1. Introduction
Increasing uses of petrochemical based plastics have created environmental and ecological difficulties due to the non-degradability of these materials. As a consequence, aliphatic polyesters such as polylactide (PLA), poly(ε-caprolactone) (PCL) and poly(3-hydroxyalkanoate) (PHA) provide a more environmentally friendly alternative to their synthetic counterparts due to their biodegradability [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. They have attracted more and more attentions aiming to at least partially replace the non-degradable plastics. These polyesters can be prepared by different methods, i.e.chemical polymerization or by biotechnology. PLA and PCL are obtained by ring opening polymerization (ROP) of lactide or ε-caprolactone respectively [13], [14], [15], [16], [17], [18] whereas PHA were produced by enzymatic polymerization [19], [20], [21], [22], [23], [24]. Although the most well-studied PHA is the poly(3-hydroxybutyrate) (PHB), over 140 constitutive monomer units have been investigated [25]. Three main types of PHAs are generally distinguished, the short-chain length PHAs (scl-PHAs) that possess alkyl side chains having up two carbon atoms, the medium-chain length PHAs (mcl-PHAs) that displays between three and eleven carbon atoms on their side chains, and unsaturated PHAs that contains alkene groups in the side chains (Fig. 1). Concerning the degradability, PLA and PCL generally degraded by a backbone hydrolysis of the ester linkage and the degradation products are naturally occurring bioabsorbable metabolites [7], [8], [16], [26], [27], [28], [29], [30], [31]. A special interest of PHAs is their direct enzymatic biodegradability due to the presence of depolymerase able to entirely degrade natural PHAs into CO2 and H2O in aerobic conditions [32], [33], [34], [35], [36], [37], [38], [39], [40], [41].
 Fig. 1. Chemical structure of aliphatic polyesters.
Fig. 1. Chemical structure of aliphatic polyesters.Many efforts have been recently made to improve the properties and make these linear thermoplastic polyesters more competitive against petroleum based polymers. For this, polymer blends, copolymerizations, composites and network formation have been extensively studied [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62]. Among the different strategies, the formation of crosslinked structure is of much interest since it offers the possibility to increase both the mechanical and thermal properties of these polymers.
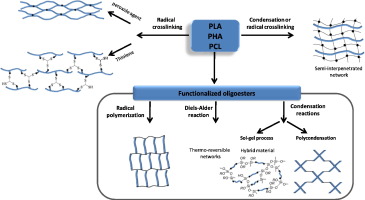
In this context, this chapter aims at reviewing the developments on the chemical crosslinking of polyesters. We can distinguish two different ways for designing the networks depending on the molar masses of the polyesters. Networks can be directly obtained from native polyesters (Fig. 2, way A). The way A describes the formation of networks in presence of a radical initiator (A1), a multifunctional co-agent as trithiol (A2) or the formation of semi interpenetrating network in which the polyester is embedded in a tridimensional network (A3). In the way B (Fig. 3), the formation of the networks first requires the synthesis of oligoesters with controlled architecture [63], [64], [65], [66], [67] that are further used in radical polymerization (B1), condensation reactions (B2) or sol-gel condensations (B3). The comparison of the different chemical strategies used to prepare networks based on PLA, PCL or PHA are detailed (Schema 1).
 Fig. 2. Chemical methods used for the crosslinking of polyesters by using native polyesters A1) in presence of peroxide initiator A2) in presence of trithiol agent A3) by forming semi-interpenetrating network.
Fig. 2. Chemical methods used for the crosslinking of polyesters by using native polyesters A1) in presence of peroxide initiator A2) in presence of trithiol agent A3) by forming semi-interpenetrating network. Fig. 3. Chemical methods of crosslinking by using telechelic oligoesters (Way B).
Fig. 3. Chemical methods of crosslinking by using telechelic oligoesters (Way B). Schema 1. Comparison of the different chemical methods used to prepare networks based on PLA, PHA or PCL.
Schema 1. Comparison of the different chemical methods used to prepare networks based on PLA, PHA or PCL.2. Networks based on native polyesters using radical initiator
Crosslinking reactions of polyesters in presence of radical initiators have been developed to create polymer radicals on the polymer backbone. The combinations of the radicals give branched or crosslinked materials with better physical and mechanical characteristics [68], [69], [70], [71]. Among the different initiators (Table 1), dicumyl peroxide, DCP was the most efficient due to its relatively high hydrogen abstraction efficiency. The peroxide efficiency depends on two criteria [72], [73]. The first criterion is the amount of peroxide required to the crosslinking reactions and the second is whether the free radicals cause more chain scissions than crosslinking reactions. The chain scissions are considered as a drawback because they are responsible of the loss of mechanical properties. In this context, the reactive extrusion process of polyesters using organic peroxides in the melt state is known to cause severe chain scissions [74], [75]. An increase in the polymolecularity index from 2.8 to 6.0 was observed when PHBHV was cured with 0.3 wt% DCP at 160 °C [76]. To counteract this negative effect, crosslinking was conducted in the presence of multifunctional co-agent as a means to introduce long-chain branching (Table 1). Co-agent modified PLA and PHA materials showed strain hardening and high thermal stability [74], [77].
Table 1. Chemical structure of different peroxides and multifunctional co-agents used during radical crosslinking.

Concerning the PHAs, the poly(3-hydroxyoctanoate-co-3-hydroxyundecenoate (PHOU) is an unsaturated polyester that contains double bonds in the side chains (Fig. 1). The addition of multifunctional co-agents such as ethylene glycol dimethacrylate and a triallyl cyanurate (Table 1) improved the crosslinking reactions and reduced the chain scission for the saturated or unsaturated polymers. However, the vinyl specific peroxides initiated the homopolymerization of the co-agent [78]. The choice of a proper initiator with favourable decomposition rate under the experimental conditions is important in the process of extrusion to improve the crosslinking degree of the materials.
The peroxide treatment was also successfully employed to enhance the compatibilization between polymers [79], [80], [81]. The role of DCP is to initiate free radical reactions between the different polymers to obtain grafted copolymers and crosslinked networks that significantly enhance the compatibility of the blends. Reactive blending of PHO/PLA has been performed to produce in situ compatibilized blends with improved properties [77] using DCP and a co-agent. The modified PHO/PLA blends had a finer morphology suggesting a compatibilization effect possibly arising from copolymer formation at the interface [77]. In addition, in order to improve the compatibilization between PCL and epoxidized natural rubber (ENR) (Table 1), Mishra et al. have synthetized PCL/ENR blends (70/30 or 50/50) by melt blending in presence of DCP. Biodegradable thermoplastic elastomers were obtained with higher tensile strength and elongation at break and fairly good elastic recovery as well as melt processability [82]. A compabilization between PHBHV, poly(butylene adipate-co-terephthalate) (PBAT) and ENR was also achieved during melt processing in the presence of DCP. Both, DCP and ENR play dual positive roles in the blend and composites, as they act as toughening agents and compatibilizers [83]. Using this process, green composites based on PHBHV, PBAT, ENR and Miscanthus fibers were prepared by reactive extrusion in presence of DCP to ensure good interfacial adhesion between the different phases of the biocomposites. Moreover, thiol-ene chemistry that involves the addition of a thiol group on a carbon − carbon double bond [84] has been employed in various applications. Ishida et al. prepared a novel material by combining the PHOU, a thiol functionalized polyhedral oligomeric silsesquioxane (POSS) and a tetrathiol crosslinker [85] (Fig. 4) to obtain an elastomeric material with excellent shape fixing and recovery. The crosslinking of poly(3-hydroxybutyrate-co-3-hydroxyundecenoate) (PHBU) was also accomplished by using a pentaerythritol tetrakis(3-mercaptopropionate) as crosslinker agent. The results have shown a unique combination of improved strength and flexibility of the polyester [86].
 Fig. 4. Synthetic scheme of PHOU-POSS network85.
Fig. 4. Synthetic scheme of PHOU-POSS network85.3. (Semi) interpenetrating polymer networks, (semi)- IPNs
IPNs are defined as the combination of two independently crosslinked polymers. Such structures have been widely studied as they may develop microphase separated co-continuous morphologies due to their particular interlocking framework. Free radical polymerization or condensation processes were used to prepare IPNs that provide an alternative response for the compatibilization of incompatible polymers and to improve the thermomechanical properties of the materials [87], [88], [89], [90], [91]. Semi- IPNs in which PLA or PCL oligomers were embedded within a functional vinylic polymer network could be effectively used as nanostructured precursors to novel functionalized (meso-)porous networks [92], [93]. Indeed, the extraction of un-cross-linked oligoesters from functional semi-IPNs (Fig. 5) constituted a straightforward and versatile strategy for engineering (meso-)porous cross-linked polymers. In this regard, the utilization of PCL/poly(methyl methacrylate-co-methacrylic acid)-based semi-IPNs afforded COOH-functionalized nano-porous networks [93]. PCL/polystyrene (PS) semi-IPNs were also designed for the development of porous capillary monoliths [94], [95].
 Fig. 5. Design of porous material by oligoester extraction from PLA/PMMA semi-IPNs.
Fig. 5. Design of porous material by oligoester extraction from PLA/PMMA semi-IPNs.Hydrogels are polymeric materials frequently employed for medical deviceapplications, but their poor mechanical properties have restricted their use. To address this limitation, semi-IPNs based on linear PCL distributed within reticulated poly(2-hydroxyethyl methacrylate), PHEMA hydrogel were prepared by free radical polymerization in presence of ethylene glycol dimethacrylate (EGDMA 1% w/w) [96]. In comparison to PHEMA, the semi-IPN exhibited greater tensile properties due to the discrete distribution of PCL into the hydrogel matrix. Furthermore, the in vitro resistance to microbial adherence is an advantageous property suggesting that the PCL/PHEMA semi-IPNs represent an interesting platform for the design of medical devices that possess enhanced physicochemical and biological properties.
Reinforcement of PLA properties was achieved by introducing 5% of semi-IPN prepared by reaction between PCL-diol, PCL-triol and toluene diisocyanate [97]. Shibita et al. had recently prepared a semi-IPN by reaction between hydroxyl-terminated 4-arm-shaped ε-CL oligomers and methylenediphenyl-4,4′-diisocyanate (MDI) in the presence of PLA [98]. According to the arm length of the precursor, different properties were obtained from amorphous to semi-crystalline materials. In a view to improve osteoconductivity of the biomaterials, hydroxyapatite HAP has been used. A semi-IPN (Linear-PCL/network-PCL)/hydroxyapatite (HAP) was prepared in three steps including i) the synthesis of a PCL-triol ii) the preparation of linear-PCL/HAP nanocomposites and iii) the crosslinking of PCL-triol with hexamethylene diisocyanate [99]. The semi-IPN was expected to produce a material with relatively high mechanical strength and desirable degradation rate. The presence of the amorphous network-PCL decreases the crystallinity degree and thus, increases the degradation rate. The introduction of linear-PCL greatly enhances the mechanical strength of the structure. The semi-IPN (linear-PCL/network-PCL)/HAP (15.8%) exhibited the higher tensile modulus due to the change from amorphous of the network-PCL to crystalline state when the HAP content increases to 15.8%.
IPNs with hydrophilic and good shape-memory properties for biomedical applications [100] were prepared using poly(ethylene glycol dimethacrylate) (PEGDMA) and poly(lactide-co-glycolide) (PLGA)/isophorone diisocyanate (IPDI). The chemical crosslinking points acted as the fixed phase to memorise the original shape, whereas the amorphous domains of miscible PLGA and PEG acted as the reversible phase. The switch temperature of the IPN could be adjusted at around body temperature for potential clinical applications.
4. Networks based on telechelic oligoesters
The preparation of these networks required two steps: first the synthesis of the oligoesters and then the radical polymerization of these oligoesters (Fig. 3, B1) or their condensation (Fig. 3, B2). PCL and PLA macromonomers are prepared in a two-step process. The macromomers are obtained by ROP of CL and LA respectively in presence of multifunctional initiators [63], [64], [65], [66], [67]and then the functionalization proceed by a condensation reaction. In the case of PHAs, various methods have been developed for preparing, in one step, telechelic PHAs having low molar mass [101], [102], [103], [104], [105], [106], [107], [108]. Among them, acid catalysis methanolysis, thermal treatment and transesterification reactions were intensively studied. Different oligoesters are used to prepare crosslinked polyesters (Fig. 3). Whatever the type of reaction used to carry out the crosslinking, the common point to these networks lies in the fact that the length of the crosslinking is directly linked to the molar masses of the oligoesters used. Although these processes are longer than the radical reactions in presence of peroxides, the structures of the network are better defined.
4.1. Radical polymerization of oligoesters
These networks can be obtained by radical polymerization of oligoesters bearing (meth)acrylate end groups (B1). The oligoesters have at least two (meth)acrylate end groups and constitute the crosslinking agents. Polymerization of the double bonds leads to the formation of an acrylic skeleton with polyester crosslinks. These materials offer the possibility to tailor their properties through the chemical structure, crosslinking density and the length of theses macromonomers. Di, tri or tetra functional initiators having hydroxyl groups have been used to initiate the ring opening polymerization of LA or/and CL to obtain bifunctional oligoesters, 3-arm and 4-arm oligoesters respectively. Condensation reactions with (meth)acryloyl chloride were then performed to introduce acrylate or methacrylate terminal end groups that have been further polymerized (Table 2) [109], [110], [111], [112], [113], [114]. PLA macromonomers with oligo(ethylene glycol) core (Table 2, PLA macromonomer) were photopolymerized by using visible light in the presence of photoinitiator to prepare degradable hydrogels used in orthopaedic applications [109]. The mechanical properties depended on both, the crosslinking density and the molar ratio of lactic acid and ethylene glycol units in the functional macromonomers. Macromonomers having high molar masses lead to rubbery networks, whereas macromonomers with low molar masses can be used to synthesize highly crosslinked glassy networks and higher glass-transition temperature. Methacryloyl terminated poly(LA-co-CL) diethylene õglycol based oligomers (Table 2, P(LA-co-CL) macromonomer) were synthesized to introduce both LA and CL in the macromonomers. The properties were correlated to the content of LA and CL, and it was shown that the presence of caproic units involved a decrease in the Tg value and an increase of hydrophobicity of the networks due to its long alkyl segments of PCL. Nevertheless, cell attachments were not affected by the change in the network composition and these biodegradable scaffolds with controlled macroscopic architecture have shown their great potential for tissue engineering applications [111].
Table 2. Multifunctional macromonomers based on oligoesters with (meth)acrylate end groups.

The effect of the incorporation of soft segment as trimethylene carbonate, TMC, into the PLA backbone was expected to increase chain mobility, thus eliminating brittleness of PLA. Poly(D,L-LA-co-TMC)-based networks (Table 2, Table 3-arm P(LA-co-TMC) macromonomer) were first prepared by ROP of LA and TMC using trimethylol propane (TMP) as initiator. The trifunctunctional prepolymer was reacted with methacryloyl chloride to give 3-arm methacrylate macromonomers and the crosslinking was achieved by using 2-butanone peroxide under thermal activation [112]. Tensile modulus, ultimate strength, and Tg increased with increasing LA content. Networks containing higher contents of LA, were strong and fairly rigid whereas networks containing lower contents of LA, showed a higher elongation to break. Another strategy consisted to incorporate fumarate units into the PCL backbone. The PCL-Fumarate macromonomer (PCLF) was synthetized through the polycondensation of PCL diol and fumaryle chloride. The photo-crosslinking of PCLF was initiated by UV light in presence of Irgacure 819. It was shown that both the crosslinking density and the molecular weight of the polymer influence the gel fraction, the melting temperature, and the crystallinity of the PCLF networks. By combining chemical crosslinks and physical associations of the crystalline domains, the crosslinked PCLF with a PCL molar mass of 2000 g ∙ mol− 1 presented high rheological and mechanical properties at 37 °C [114].
Table 3. Structures of crosslinking agents used during condensation reactions.

4.2. Networks prepared by condensation reactions
The networks are essentially obtained by reaction between telechelic oligoesters and diisocyanates, diepoxides or triethoxysilanes units (Fig. 3, B2). The first step consists therefore in synthesizing the telechelic oligoesters having a strictly controlled molar mass. After crosslinking in the presence of a multifunctional agent, the critical molar mass between network crosslinking nodes is directly related to the molar mass of the oligoesters that is a real advantage over free radical crosslinked networks.
Thus, this process offers the possibility to controlled macroscopic architecture of the scaffold and to obtain materials with increasing properties. A series of Shape Memory PolyUrethanes SMPUs were successfully achieved by condensation reaction between oligomeric PCL-diol, butanediol, dimethylol propionic acid and diphenylmethane diisocyanate (MDI) or toluene diisocyanate (TDI) [115], [116] (Table 3). The crosslinking decreased the crystallization of the materials but they have enough elasticity to recover to their original shape when they are heated. Polyurethane networks for medical applications were prepared through the synthesis of polyester-based polyurethanes via the reaction of hydroxy-terminated polyester and polyfunctional isocyanates [117], [118]. Storey et al. prepared amorphousbiodegradable polyurethane networks from difunctional hydroxy-terminated poly(ε-caprolactone-co-δ-valerolactone) P(CL-co-VL) oligomers and triphenylmethane triisocyanate (TTI) [117]. It was demonstrated that the modulus of the networks decreased while the strain at break increased with the molar mass of the prepolymer due to the increase of the molar mass between the crosslinks. Trifunctional hydroxy-terminated polyesters were prepared by triol-initiator, ring-opening polymerization of D,L-lactide, ε-caprolactone and/or trimethylene carbonate and they were further used as precursors for the synthesis of bioabsorbable polyurethane networks by reaction with toluene diisocyanate [118]. The use of aromatic diisocyanates or aliphatic diisocyanates (Table 3) are limited due to the toxicity of their ultimate hydrolysis product. To overcome this drawback, nontoxic bioabsorbable PU were prepared by using l-lysine diisocyanate (LDI) [119], [120].
Degradable networks with only ester linkages were also prepared by polycondensation of PCL-diols (from 530 to 2000 g ∙ mol− 1) and multifunctional aliphatic carboxylic acids. Materials based on PCL-diol with Mw of 1250 g ∙ mol− 1 showed good elastomeric properties with high ultimate elongation (540–590%), and low Young's modulus (2.5–3.3 MPa). Another way to obtain crosslinks based on ester linkages is to use the dicarboxylic end groups of PCL and the epoxide groups of epoxidized natural rubber [121]. Crosslinked networks were formed during thermal molding of the blends, which is supposed to occur via intermolecular reactions between the epoxide groups of ENR and carboxylic groups of PCL. The degree of crosslinking and crystalline melting transition temperatures depended upon both the blend compositions and the molecular weight of the PCL segment. It can be seen that the gel content increased with increasing content of ENR. Moreover, at the same composition, the gel content increased with the decrease in the molecular weight of PCL.
4.3. Hybrid networks
Hybrid materials were successfully produced by sol-gel process that consists in two-step hydrolysis-condensation reaction of metal alkoxides (Fig. 3, B3). The interest of those materials is the possibility to tailor the solid-state properties in relation to the nature and content of the constitutive components. Inorganic-organic hybrid networks based on biodegradable polyesters such as PHA and PCL have been prepared [44], [122], [123], [124], [125], [126], [127], [128]. PCL and PHA were first end-capped with triethoxysilane TEOS and then used to design hybrid network by sol-gel process [123]. It was demonstrated that triethoxysilane end groups were more efficient than hydroxyl end groups in incorporating polyesters chains into silica network [124]. A triethoxysilane functionalized PCL oligomers were used in the preparation of new transparent hybrid materials [122], [124]. PCL remained completely amorphous because it was intimately incorporated into the networks. The organic PCL and the inorganic SiO2 components were associated by covalent silsesquioxane domains but also by hydrogen bonds. Biodegradable PHA-silica hybrid network was synthesized by sol-gel technique under acidic, basic conditions or UV-curing [44]. The UV treatment was the more efficient and rapid route to prepare the PHA-silica network hybrid and promote higher degree of condensation compared to the conventional sol gel processes using acidic or basic catalysis. The lipase enzymatic degradation produced a decrease of the weight loss of PHA (14%) after 10 days proving that this novel material may be envisioned as a novel biodegradable material for tissue engineering application.
5. Reversible networks
In order to cumulate advantages of crosslinking while preserving the possibility of re-processing, the thermo-reversible Diels-Alder (DA) reaction can be used for the networks formation (Fig. 2, B3). One of the most relevant aspects of the Diels-Alder reaction is its thermal reversibility via the retro Diels–Alder reaction [129]. DA has been studied to crosslink PLA or PCL networks with remarkable shape-memory properties [130], [131], [129], [132], [133], [134], [135], [136], [137]. The polymers were used as soft segments and the reversible crosslinks based on the DA adducts defined the permanent shape at low temperature and allowed shape recovery above the retro-DA temperature. The networks were successfully synthesized from star-shaped PCL or PLA-based precursors bearing furan or maleimide end-groups [130], [131], [132], [138]. The thermo-reversible networks were obtained through furan-maleimide adducts formation (Fig. 6) with excellent memory properties. The mechanical properties of these materials depended on the structure of the precursors. Indeed, four-arm star-shaped PCL bearing furan or maleimide functions with oligomers chain of n = 10 were semi-crystalline and showed higher tensile strength and modulus and those composed of oligomer chains of n = 5 that remained amorphous [131]. The materials showed thermo-reversible sol-gel transition in response to repeated heatings [133], [134].
 Fig. 6. Thermo-reversible network prepared with maleimide linker and furan modified-polymer.
Fig. 6. Thermo-reversible network prepared with maleimide linker and furan modified-polymer.Biocompatible gels were prepared by blending furan-terminated diblock copolymers of F-PEG-PLA and triblock copolymers of PLA-PEG-PLA synthesized by ROP of L and D-lactide using furanylated PEGs as initiators. Crosslinking was achieved by two mechanisms: physical crosslinking by stereocomplex formation between PLLA and PDLA blocks and DA reaction between furanyl functions present on PEG chains and 1,8-bis(maleimido)diethylene glycol agent. The resulting sol-gel systems demonstrated their potential as scaffolds in the tissue engineering field [139].
Although these networks combine attractive shape memory behaviour, possible melt-processing and recyclability, the multiple-step preparation could limit their potential use on an industrial scale. Djidi et al. described a one-step facile and efficient approach to prepare thermally-reversible PLA network by simultaneous coupling dihydroxy-telechelic PLA with well-controlled molar masses, a dihydroxy-Diels-Alder adduct and multi-alcohols with diisocyanate [135]. The thermo-sensitive networks obtained by DA cycloaddition are promising candidates in supramolecular engineering and for preparation of thermo-retractable films applicable in packaging.
6. Conclusion
This chapter aims to give an overview of the chemical ways to prepare crosslinked aliphatic polyesters to achieve materials with improved mechanical and/or thermal properties over the natural polyesters. Different approaches were used to develop the networks leading to various structures whose properties can be adapted to the intended applications. The networks can be obtained by radical crosslinking, condensation reaction or thermo-reversible Diels-Alder reaction from native polyesters with high molar masses or by using functionalized oligoesters. Radical reactions are generally faster but lead to less controlled structures and secondary reactions such as scission of macromolecular chains may occur. This type of crosslinking can be carried out without solvent, directly in the solid state by mixing the polymer and the initiator. This process is an efficient method to compatibilize different polyesters. Another method to compatibilize incompatible polymers consists in the formation of semi interpenetrating networks. The networks obtained by condensation reactions require two steps but the structure is better defined because the molar mass between the crosslinking nodes depends closely on the length of the oligoesters. Wide range of renewable materials with higher added value can be obtained including shape memory polymers, polyesters with controlled porosity, hybrid networks and those which have the particularity of being reversible. The resins formed are crosslinked and therefore it is necessary to study very precisely the degradability of these new structures and the next challenge will be to prepare networks that will be totally degradable.