions either incorporated into its lattice or adsorbed onto the crystal surface, characterized by a low crystallinity and a small crystals size [19]. Further, together with HA, other calcium phosphate phases, such as octacalcium phosphate (OCP), brushite (dicalcium phosphate di-hydrate, DCPD) and amorphous calcium phosphates (ACP) can be found in the human body [19].
Among the ions that are present in different concentrations in biomineralized tissues, some (namely carbonates, magnesium, fluorine, strontium, silicate, zinc and manganese) have great biological relevance and hence have been extensively investigated with aim of obtaining ion substituted-HA biomaterials [19], [21]. The weight concentration of each of these ions in mineralized tissuesis reported in Table 1. Composition, crystallinity degree and crystal dimensions can significantly vary among different mineralized tissues. Even within bone tissue, composition can vary to a certain extent, depending on animal species, anatomic site, age, etc. [21].
Table 1. Amount of relevant ions in mammals mineralized tissues. All values are to be considered indicative (a, b [22], c [19]).
| Amount | ||||
|---|---|---|---|---|
| Bone | Enamel | Dentine | ||
| CO32 − | wt.% | 7.4a, b | 3.5a, b | 5.6a, b |
| Mg | wt.% | 0.72a, b | 0.44a, b | 1.23a, b |
| F | wt.% | 0.03a, b | 0.01a, b | 0.06a, b |
| Sr | wt.% | 0.05c | 0.03c | 0.04c |
| Na | wt.% | 0.9a, b | 0.5a, b | 0.6a, b |
| Cl | wt.% | 0.13a, b | 0.30a, b | 0.01a, b |
| K | wt.% | 0.03a, b | 0.08a, b | 0.05a, b |
| Si | ppm | 500c | ||
| Zn | ppm | 263c | 173c | |
| Mn | ppm | 0.17c | 0.6c | 0.6c |
Wet deposition techniques are, by far, the most investigated methods to fabricate ion- substituted HA coatings, due to the possibility to easily incorporate significant ions during a preliminary wet chemical synthesis step, the possibility to cover complex shaped morphologies and the relatively low costs. However, the wet deposition process is strongly dependent on many parameters, such as pH, temperature, deposition time, precursors impurities, etc. that hinder a good control over the composition and stoichiometry of the final material as well as the reproducibility of the process on a large scale. Further, wet processes are time consuming also because they often require post-deposition heating treatments. Due to these drawbacks, wet deposition methods are not industrially feasible [21].
Commercial HA coatings are fabricated by plasma-assisted techniques, mostly plasma spraying. However, despite the above-mentioned difference in the characteristics of synthetic and biological HA, the vast majority of the studies on plasma-assisted techniques focus on the deposition of stoichiometric HA. Instead, the literature regarding plasma-assisted deposition of ion-substituted coatings is still scarce, especially for what concerns in vivo or clinical results, and is the subject of the present review.
1.3. The rationale of the review process
Due to the importance of incorporating biologically significant ions into the structure of HA, the state of the art concerning the fabrication of ion-substituted HA by plasma-assisted deposition was reviewed. However, the survey was not limited to plasma spraying methods, as the actual research trend is focusing on alternative plasma-assisted methods able to overcome commonly-accepted limitations of sprayed coatings such as scarce adhesion to the substrate and tendency to crack and form wear debris [15], [23], [24]. Limitations, potentialities and future perspectives of plasma-assisted methods are highlighted and discussed.
The review is split into two main sections: in section I, an overview of plasma-assisted techniques for deposition of calcium phosphate coatings(independently of the ion-substitution) is presented; in section II, the biological role of the most important ions present in substituted HA is first considered and then the effects of their incorporation in the HA structure by plasma-assisted methods are discussed. In particular, the authors focused on the in vitro and in vivo behavior, in order to analyze the actual significance of ion addition in improving the performances of the coatings.
2. Section I
2.1. Plasma-assisted methods for calcium phosphate (CaP) deposition
Plasma-assisted techniques generally allow for high deposition rates when compared to wet methods. Moreover, it is possible to obtain either dense, non-porous, homogeneous coatings, that show good biocompatibility and impede the release of toxic ions from the substrate, or rough and porous films that can enhance osteogenesis [14], [15]. Among them, commercially available HA coatings are usually made by plasma spraying (PS). Food and Drug Administration (FDA) guidelines and the ISO standards regulated the main parameters that a HA coating should possess, in terms of thickness, mechanical properties, microstructure and surface roughness, as well as crystallinity, purity and composition [15], [18], [23]. Nevertheless, the high thickness, characteristic of PS coatings (50–200 μm), gives rise to incompatibilities such as fatigue under tensile stress and cracking at the interface with the coating due to high residual stresses, energy release and presence of voids. Further, PS coatings also lack uniformity (in terms of thickness, phase composition and microstructure), are prone to wear and might be scarcely adherent, leading to coating detachment and possibly implant failure, to such an extent that the real clinical advantage of PS-coated against uncoated implants is debated [15], [24]. Due to the above-mentioned issues, a strong interest is raising over the fabrication of nanostructured thin HA films by alternative plasma-assisted techniques, namely radio-frequency magnetron sputtering (RF-MS), pulsed laser deposition(PLD), matrix-assisted pulsed laser evaporation (MAPLE) and pulsed electron deposition (PED), that will be discussed in the following. A schematic representation of the deposition techniques is reported in Fig. 1.
 Fig. 1. Scheme of the main plasma-assisted deposition techniques used for CaPs deposition a) plasma spray, b) magnetron sputtering, c) pulsed laser deposition/, d) matrix assisted plasma deposition (2-column fitting image).
Fig. 1. Scheme of the main plasma-assisted deposition techniques used for CaPs deposition a) plasma spray, b) magnetron sputtering, c) pulsed laser deposition/, d) matrix assisted plasma deposition (2-column fitting image).2.2. Plasma spray (PS)
The term “plasma spraying” in reality accounts for a library of techniques that differ for deposition conditions and target type, such as atmospheric plasma spraying (APS), vacuum/low pressure plasma spraying (VPS), powder or suspension plasma spraying, liquid or gas plasma spraying [15], [18]. Powder plasma spray is normally the most used among these methods [15]. Economically favorable, it allows for high deposition rates and coating of quite extended areas [15], [18]. In PS deposition, the material to be deposited is inserted in a plasma gun and heated by means of a plasma jet, having high temperature and speed. The material, either melted or softened (also depending on the grain size of HA powders), is turned into droplets that deposit on the substrate [18]. High temperatures reached by the material, as well as fast cooling of the droplets once they get on the substrate, strongly affect the final thickness, morphology, crystalline phase distribution, composition and hence the mechanical and biological properties of the coating. Moreover, high temperatures can cause decomposition of the material and prevent incorporation of biological molecules [18].
The surface morphology of PS coatings is usually characterized by high roughness, no matter the nature of the substrate [15]. Significant formation of cracks occurs due to the fast cooling of the droplets [15]. In general, both morphology and microstructure of the films are scarcely uniform. In fact, coatings are characterized by the presence of unevenly distributed cracks and porosity; as a result, the properties of the coating might differ from those of the bulk target [15], [18]. The typical thickness of the coatings is quite high, comprised between 30 and 200 μm [18], also depending on the deposition technique. The high thickness causes the coatings to exhibit scarce tensile and shear strength and scarce stress relief, that might induce cracks, wear, long-term instability and failure at the bone-implant interface [25]. Moreover, layers obtained are scarcely uniform in thickness [15], [25].
Rapid cooling of the sprayed material causes the formation of amorphouscoatings and also leads to the formation of metastable phases that affect the solubility of the coating [18], [26]; the distribution of these metastable phases and of by-products is generally not homogeneous, thus non-uniform properties can be found inside one same coating.
The obtained films may exhibit high adhesive strength [15]; however the bond between the coating and the substrate is entirely mechanical and occurs due to interlocking between HA and the substrate, hence bond strength is not negligent of surface roughness [26], [27]. For this reason, deposition procedures that do not require surface preparation might be desired. As the coating exhibits micro-roughness and high porosity due to the combination of high temperatures and fast cooling, it is expected to enhance adhesion and proliferation of osteoblasts and to favor bone regeneration [15].
2.3. Magnetron sputtering (MS)
In magnetron sputtering, a target material is ionized in vacuum by negatively charged magnets and deposited onto the substrate [18]. Sputtering can be obtained by direct current (DC) or radio-frequency (RF) mode; CaP coatings, however, can be deposited only in RF mode, as DC mode requires the to-be-deposited materials to be conductive [18]. Multi-component ceramic targets as well as multiple targets can be used for the deposition of composite coatings[27].
Substrate temperature and deposition time are critical in determining the characteristics of the produced CaPs [15]. However, films can be deposited at room temperature, allowing co-deposition of biological molecules and coating of heat-sensitive substrates [18], [26]. Dense, pore-free coatings are normally obtained [15]. Post deposition annealing, however, can be performed to control morphology and roughness of the surface [27]. Films that are obtained can be very thin (typical thickness being comprised between 40 and 50 nm and 3.5 μm), very uniform in thickness and can effectively cover surface irregularities [15], [18].
As deposited coatings are amorphous, unless the deposition is performed at high temperatures (about 450–550 °C) or post deposition annealing is performed [18], [27]. The conversion from amorphous to crystalline coatings, in fact, depends on annealing temperature and absence or presence of water vapor in the chamber [27]: annealing at 500 °C in absence of water vapor and around 450° in presence of water vapor have been reported for producing CaP coatings with 60–70% crystallinity (i.e. that normally required to obtain the desired solubility) [26], [27].
High purity coatings are generally obtained [15], [18]. Their composition, however, might differ from that of the bulk target, also depending on sputtering system and parameters [27]. In particular the Ca/P ratio of the films might vary in a quite wide range (1.6 up to 2.6) and is normally higher than that of the HA target, due to preferential sputtering of calcium and/or to evaporation of P2O5[15], [27].
Magnetron-sputtered coatings generally exhibit high adhesion to the substrate (bonding strength > 30 MPa) and suitable bioactivity [15], [18], [28].
2.4. Pulsed laser deposition (PLD)
In PLD, the material to be deposited (normally a solid target) is placed in a vacuum chamber, either in ultra high vacuum or in presence of a background gas, and hit by a high-power, focused, pulsed laser beam [15], [29]. As a result, part of the target (normally kept rotating to ensure a uniform rate of ablation), is ablated and atoms, ions, molecules and, eventually, target fragments, create a gaseous cloud or “plasma plume” that causes deposition of target material onto the substrate [15], [18]. Provided that the energy density of the laser is high enough, one plasma plume is created at each pulse: about 0.02 to 0.05 nm of target material are deposited for each shot, so very thin layers, 50 nm up to 5–10 μm thick, can be obtained [15], [18].
Deposition parameters, such as wavelength of the laser, duration of each pulse and energy density, are fundamental for the final morphological, compositional and crystalline outcome of the coating [14]. Either dense or porous coatings can be obtained by properly selecting the deposition parameters [15], [18]. Even for PLD, substrate temperature strongly affects the final outcome of the deposition: indeed, by varying the deposition temperature it is possible to finely tailor the texture and roughness of the coating surface and its crystallinity degree. Notably, the possibility to work at room temperature allows to deposit onto polymers and heat-sensitive materials [14]. Both crystalline and amorphous features can be obtained [15], [18], [29], depending on the deposition temperature, laser energy density, working gas and presence or absence of water vapor [14]. In general, low temperature deposition causes the coatings to be amorphous. Highly pure and crystalline coatings, instead, can be achieved when the substrate is heated to temperatures between 350 and 400 and 600 °C, recent studies suggesting that the transition between amorphous and crystalline phases occurs at 340 °C [14], [15], [18]. Post deposition annealing can also be performed to increase the crystallinity of the coating [14], [15].
Like the crystallinity degree, also the stoichiometry of the coatings is largely influenced by deposition parameters and, in particular, by the temperature of the substrate. Some studies highlight the dependence of the formation of HA or other calcium phosphates on temperature [30]. As a precise control of the stoichiometry is possible, mixtures of different CaPs and multicomponent stoichiometric coatings from one same target can be deposited [15], [18], [29], [31]. Finally, PLD coatings are generally highly adhesive to the substrate [18] and exhibit good biocompatibility and bioactivity [32], [33].
2.5. Matrix assisted pulsed laser evaporation (MAPLE)
MAPLE is a recent modification of the PLD specifically developed for the transfer of delicate organic and/or biological material that would be damaged by laser-based transfers. These delicate organic materials can be deposited together with CaPs to form the coating. Thermally unstable phases (such as octacalcium phosphate) can be deposited as well, due to the possibility of preserving the stoichiometry of the target [18], [34], [35].
MAPLE deposition is obtained by means of a solid target, put into the deposition chamber and kept frozen during laser irradiation by means of a cooling system inside the chamber, so as to prevent evaporation. Solid targets are obtained by instant-freezing (generally by immersion in liquid nitrogen) a mixture of the “active” biomaterial (up to 5 wt.%) in volatile solvent. As deposition proceeds, the solvent is gradually removed by a vacuum system.
Because the active material concentration is low and the solvent is chosen to absorb the laser wavelength, direct interaction between the laser and the active material is prevented, so that eventual delicate matter can be released without suffering damage and can reach the substrate and form the film. Moreover, also the intensity of the laser can be tailored so as to prevent damage of the target. The substrate is kept parallel to the target and can be heated to a selected temperature by a laser source [34], [35].
2.6. Pulsed electron deposition (PED)
PED has been emerging as an effective and cheaper alternative to PLD, being the target material ablated by a high energy pulsed electron beam instead of a more expensive pulsed laser beam [36], [37], [38], [39], [40]. PED working principle is similar to that of PLD, but because of the different ablating source, it allows for some advantages. Among the others, the most important are the higher pulse energy frequency, the higher energy efficiency and the possibility of ablation of wide band-gap and highly reflective targets. Moreover, a higher capability of being scaled for large-area deposition is expected due to the possibility to obtain multiple systems at a relatively moderate cost.
PED has been developed in the 2000s for film deposition for photovoltaic and organic electronics applications but it has been recently proposed for the deposition of thin ceramic CaP films [37].
As-deposited CaP films are usually amorphous, but post deposition annealing allows to tailor the crystallinity degree, so that highly crystalline coatings can be achieved by heating the substrate at suitable temperatures [37].
High adhesion can be achieved, that can be further enhanced by post deposition annealing. Interestingly, mechanical properties comparable to those of plasma sprayed coatings have been reported, despite the remarkable differences in films thickness [37].
Finally, in vitro tests show good biocompatibility and bioactivity, possibly to be ascribed to the morphology of the surface [37].
3. Section II
3.1. Ion-substituted hydroxyapatite
Cationic and anionic incorporations in the HA lattice are very frequent in biological apatites [19], the most diffused being CO32 − for OH− (A-type substitution) or for PO43 − (B-type substitution), and Cl− and F− for OH− [19]. Together with these ions, also magnesium, strontium, zinc and manganese substitutions for calcium and silicates for phosphates can be found in human mineralized tissues [20], [21], [41], [42]. Ionic substitutions can alter crystallinity degree and lattice parameters on HA, thus determining its solubility [21]: opposite effects can be exerted on HA crystallinity and solubility by different additions, as in the case of carbonate groups and fluorine, where carbonates inhibit crystal growth (thus boosting solubility), that, conversely is enhanced by fluorine. This allows to obtain co-substituted HAs with tailored properties. It is noteworthy that the ability to be incorporated in the lattice and the effects that the ionic substitutions exert on HA might be remarkably different from those registered for other calcium phosphates (i.e. OCP) [19].
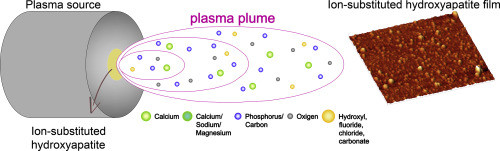
A schematic view of doping ions in substituted HAs and of the techniques by which they are deposited is in Fig. 2.
 Fig. 2. Schematic view of the main ionic additions present in the human bone and used for obtaining ion-substituted HAs and of relevant techniques used for their deposition (1.5-column fitting image).
Fig. 2. Schematic view of the main ionic additions present in the human bone and used for obtaining ion-substituted HAs and of relevant techniques used for their deposition (1.5-column fitting image).3.1.1. Carbonate ions.
Carbonates are the most diffused substituents in bioapatites [21]. In fact, biological apatite consists in a carbonated apatite, with a variable CO2concentration (3.5 up to 7.4 wt.%), also depending on the tissue that is under examination (see Table 1) [19]. Substitution of carbonate groups for phosphates, leading to the formation of B-type carbonated hydroxyapatite, is more diffused than A-type. B-type substitution results in a decrease in a-axis and decrease in c-axis of HA: unless other ions are co-substituted, the so-obtained structure is less crystalline and hence more soluble and more bioactive [19], [21], [43].
Deposition of carbonated hydroxyapatite (CHA) has been recently reported by magnetron sputtering and PLD [43], [44].
Highly adherent coatings have been obtained on different substrates by magnetron sputtering; co-sputtering of titanium allows for further increased surface adhesion [44].
As-deposited films are amorphous, though post deposition annealing allows to increase film crystallinity (up to a crystallinity degree of about 90%). Nearly stoichiometric films can be obtained (Ca/P 1.80) [44].
Sputtered CHAs are not cytotoxic; suitable adhesion, viability and proliferation of osteoblasts have been reported. Further in vitro tests using human bone marrow derived MSCs have shown mineralization after 21 days immersion in simulated body fluid (SBF), due to differentiation of MSCs in human osteoblasts-like cells (hOBs).
CHA films obtained by PLD are nearly stoichiometric too, however, Ca/P ratio increases for increasing deposition temperature (from 30 to 700 °C), and so do crystallinity and mechanical properties of the coatings [43]. On the contrary, surface morphology seems not to be affected by deposition temperature in the 30–500 °C range, whereas significant alterations are observed above 700 °C.
3.1.2. Magnesium
Magnesium is present in concentrations of about 0.4–1.2 wt.% (see Table 1), in human enamel, dentine and bone; its concentration tends to be higher during the initial steps of osteogenesis, but gradually decreases as calcification proceeds [19], [21]. Magnesium is the most diffused substitute for calcium in HAP [45] and it causes a decrease in the c-axis of the lattice, acting like a suppressor for HA nucleation and crystallization and destabilizing the HA structure. By making HA less crystalline, Mg substitution increases its solubility [19], [21], [45]. From a biological point of view, Mg is an essential element for living beings, as it is fundamental in the activity of > 100 enzymes [45]. Mg influences the metabolic activity of bone and its growth due to its influence on osteoblast/osteoclast cells and its absence can cause osteopenia or fragility [45].
Magnesium-doped hydroxyapatite (Mg-HA) coatings have been deposited by plasma spraying, magnetron sputtering and PLD, starting from targets with variable magnesium content.
Huang et al. have demonstrated that Mg-doping of HA coatings obtained by liquid Plasma spraying exhibit lower crystallinity degree and crystal size compared to un-doped HA [46].
Even if in vitro tests on sputtered Mg-HA coatings suggest high osteoblast adhesion and cells viability [47], no significant differences have been detected in cells attachment with respect to HA. In the paper, this result is ascribed to the amorphous nature of as-deposited HA and Mg-HA coatings, which, in turn, induces fast dissolution of both coatings, leading to similar biological response.
Mroz and co-workers deposited Mg-HA coatings by PLD, highlighting a progressive decrease in crystal size and crystallinity degree for increasing Mg content (0.6 up to 1.8 wt.%) [45], [48]. Interestingly, effective incorporation of Mg in the HA lattice is demonstrated even for the highest content, but distribution of Mg is not homogeneous.
When cultured with osteoblast, cells tend to aggregate over Mg-rich areas, instead of uniformly distributing on the whole surface of the coating [45].
The osteoconductive properties of Mg-HA and Mg-OCP (magnesium-doped octacalcium phosphate) coatings (Mg = 0.6 wt.%) deposited by PLD have been tested in vivo in femoral condyles of rabbit hindpaws [30]. At 6 months from surgery, both coated and non-coated Ti control implants exhibit good integration and new osteogenesis, with absence of any inflammatory or foreign body reactions. Both Mg-HA and Mg-OCP coated implants exhibit improved efficacy compared to Ti implants in terms of bone volume, length of bone-implant interface and ratio of contact length to implant circumference, as indicated micro-computer tomography (μCT), with Mg-HA performing better than OCP.
3.1.3. Fluorine
F− substitutes for OH−, thus causing a contraction of the a-axis lattice parameter, and a contraction of the c-axis, though by an order of magnitude lower than that of a-axis. Fluorapatite (FA) crystal lattice shows higher symmetry compared to HA, thus higher stability and lower solubility in acidic solutions and body fluids [21]. Thermal stability is also increased upon F− substitution. The effect on cells proliferation is still debated, as high F− content favors cells attachment; however, due to reduced Ca2 + release deriving from lower solubility, cell proliferation is expected to decrease [21]. Notably, fluorine is expected to exert a positive influence against osteoporosis [21], [49].
Deposition of FA by plasma spray has been reported starting from 1986 [50], [51] for both orthopedic and dentistry applications. As fluoride substitution for OH− makes the lattice more stable, also coatings are more crystalline and less soluble than HA. As a consequence, FA coatings generally show reduced mineralization in SBF compared to HA [49], [50].
Several in vivo experiments on plasma sprayed FA and FA/HA double layers are reported in the literature. By comparing uncoated, HA-coated and FA-coated implants, different outcomes are observed, even within the same animal model and experimental time. Some authors, evaluating short time response (3, 7, 14 and 28 days) of implantation in cortical bone of rabbits tibiae do not highlight remarkable differences between the behavior of HA and FA coated implants and, more in general, between coated and uncoated ones, response being more dependent on the recipient than on the implants themselves [52]. Other studies, instead, while not evidencing significant differences between the implants at 7 days, indicate that osteoid deposition at 14 days (when first signs of bone remodeling are noticed) is more marked for coated than for uncoated implants. In such case, both FA and HA coatings are highly crystalline, which might be the reason behind their similar behavior and for the absence of evident clues of degradation of either HA or FA coating at the end of the trial.
Implantation of dual FA/HA coatings has also been investigated on larger animal models, namely goats, in both short- and long-term studies. Short-term trials (3, 12 and 24 days) have been performed comparing FA/HA double coatings, magnetron sputtered thin amorphous CaP films and uncoated implants, all inserted in the trabecular bone of the femoral condyles of the animals [53]. At 12 days, bone formation is more significant in both coated implants, with respect to the uncoated references. Similarly, at 24 days evidences of better bone contact is registered for FA/HA coatings with respect to uncoated implants (differences detected for sputtered CaP coatings are considered not statistically significant).
Long-term results (3 months of follow up) do not highlight any significant difference between the torsional failure resistance of the plasma-sprayed and sputtered coatings. However, no residual parts of the sputtered coatings are detected by SEM analyses, due to their smaller thickness and to faster dissolution caused by lower crystallinity. On the contrary, some remaining of the sprayed coating is still visible at the end of the trial. Concerning bone formation induction, FA/HA double coatings exhibit higher bone contact but lower bone mass around the implant with respect to sputtered coatings [54]. At longer experimental time (3 to 6 months), a general increase of bone contact of coated-implants compared to uncoated ones is detected, but no significant differences are assessed between the two coatings.
Significant differences, instead, arise when implants are tested on low-density bone (such as goat maxilla), where failure occurs for uncoated and sputter coated implants (3 and 1 out of 16, respectively) but not for plasma-sprayed FA/HA [55].
The specific effect of fluoride addition, on the ability of CaP-coated implants to fill the bone-implant gap, has also been investigated: uncoated, FA-coated (highly crystalline) and HA-coated (both amorphous and crystalline) implants have been implanted in goat model and evaluated at 6 weeks from implantation. Bone apposition is found to be strongly dependent on gap size [49]: for uncoated implants, bone apposition is obtained only for gaps smaller than 0.25 mm, whereas gaps up to 1 mm can be sealed when using CaP-coated implants. However, whereas at 6 weeks from implantation the healing process appears not to be affected by coating composition and/or crystallinity, at longer experimental time (3 months), higher bone apposition is found for crystalline coatings compared to amorphous ones.
FA coatings have been deposited by right-angle magnetron sputtering (RAMS) on Ti and Ti/Si substrates [51]. FA crystallization has been found to be negligent of the substrate to which the coating is applied and, in general, fluorine reduces grain size of magnetron sputtered HA coatings [51]. Interestingly, heating of the substrate prior to deposition (at 100 °C, 200 °C, 300 °C) allows to obtain crystalline coatings also when depositing at room temperature [51]. Short deposition time (~ 5 min) leads to disordered nanocrystalline apatite phases, whereas longer deposition time allows to obtain more crystalline coatings. Pre-deposition heating allows to achieve the required crystallinity for lower thickness [51]. Finally, by pre-heating, a suitable crystallinity can be obtained at temperatures of about 300 °C, which are lower than those required for post deposition annealing or deposition temperature.
3.1.4. Strontium
Sr is present in concentrations of about 0.03–0.05 wt.%, (depending on the tissue that is considered) and is present in remarkable concentrations in regions of high metabolic turnover [1], [17], [19], [21]. Both ion exchange and ionic substitution are possible mechanisms for Sr incorporation in bone [19]. The most frequent ion-substitution is Sr for Ca, and its effect depends on the Sr dose: if Sr content is high, crystals dimensions and crystallinity in general tend to increase, while for low concentrations crystallinity and crystalline length decrease and the lattice is distorted [19]. As a result, Sr-substituted HA, for low Sr concentrations, exhibits higher solubility [19], that can be further increased if Sr is substituted to CHA instead of stoichiometric HA. Sr is also known to increase the mechanical strength of bone [21]. Sr substitutions are widely investigated due to their positive effect against osteoporosis [19], [20], [21]. In fact, initially considered noxious due to its high dosage toxicity [56], strontium has recently drawn attention in view of its biological relevance, because of the introduction of strontium ranelate as a drug; strontium ranelate, in fact, can reduce fractures due to osteoporosis thanks to its ability to boost osteoblast activity while inhibiting osteoclasts [21], [41], [56], [57].
Strontium can interact with both osteoclast and osteoblast activity by decreasing the number and the activity of osteoclasts without affecting osteoblasts, whose replication is favored. Sr effect on osteoblasts is due to their ability to interact with their Ca-sensing receptor, thus boosting cells division by interacting with the mitogenic signals in the protein kinase c/d [20], [24], [41], [42].
In vivo it prevents bone resorption and favors bone formation and regeneration, as it enhances the synthesis of both collagenous and non-collagenous proteins [17], [20], [41], [42], [56]. Similarly to what happens for crystallinity and solubility, Sr effects on bone mass and strength and in vitro performances oh Sr-HA depend on its content, in particular osteoclast proliferation decreased as Sr concentration increases [19], [41]. The optimal concentration for Sr additions has been reported to be around 3–7% [42].
Deposition of Sr substituted HAs has been reported by plasma spray, magnetron sputtering, pulsed laser deposition and Combinatorial Matrix-Assisted Pulsed Laser Evaporation (C-MAPLE).
In the case of plasma spray, powders with different mol% substitution of Sr for Ca have been deposited on titanium alloys at atmospheric conditions (atmospheric plasma spray, APS). Precursors can be prepared by following different chemical or sol-gel routes. Strontium is effectively incorporated into the apatite lattice, however it does not have remarkable influence on the morphology and crystal structure of the coating, nor on its physical properties [20], [24]. XRD patterns indicate a low crystallinity level for APS Sr-HA [41]. Studies on RF plasma sprayed Sr-HA evidence an improved adhesion to the substrate (as evaluated by ASTM C633) and a cohesive failure [24].
Mineralization studies indicate apatite formation after only 1 day of immersion in SBF and hence a possible high bioactivity of plasma-sprayed Sr-HA [41]. High bioactivity should be related to fast dissolution of the coating, caused by its low crystallinity and by the increased solubility due to Sr substitution for Ca [41]. In vitro cell studies evidence suitable biocompatibility with respect to human osteoblasts [20], [24], [41]: Sr-HA is found to promote cells adhesion, spreading and proliferation to a higher extent than stoichiometric HA, with no detrimental effects on extracellular matrix formation and mineralization [41].
In vivo studies on plasma sprayed Sr-Ha coatings implanted in dogs provide controversial results, with no clear benefits due to strontium substitutions [58].
Magnetron sputtered Sr-Ha coatings can be deposited starting from targets with variable Sr/(Sr + Ca) ratio or from separate targets (co-sputtering), either in the form of powders or coarse targets (normally disks) [1], [24], [42], [57]. Deposition on titanium, titanium alloys and silicon have been reported in the literature [1], [42], [57]. Interestingly, Sr content in the target influences sputtering deposition rate [1].
Successful incorporation of Sr in HA has been highlighted [57]. As-deposited films have low crystallinity [57], hence post deposition annealing might be desired to tailor the crystallinity of the coating. Annealing of the films, in turn, influences several parameters, including surface morphology and adhesion to the substrate, (Sr + Ca)/P ratio, presence or absence of undesired byproducts (such as possibly cytotoxic CaO and Sr(OH)2). Regarding Ca/P and (Ca + Sr)/P ratios, values around 1.18 and 1.26, respectively, have been recorded for as-deposited coatings. This is because, during sputtering, fewer Sr atoms are sputtered compared to Ca, due to the differences in their atomic weight: for this reason, Sr/(Sr + Ca) ratio after deposition tends to be lower than that of the target. The ratio can be raised by annealing (as in the case of hydrothermal treatment), that provides additional Sr, or by using multiple deposition targets [1], [42], [57].
Adhesion of the coatings to Ti substrates has been evaluated, indicating remarkable adhesion strength, improved with respect to pure HA, and cohesive failure [24]. As in the case of plasma spraying, Sr substitution enhances osteoblasts activity and inhibits osteoclasts, thus resulting in increased proliferation and differentiation of cells with respect to HA [57].
In vivo studies on magnetron sputtered Sr substituted HA (1 wt.%) implanted on rats (after 7, 12 and 16 weeks from implantation), indicate that Sr presence in the coating accelerates bone formation at the early stages, compared to both uncoated and HA-coated implants, and enhances mineralization with respect to uncoated implants [59].
Pulsed laser deposition of strontium substituted hydroxyapatite (Sr-HA) [17] and carbonate apatite (Sr-CHA) [56] have been reported. High temperature deposition and/or post deposition annealing can be used to obtain crystalline coatings [17]. Powders have been prepared by mixture of commercial powders with or without subsequent calcinations. Thin (200 nm up to 1 μm) films of Sr-HA have been successfully obtained [17], [56] and Sr incorporation in the apatite lattice can be clearly determined by EDS; however, as for plasma sprayed coatings, the presence of strontium does not remarkably influence the morphology of the coatings [17].
As a consequence of Sr incorporation, osteoblast adhesion is improved since the early phases, cell adhesion increasing for increasing Sr content [17]. As for cells adhesion, their proliferation and viability increase for increasing Sr content, and so do osteoblast activity and differentiation [17]. Spreading and flattening of osteoblasts are more marked on Sr-HA than on HA coatings. Osteoclast proliferation, instead, is inhibited, mostly for high Sr content (3–7%).
Strontium-doped octacalcium phosphate has also been obtained by pulsed laser evaporation, as well as composites of hydroxyapatite and drugs (in the specific case strontium and zoledronate), thus confirming the possibility to deposit both metastable phases and/or drugs [34], [60]. Ion doping with Sr improves OCP behavior by enhancing cells proliferation, activity and differentiation. Mixtures of strontium zoledronate and HA favor osteoblasts adhesion and boosts proliferation compared to plain substrates.
No further data are present in literature about in vivo evaluation of Sr-HA, to the authors' best knowledge.