1. Introduction
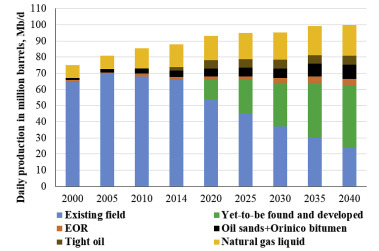
The consumption of crude oil is expected to contribute 26% of the world's energy until 2040 (I. E. A, 2014). Although the average recovery for major oil reservoirs is only between 20% and 40% of the resource present in mature reservoirs, and about half of the unrecoverable oil (∼200 billion barrels) in the United States is at reasonable depths where enhanced oil recovery (EOR) techniques could be applied. Such techniques improve the amount of oil extracted and offset production declines from mature reservoirs, and by doing so help to meet the growing demands and constraints on energy resources. They also overcome some of the difficulties associated with discovering and developing new oilfields. As the production of existing oil fields continues to decline, EOR has the potential to offset this decline (Fig. 1). The IEA forecasts that the global oil produced from EOR will expand to about 4 Mb/d in 2040 (I. E. A, 2015). However, history shows that it is only the cost-effective EOR techniques that tend to be commercially applied during periods when oil prices have been sufficiently high to render these techniques economically attractive (Lake et al., 2014; Koottungal, 2014).
 Fig. 1. Global production of oil and gas in World Energy Outlook (2015) by IEA (I. E. A, 2015).
Fig. 1. Global production of oil and gas in World Energy Outlook (2015) by IEA (I. E. A, 2015).Chemical and thermal EOR projects made a decreasing contribution to the world oil production from 1986 to 2004 (Fig. 2). However, as reported by Alvarado and Manrique (2010) (Alvarado and Manrique, 2010), interest in chemical flooding projects in US and Canada grew from 200 to 2010 (Fig. 2), especially in heavy oil and offshore oilfields. Gas injection EOR projects also grew from 2000 to 2010 driven in part by a greater uptake of CO2-injection projects. The numbers of EOR projects (including thermal, chemical and gas injection recovery) are highly sensitive to production costs and crude oil prices. Despite some delay in investors reacting to shifts in crude oil prices (almost 3–4 years' time lag), the initiation and persistence of EOR projects numbers shows a strong positive correlation with crude oil prices, reflecting the willingness and confidence, or otherwise, of investors regarding EOR projects. Hence, the profit-motivated oil companies are constantly seeking more advanced and cost-effective EOR technologies that can sustain profitability in volatile-oil-price markets (Muggeridge et al., 2014). During periods of low oil prices there is increased competition for available sources of finance and only the most profitable projects tend to be sanctioned. In such circumstances, it is particularly desirable to improve the efficiency of enhanced oil recovery by minimizing its potential risks and costs, and by providing sustainable production improvements.
 Fig. 2. The evolution of EOR projects with changes of oil price (US$/barrel) in the United States from 1970 to 2010.
Fig. 2. The evolution of EOR projects with changes of oil price (US$/barrel) in the United States from 1970 to 2010.Enhanced oil recovery (EOR) is the implementation of various techniques for increasing the efficiency of crude oil recovery from an oil field. It includes enhanced oil recovery (thermal recovery, gas injection and chemical flooding), secondary recovery (water and gas injection), hydraulic fracturing and the drilling of horizontal and multi-lateral wells (Walsh and Larry, 2003). Traditionally, the oil and gas industry has not linked improved oil recovery techniques with formation damage in high-oil-price environments, although formation damage is frequently a consequence of the implementation of EOR. Civan (2015) summarized the relevant causes of formation damage and its consequences, and various approaches and techniques for formation assessment, control and remediation (Civan, 2015). Formation damage refers to the impairment of physical, chemical or mechanical properties of petroleum-bearing formation, which primarily involves permeability damage during various processes of oil and gas recovery. The changes of chemical-physical-thermodynamic conditions associated with EOR techniques can result in various types of formation damage, such as, water and gas bubble blockage, fines/sands migration, fluids-rock incompatibility, organic and inorganic precipitation and deposit, alterations of pore surface properties, pore structures and mechanic characteristics. In some cases, formation damage may itself lead to some benefits that enhance oil recovery, for instance, improving sweep efficiency thorough selected blockage of high-permeability regions caused by fines migration (Yuan and Moghaloo, 2017; Bedrikovetsky et al., 2011a); however, more usually, it reduces the efficiency of secondary and tertiary recovery from the reservoir and impairs well injectivity and/or productivity dramatically (Bedrikovetsky, 1993). Hence, in this work, formation damage will not simply be addressed as a “problem”, but rather as an “issue”, reflecting its potentially positive and negative effects on well productivity and economic performance.
Porter (1989) stated that it is better to avoid formation damage in advance than to make tremendous efforts to remediate it after it has occurred (Porter, 1989). However, different types of formation damage may be realized by different EOR methods, meaning that studies of a formation's susceptibility to specific type of damage have limited practical value if conducted without consideration of the associated engineering activities which may, or may not, lead to that specific type of damage. It is beneficial for oil operators to understand formation damage specific to various EOR approaches, because it enables them to maximize oil recovery both technically and economically by optimizing EOR techniques in different types of reservoir paying due consideration to relevant formation damage issues. As an extension and short summary of our previous work (Yuan and Wood, 2018), the intention here is to provide a better understanding of the pros and cons of formation damage as it occurs during the application of diverse EOR techniques. We also provide guidelines on how to optimize the design of EOR projects through controlling or taking advantages of formation damage.
Rather than concentrating on the fundamental theories related to individually to EOR techniques and formation damage, here we link the series of formation damage issues with various EOR techniques in different types of sub-surface reservoir, including conventional and unconventional oil/gas reservoirs. To do that, a comprehensive investigation is conducted on numerous published modelling and simulation data, laboratory experiments and case studies. The results are applied to characterize risks and opportunities of formation damage, and propose a summary of new advanced technologies and methodologies to control formation damage issues during EOR processes in both conventional and unconventional reservoirs. Moreover, because the extent and occurrence probability of formation damage issues remain very uncertain in EOR cases, an integrated risk and opportunity assessment and management framework is proposed to assist the search for optimum outcomes when applying improved oil recovery techniques.
2. Formation damage during EOR: causes, characterization and control
In the most general sense, formation damage can be defined as the various damage mechanisms affecting the properties of a reservoir formation (matrix and fractures) and through which the transport efficiency of multi-phase fluids (oil, gas, water, particles, droplet, foam and emulsion) is altered. It is usually diagnosed as the changes of well performance in terms of well injection/productivity and oil recovery (Harper and Buller., 1986). The major damage mechanisms can be categorized into four types, such as, mechanically, chemically, biologically and thermally induced (Faergestad, 2016). Among them, the types of chemical damage mechanisms (Bennion, 2002) can be further classified as, 1) fluid-fluid incompatibility (such as, inorganic scale deposition, organic asphaltene deposition, foam/emulsion blockage, and hydrate formation); 2) rock-fluid incompatibility (such as, clay swelling/deflocculation, wettability alteration, and ionic/surfactant/polymer adsorption). The mechanically induced damage mechanisms (Sharma et al., 1992; Bedrikovetsky et al., 2011b) mainly include fines/sands or any other types of particles migrating through the porous/fractured media, phase trapping caused by high capillary force in multi-phase flow (Mirzaei-Paiaman et al., 2012), and rock compaction or dilatation caused by pressure changes. Temperature change can also lead to the dissolution of minerals, transformation of minerals, and temperature-dependent wettability alternation due to the loss of thermal equilibrium (Romanova et al., 2015). In addition, the biological activities of bacteria in reservoirs (Ezeuko et al., 2013) can cause the souring of crude oil, erosion of minerals, and blockage of pore-throats (with positive and/or negative impacts).
During the application of different improved oil recovery technologies, the changes of physical, chemical, thermal-electrical, mechanical and biological environment may vary. The following sections of this review link the mechanisms of formation damage with diverse EOR techniques in conventional sandstone/carbonate reservoirs (Fig. 3).
 Fig. 3. The potential formation damage mechanisms linked with specific improved oil recovery techniques and types of reservoirs.
Fig. 3. The potential formation damage mechanisms linked with specific improved oil recovery techniques and types of reservoirs.2.1. Low-salinity water flooding (LSWF) for oil recovery: from novel to mature
LSWF impacts on reservoirs were first observed when Martin (1959) discovered the increase of oil production while manipulating the salinity of injected water (Martin, 1959). Subsequently, extensive theoretical and experimental research has focused upon the mechanisms responsible this phenomenon. The uptake of LSWF in a wide range of reservoirs is significant and rising for several reasons: (1) low capital expenditure and minimal incremental operating costs for those reservoirs already developed with water injection facilities and wells; (2) ease of injection into most oil reservoirs; (3) high incremental recovery gains for light to medium gravity oil reservoirs; (4) reduction in the scaling and corrosionof wellbore tubulars and surface-water-handling facilities and, (5) potential avoidance of reservoir souring (Collins, 2011).
LSWF has proved to be an effective EOR method for both secondary (initial water flooding) and tertiary (residual oil) modes (Gamage and Thyne, 2011). Despite its success in core experiments and field-scale pilot studies, the multiple mechanisms induced by LSWF impacting reservoirs and oil recovery remains poorly understood and controversial (Al-Shalabi and Sepehrnoori, 2016; Atthawutthisim, 2012). This is particularly so for the induced formation damage associated with the technique. The major damage mechanisms associated with LSWF (a few with positive consequences for oil recovery) are described in the next sections.
2.1.1. Formation damage mechanism associated with LSWF enhanced oil recovery
(1) Clay swelling, fine particles migration, detachment and straining
Clay swelling is a widely recognized phenomenon related to formation damage and can have significant negative impacts on reservoir permeability and fracture conductivity, as well as on the effectiveness of LSWF (Sanaei et al., 2016). Mohan et al. (1993) indicated that water sensitivity of various reservoirs to LSWF is highly dependent on the composition of the clays, and the total clay content and distribution of clays grains within a formation, such as swelling (smectite) and non-swelling (kaolinite and illite) clays (Mohan et al., 1993). To study clay detachment and pore blocking, Song and Kovscek (2016) constructed clay-functionalized, etched-silicon micromodels to visualize directly the mobilization of clay-fines in both kaolinite-rich and montmorillonite-rich system (Song and Kovscek, 2016). Fines migration that preferentially accumulate and block the high-permeability channels is very pronounced in kaolinite-rich systems, but can bring improvements of oil recovery by positive adjustments to reservoir sweep efficiency. However, the mobilization of swollen montmorillonite typically damages permeability without increasing oil recovery under LSWF condition.
Fine particles in reservoirs exist in mechanical equilibrium balancing the drag, lift, electrostatic and gravitational forces acting upon them. By weakening the electrostatic forces LSWF can, in certain conditions, cause the loss of mechanical equilibrium leading to fine particles being dislodged from the pore linings and dragged along with flowing fluids into pore throats. The formation damage mechanisms related to fines migration (Fig. 4) include fines surface deposition and/or attachment, fines bridging or straining into pore-throats, fines internal cake formation, and fines infiltration sedimentation (Nguyen et al., 2007; Yuan et al., 2016).
 Fig. 4. Formation fines migration, detachment and straining phenomenon caused by LSWF, and the mechanism of nanoparticles to mitigate fines migration.
Fig. 4. Formation fines migration, detachment and straining phenomenon caused by LSWF, and the mechanism of nanoparticles to mitigate fines migration.Tang and Morrow (1999) defined the release of fines by the invasion of low-salinity water, and identified improvements of sweep efficiency in reservoirs by the selective fines blockages of high-permeability zones as a mobility controlmechanism (Tang and Morrow, 1999). Fines migration associated with LSWF may carry small amounts of residual oil as a consequence of the detachment of oil-coated particles from rock grains, which thereby improve oil displacement efficiency (Aksulu et al., 2012). However, fines migration and it associated particle size exclusion effects typically results in severe damage to reservoir permeability near wellbores causing the decline of well injectivity (and productivity in case of production well). In particular, during LSWF, high-magnitude pressure drops realized near the injection/production wells can exaggerate the problems of fines migration. The best strategy to avoid fines migration is to keep the fines stagnant at their original location/sources. This can be achieved by either limiting flow rates (to less than the critical rates) or somehow enhancing the rock capacity to retain the free particles.
A number of fines-migration mitigation techniques are employed. Different types of acid systems have been developed to remove the formation of fines plugged in the near-wellbore region, gravel packs, and/or sand-control screens under various downhole conditions (Khilar and Fogler, 1998). The distance between two structural layers in clay minerals depends upon the exchangeable cations (salts), the formation fluid composition, and the composition of clay minerals. Amorim et al. (2007) studied the performance of various salts to inhibit clay swelling on various clays, including samples from the Calumbi Formation (Sergipe-Alagoas Basin, NE Brazil), and determined the CaCl2concentrations most effective in inhibiting clay swelling (Amorim et al., 2007). For example, they defined the critical salt concentration (CSC) to inhibit clay swelling in the samples studied with different salt combinations, such as 0.5 M for NaCl, 0.4 M for KCl but only 0.2 M for CaCl2 (Amorim et al., 2007). Nanoparticles can effectively mitigate formation damage caused by fine particles clogging pore-throats through enhancing attractive forces among fine particles and mineral grains (Arab and Pourafshary, 2013). Yuan et al. (2018)introduced a series of analytical models to characterize the mutual interactions among nanoparticle/fines transport in porous media and justified the positive effects of nanoparticles treatment (both pre-flush and co-injection) to control fines migration (Yuan et al., 2018).
(2) Wettability alteration, ion exchange, pH increase and dissolution of organic components
Wettability alteration has been widely recognized as the primary mechanism to enhance oil recovery with LSWF. The wettability alteration in sandstone rocks depends heavily on the presence of clay minerals, composition of oil and formation water and the salinity levels of injected water (Alagic and Skauge, 2010). The enhanced negative charges for both oil-brine and rock-brine interfaces improves the stability of the adsorbed water film and water-water state associated with the rock surface. It does this by increasing the repulsive forces in the double-layer, which is referred to as double-layer expansion effects (Nasralla and Nasr-El-Din, 2012). At the nano-scale, a decrease of adhesion forcebetween quartz grains and carboxylic acid is associated with the decrease of water salinity during LSWF improved oil recovery (Hassenkam et al., 2012). LSFW is also confirmed as an effective approach to enhance oil recovery in carbonate reservoirs as a result of wettability alterations, impacted by the concentration of calcium and magnesium ions in the presence of sulfate (the divalent ions: Ca2+, Mg2+, SO42−) and the salinity level of the injected water (Hamouda and Gupta, 2017; Zhang et al., 2006). However, as a negative effect, wettability alteration in carbonate rocks can also lead to CaSO4 precipitation that damages reservoir permeability and well injectivity (Zhang et al., 2007). In addition, the performance of enhanced oil recovery can in some cases be reduced by the discontinuity of oil throughout the reservoir (i.e., isolated pockets of residual oil) in case of LSWF applied in the tertiary recovery mode rather than the second recovery mode.
The increase of pH eases desorption of both acidic and basic materials, which is caused by the induced interactions among brine and rocks to compensate for the loss of cations, caused by the replacement of Ca2+ by H+ during LSWF (Austard et al., 2010). The loss of thermodynamic equilibrium among water, oil and rock can also result in the phenomenon of “salt-in” which enhances the solubility of organic components. Doust et al. (2009) confirmed the effectiveness of decreasing water salinity below the critical ionic strength in stimulating an increase in the solubility of organic components into flowing aqueous phase, which is commonly referred to as “salt-in” (Doust et al., 2009). In addition, analogous to surfactant flooding, low salinity water can generate surfactants in the environment of low salinity and high temperature. As a result, the formation of oil-in-water emulsions can improve the mobility control of the oil phase by increasing the viscosity of aqueous phase. The interface phenomenon may also result in phase trapping in some low-permeability reservoirs due to high capillary forces, which will impair the oil recovery performance of LSWF in such circumstances.
2.1.2. Potential to combine LSW with other EOR techniques to minimize damage effects
The synergistic benefits of combining various EOR techniques are well documented. Hence, it is often attractive to combine LSWF with other EOR techniques in order to minimize the negative effects and enhance positive consequence derived from such combinations of methods.
Discontinuous oil in the reservoir is more likely to be trapped in the reservoir at conditions of high capillary pressure due to phase trapping. LSWF combined with surfactant flooding can reduce the capillary pressure in the swept reservoir, thereby reducing the isolated pockets of trapped oil. Skauge et al. (2011) confirmed that surfactant-LSWF flooding performed better than that of surfactants and low-salinity water methods applied in isolation, in terms of reduction of IFT and capillary number (Skauge et al., 2011).
Dang et al. (2016) (Dang et al., 2016) identified that applied in secondary-recovery mode LSWF followed by LSWF combined with water-alternating-CO2 flooding achieves better performance than high-salinity WAG, or continuous LSWF and CO2 flooding applied on a standalone basis. This improvement is considered to be due to several synergistic effects of the combined method, including, solubility of CO2 in various water-salinity-dissolution states, ion exchange, carbonate minerals and clay distribution, and wettability alteration.
The motivation for combining LSWF with polymer flooding is that the introduction of larger molecular-weight, water-soluble polymers into injection water increases the viscosity of the displacing fluid and improves oil displacement and mobility control. Shiran and Skauge (2013) evaluated the synergistic effects on residual oil mobilization and final oil recovery when combining LSWF with polymer injection in both secondary and tertiary modes (Shiran and Skauge, 2013).
Fines migration induced by LSWF can in some reservoirs improve mobility control by diverting the injected water from high-permeability channels into low-permeability areas. However, the straining effects of fines typically lead to significant damage to the formation's permeability and result in a decline of well injectivity. This is particularly so in the near-wellbore region of a radially flowing reservoir fluid system with very severe fines migration problems induced by high fluid flow rates. In order to take advantage of fines migration in the deep reservoir zones away from injection wellbores it is necessary to prevent, or at least minimize, the problems of fines migration in near-wellbore region. Yuan and Moghanloo (2018) introduced nanofluid slugs with the objectives of: 1) retaining fines in the near-wellbore zones during LSWF, and, 2) improving of sweep efficiency for layered heterogeneous reservoirs. That work established that the treatment of the injection wells with nanofluid slugs can help mitigate the loss of injection pressure, but also increased the risk of accelerating the breakthrough of injected water (Yuan and Moghaloo, 2018).
2.2. Formation damage by chemical EOR techniques
Among various techniques of EOR, chemical enhanced oil recovery (CEOR) has drawn of great interest by oil companies and academics over the past decade or so. CEOR involves injecting chemical agents into oil reservoirs in isolation or in various combinations. CEOR methods include: surfactant flooding (S), alkaline flooding (A), polymer flooding (P); binary combination chemical floodinginvolving alkaline-surfactant (AS), or alkaline-polymer (AP), or Surfactant-polymer (SP); and, tertiary combination chemical flooding involving alkaline-surfactant-polymer (ASP) combination. However, despite the excellent potential of CEOR to enhance oil recovery, the formation damage itself typically induces can not only impair the oil recovery performance, but also bring additional technical and cost challenges to oilfields facilities and operations (Dai et al., 2018).
2.2.1. Surfactant flooding
The performance of surfactants depends on the interaction efficiency between crude oil and brine, reservoir conditions, and other stringent requirements associated with the specific surfactants injected, such as low retention, compatibility, and thermal and aqueous stability. Hence, the associated formation damage mechanisms with surfactant flooding are dominated by:
(1) Wettability alteration and water-in-oil emulsion blockage
Surfactants can be extensively emulsified into oil or water causing a decrease in the interfacial tension (IFT) of the emulsified fluid. This, in turn, leads to dispersion and scaling off of the oil from rock surfaces, causing the surfaces of rock grains to become more water-wet. The formation of oil-in-water emulsion, on one hand, can improve the mobility control and oil productivity performance of the reservoir by increasing the viscosity of aqueous phase. However, can also sometimes lead to severe blockage of the pore throats by emulsion, which impairs the formation's permeability, especially in low-permeability reservoirs (Feng et al., 2011). On the other hand, the formation water emulsions in oil (by mixing surfactant with crude oil), such as in conditions of high-salinity, increases the viscosity of the emulsified fluid in comparison to clean oil. Such changes impair the flowing efficiency of oil towards the wellbore (Xu et al., 2011).
(2) Surfactant precipitation and phase trapping
The contact of anionic surfactant with cationic fluids and minerals in the formation results in the loss and precipitation of surfactant (Stellner and Scamehorn, 1986). Sulfonate precipitates, such as calcium sulfonate and magnesium sulfonate, can be generated by the incompatibility of petroleum with the multivalent cations in both formation water and clays to form. This results in the loss of surfactants and the blockage of the pore-throat system, thereby impairing the performance of surfactant flooding. Additionally, the instability and separation between the oleic and aqueous phases may result in phase trapping caused by the multi-phase-fluids transport with high capillary forces (Nelson and Pope, 1978). The precipitation of surfactants is controlled by the ionic strength, degree of mineralization, temperature and pH value in a formation (Martin et al., 1985). Hence, the mitigation of surfactants precipitation can be achieved by increasing fluid temperatures, increasing pH values, and decreasing the degree of mineralization. For example, by reducing the contents of Ca2+ and Mg2+ in the reservoir fluids precipitation of calcium sulfonate and magnesium sulfonate can be prevented or significantly reduced.
(3) Excessive adsorption and retention of surfactants
A primary objective of surfactant EOR is to achieve appropriate levels of surfactant adsorption onto the rock grains. However, excessive adsorption of surfactants can occur in certain conditions which typically results in permeability damage in sandstone reservoirs (Panga et al., 2006). The excessive adsorption or retention of surfactants by phase trapping (Hirasaki et al., 2011) tends to lead to a decrease of porosity and permeability, and lowers the efficiency of surfactant adsorption along the oil-water interfaces, and limits enhancements in oil recovery. Decreasing fluid salinity and introducing alcohol-based methanol into water-based surfactant solutions are recommended to alleviate the excessive adsorption, aggregations and precipitation of surfactants.
2.2.2. Polymer flooding
The primary mechanism involved in polymer flooding is the increase in viscosity of the displacing phase, which improves mobility control for further oil recovery. However, in cases of harsh reservoir conditions, such as high salinity fluids, the presence of calcium, high temperatures and long injection times, formation damage often occurs. Problems and limitations commonly arise with water-soluble polymers, including decrease in the polymer's thickening capability and polymer flocculation (I. E. A, 2015). Hence, it is important to identify potential formation damage issues associated with specific reservoirs before initiating polymer flooding. These tend to be:
(1) Polymer retention and particle plugging
Appropriate retention of polymers can reduce the water-phase permeability, which is beneficial to decrease the mobility ratio and improve sweep efficiency. However, the excessive adsorption and even plugging of the pore throats with polymer leads to the damage of rock porosity and permeability. Three types of mechanisms of polymer retention exist, including physical adsorption (Tang et al., 2001), mechanical trapping (Garti and Zour, 1997), and hydraulic retention (Bhardwaj et al., 2007). Adsorption retention causes the polymer molecules to accumulate on the surface of rock grains by physical interactions, such as electrostatic interactions and hydrogen bonding with the hydroxyl groups present at the surfaces of the rock grains. Mechanical trapping refers to the trapping of polymer within the smaller-diameter pore throats mimicking particle filtration behavior. The retention of polymer can also be exaggerated by the alteration of fluid-flow directions or increases in fluid-flow velocity, which is called “hydraulic retention”. Dai et al. (2017) (Dai et al., 2018), proposed the following strategies to offset problems of excessive polymer retention: decreasing the degree of mineralization, utilizing appropriate low temperatures and molecular weights of injected fluids, applying optimal polymer concentrations and injection rates.
(2) Inorganic precipitation and organic deposition
Cations of inorganic salt particles with strong electro-philicity can form ionic pairs with the carboxyl groups on the polymer molecular chains, which reduces the electrostatic repulsive force among polymers. As a result, the contraction of polymer coils and generation of inorganic precipitation is likely to occur (Yan et al., 2000). In addition, ferric ions (Fe3+) in the formation water tend to establish cross-linking with polymers to generate hydrogels that block the reservoirs (Fletcher et al., 1992). The decrease of formation temperature by the injected fluids is also likely to accelerate inorganic precipitation that tends to block the formation. The commonly used additives of deoxidants such as Na2SO3 and NaHSO3 react with cations in the formation water to generate inorganic precipitates. The paraffin molecules in crude oil can precipitate out as waxes as the formation temperature is decreased by the injected polymers. Water-wet rocks are prone to enhance the adsorption and precipitation of polymer molecules. The adsorption of polymers in the smaller pores will generate resistance to the flowing capacity of fluids. In addition, the mitigation of fines or sands particle lifting into the formation fluid is achieved in part by the adsorption of polymer onto clay particles (Borchardt, 1989). Formation damage caused by polymer flooding can be at least partly mitigated by: narrowing the temperature gap between injected fluids and formation fluids, screening out the suspended particles in polymer solution prior to the injection, and optimizing the mixture of selected additives with the polymer to prevent incompatibility with the in-situ formation fluids.
Before a polymer is injected into a specific reservoir for improved oil recovery, it should be subjected to a number of laboratory screening tests, including polymer injectivity, adsorption and brine compatibility tests and to establish its microbial, mechanical and chemical degradation characteristics.
2.2.3. Alkaline flooding
Alkaline flooding, also referred to as caustic flooding, injects alkaline solutions, such as sodium orthosilicate, sodium carbonate and sodium hydroxide, to displace the formation fluids. The primary EOR mechanism at work is the generation of soap and surfactants by the reactions between alkaline and crude oil (Liu, 2006). However, the incompatibility of alkaline-formation water with alkaline-rocks leads to the following severe formation damage often associated with alkaline flooding EOR:
(1) Migration and blockage of fine particles
Alkaline can dissolve clays and other minerals. Sometimes, the dissolution may increase formation permeability. However, typically fines migration and blockage ensue damaging reservoir permeability. Under alkaline conditions, the interactions between clays and the surfaces of the mineral grains are also disturbed resulting in fines migration and/or clay swelling through changes in the electrostatic forces among particles (Assef et al., 2014). Moreover, the formation of oil emulsions with different sizes of water phase at different alkaline concentrations can lead to the blockage of fluid flow paths and reduced reservoir permeability. As alkaline concentration increases, the type of emulsion evolves to become water-in-oil emulsion (Fig. 5), which impairs the flowing efficiency of oil by increasing the viscosity of oil phase (Ge et al., 2012).
 Fig. 5. Evolution from oil-in-water emulsion to water-in-oil emulsion with the increase of alkaline concentration [revised after 66].
Fig. 5. Evolution from oil-in-water emulsion to water-in-oil emulsion with the increase of alkaline concentration [revised after 66].(2) Carbonate scale, hydroxyl scale, silicate scale, or sulfate scale
The incompatibility of alkaline conditions with formation water depends on formation water composition and temperature (Moghadasi et al., 2004). Formation water typically includes cations of K+, Ca2+, Fe2+, Mg2+, F3+, and Na+, and anions of HCO3−, CO32−, SO42−, and Cl−. As alkaline concentration increases, CO32−, OH−, and SiO32− concentrations become elevated, generating carbonate scale, hydroxyl scale, and silicate scale (Fig. 6), depending upon the availability of metal ions in the formation water and rock minerals (Sheng, 2011). As reservoir temperature increases, the solubility of inorganic scale would decrease potentially leading to precipitation (Sheng, 2016). The precipitation and deposition of all those scales lead to significant reduction of permeability, and decreases the efficiency of alkaline flooding. It can also lead to flow line restrictions, choke and safety valve failures, pump wear, and corrosion beneath the scale in pipework and surface facilities. In the Wilming field, California, after the deposition of CaCO3 and Mg2SiO4 scales, no acid treatments could remove them, leading to the suspension of the alkaline flooding project. If injected seawater is rich in SO42−, sulfate scale is also likely to be generated. Sulfate scales are very difficult and expensive to remove, because they are usually acid insoluble. Lakatos et al. (2007) evaluated the performance of the scale-mitigation additive polyamino carboxylic acids to dissolve both barium sulfate and calcium sulfate, including Dioxaoctamethylene dinitrilo teraacetic acid (DOCTA), Hydroxyethyle)ethylenediamin triacetate acid (HEDTA), Triethyleneteramin hexaaxetic acid (TTHA), Nitrilotriacetic acid (NTA), Ethylenediamin tertaacetic acid (EDTA), Cyclohexylene dinitriloacetic acid (DCTA), and Diethylenetriamin pentaacetic acid (DTPA). Sodium metaborate was proposed as a weaker alkali to replace strong alkalis, and/or the replacement of inorganic alkalis by organic alkalis, to reduce formation damage by scales (Flaaten et al., 2009). Sometimes it is possible to take advantage of scale precipitation by injected alkaline solutions to improve sweep efficiency by decreasing the permeability in high-permeability zones, and diverting the injected water and flowing fluids into lower-permeability pathways (Sarem, 1974).
 Fig. 6. Inorganic scale formation with alkaline injection.
Fig. 6. Inorganic scale formation with alkaline injection.2.2.4. Binary combinations of chemical flooding
In order to achieve the benefits of combination chemical flooding, it is essential that the chemicals involved are compatible and stable in their mixture. If they are not severe formation damage is likely to ensue.
(1) Alkaline-surfactant flooding (AS)
Addition of surfactant makes alkaline flooding more efficient. Rudin et al. (1994) found that the addition of surfactants into alkaline injection fluids further decreased the equilibrium interfacial tension and created emulsions with higher interfacial resistance (Rudin et al., 1994). The more stable emulsions can carry more oil in flowing water, but are also prone to block the pore throats with the accumulation of emulsions. The adsorption of sulfonate and surfactants onto kaolinite can be decreased with the addition of alkaline as the charges of the mineral surfaces become more negative (Hanna and Somasundaran, 1977). The decreased adsorption of surfactants is likely to improve oil displacement. However, the detachment of sulfonate can enhance damage to the reservoirs.
(2) Alkaline-polymer flooding (AP)
One major problem of alkaline flooding is its lack of mobility control, due to the high mobility ratio of displaced phase to displacing phase. The addition of polymer has the potential to solve this issue. However, as alkaline concentrations increase, the polymer hydrolysis and polymer viscosity reduces, causing the sodium ions in polymer solution to neutralize the carboxyl groups resulting in polymer coil up (Green and Willhite, 1998). In the alkaline condition, the adsorption of polymer can be reduced by the increasing negative charge of the mineral surfaces (Krumrine and Falcone, 1983).
(3) Surfactant-polymer flooding (SP)
The addition of surfactants into polymer flooding can further reduce the interfacial tension, and surfactants can form chelation structures with polymers to increase the viscosity of the displacing phase. As sacrificial agents, polymers react with the divalent ions on the mineral surfaces, which reduces the loss of surfactants by adsorption, and the precipitation of surfactants which enhances the stability of the emulsions (Cui et al., 2011). However, under certain conditions, the emulsification of surfactants into the oil phase may restrict the flowing efficiency of oil into the wellbores, as a negative effect. Separation of the polymer-rich phase and surfactant-phase may occur, which is not desirable for the movement of fluids in reservoirs (Pope et al., 1982).
2.2.5. Alkaline-surfactant-polymer flooding (ASP)
ASP systems mix with the formation water and oil through physico-chemical reactions, which leads to the corrosion and dissolution of minerals in reservoirs. Kalwar and Elraies (2013) reported the fluid-fluid incompatibility and scale precipitation for an ASP case with total salinity of 59,940 ppm and 2762 ppm of Ca2+ and Mg2+, and the success of preventing scale precipitation by the addition of acrylic acid (Kalwar and Elraies, 2013). Liu et al. (2007) described a field example in which scale production consisted of a mixture of silicate scale, carbonate scale and organic matter (Liu et al., 2007). For ASP flooding, chemical anti-scaling techniques and physical anti-scaling techniques have been proposed to mitigate scale problems (Wang et al., 2013; Xu et al., 2001; Li et al., 2009).
2.3. Formation damage by thermal techniques for heavy oil recovery
About 70% of world oil reserves are heavy crude oil (Giacchetta et al., 2015), which tend to have low primary recovery factors. The thermal recovery of heavy oil mainly includes, in-situ combustion (ISC), steam flooding (SF), cyclic steam stimulation (CSS), and steam-assisted-gravity-drainage (SAGD) techniques. Most heavy oil reservoirs exist at relatively shallow depths and reside in unconsolidated sandstone formations. The poor consolidation makes heavy oil reservoir more susceptible to formation damage. Thermal recovery methods can further exacerbate the extent of formation damage by the alteration of the prevailing physical-chemical-thermal system in the formation. The associated formation damage mechanisms with thermal recovery in heavy oil mainly include (Chen and Chen, 2018), sands and fines migration, clay swelling and deflocculation, organic precipitation, wettability alteration, phase trapping, foam and emulsion formation, mineral transformation, dissolution and precipitation, and types of biologically induced damage.
2.3.1. Mechanical formation damage during the thermal recovery of heavy oil
(1) Migration of fine particles in both sandstone and carbonate reservoirs
Most of heavy oils are located in poorly consolidated sandstone reservoirs which are typically associated with large amounts of mobile fines and particles, such as in-situ kaolinite, detrital rock fragments, pyrobitumen and other mobile particulates. In carbonate formations containing heavy oil, the migration of dolomite or carbonate fines, and pyrobitumen is also likely to occur (Bennion et al., 1995). Migration of fines is affected by: pH, temperature, rock wettability, clay mineral composition, flowing rates, and fluid salinities. The migration of fines and particles can lead to severe damage of permeability through blockage and plugging of pore throats. In heavy oil, the abrupt changes of formation temperature, mineral composition, and fluid properties is likely to exaggerate the problems of fines migration. Yuan et al. (2018) described several approaches to potentially mitigate fines migration, including nanofluids and commercial clay stabilizer CS-38 (Yuan et al., 2018).
(2) Inevitable amounts of sands production along with production of bitumen and heavy oil
Changes to the in-situ effective stress make it inevitable that formation sand particles and fines are produced along with the heavy oil, especially in the more poorly consolidated reservoirs. The hydrodynamic forces exerted by the flowing fluids during production enhances the movement of clay particles (Muecke, 1979). The excessive contact of sand and fines with high-temperature steam can also promote the migration of sands and fines (Tague, 2000). Gravel packs and frac packs have been intensively investigated to prevent sand production (Ghalambor et al., 2009). However, the plugging of fines into gravel packing can also lead to a significant decrease of permeability and well productivity (Bennion et al., 1994). Huang et al. (2008) investigated the effectiveness of nanofluids to control fines migration and plugging of Frac-packs (Huang et al., 2008).
(3) Water-phase trapping, gas-in-oil foam and water-in-oil emulsions
The invasion of water-based fluids into oil-bearing formations can result in adverse damage to the relative permeability of oil, which is referred to as the water-phase trapping phenomenon (Bennion et al., 1996). Such damage is usually not permanent, and can be cleaned up over time if a sufficient pressure gradient is established to push the trapped phase through the pore throats. Suspended “skim” oil in the injection water can also have negative effects on the relative permeability, as it is typically difficult to completely remove heavy oil from the produced water (Bennion et al., 1998).
Foamy oil emulsions can be generated under conditions of high fluid viscosity, and strong interfacial tension between gas and heavy oil, and once the reservoir pressure has fallen below the bubble-point pressure. Although the swelling of heavy oil is beneficial to oil production, the enhancement of a foamy oil solution in the gas phase can increase its viscosity and the critical gas saturationrequired for mobility, which can lead to a decline in well productivity (Chen et al., 2015). The stable interfaces between the oil and gas phases can help the transport small fines particles, which may exaggerate the problem of fines migration, sands production, and damage to a formation's permeability (Simith, 1988). Water-in-oil emulsion can also be formed in heavy oil, significantly increasing the viscosity of the oil phase, which impairs the flowing efficiency of oil towards and into wellbores (Czarnecki and Moran, 2005).
2.3.2. Chemical damage during thermal recovery in heavy oil reservoirs
(1) Clay swelling and deflocculation
Most heavy oil reservoirs contain problematic clay minerals, including the swelling clay montmorillonite and the migrating clays illite and kaolinite. Clay swelling and deflocculation are caused by an abrupt change of brine chemistry, such as fresh or low-salinity water, which lead to pore constriction, bridging and blockage to damage formation permeability and well productivity (Zhang et al., 2015).
(2) Wax and asphaltene precipitation, accumulation and deposition
Wax and asphaltene problems in heavy oil include (Permadi et al., 2012): 1) asphaltene precipitation as solid particles once destabilized from crude oil due to either reductions of temperature and pressure or by contact with precipitation agents, such as unsequestered hydrochloric acid, LPG and carbon dioxide gas; 2) the formation of crystalline wax caused by the reduction in temperature. Asphaltene precipitation refers to the process where asphaltene suspend as a separate phase in the crude oil, in small quantities and in small particle sizes, can no longer be supported by the fluid phase. Precipitated asphaltene particles tend to aggregate together forming larger particles, referred to as “flocs”, which then attach onto various surfaces (i.e., mineral grains, pipework, within surface facilities) (Alian et al., 2011).